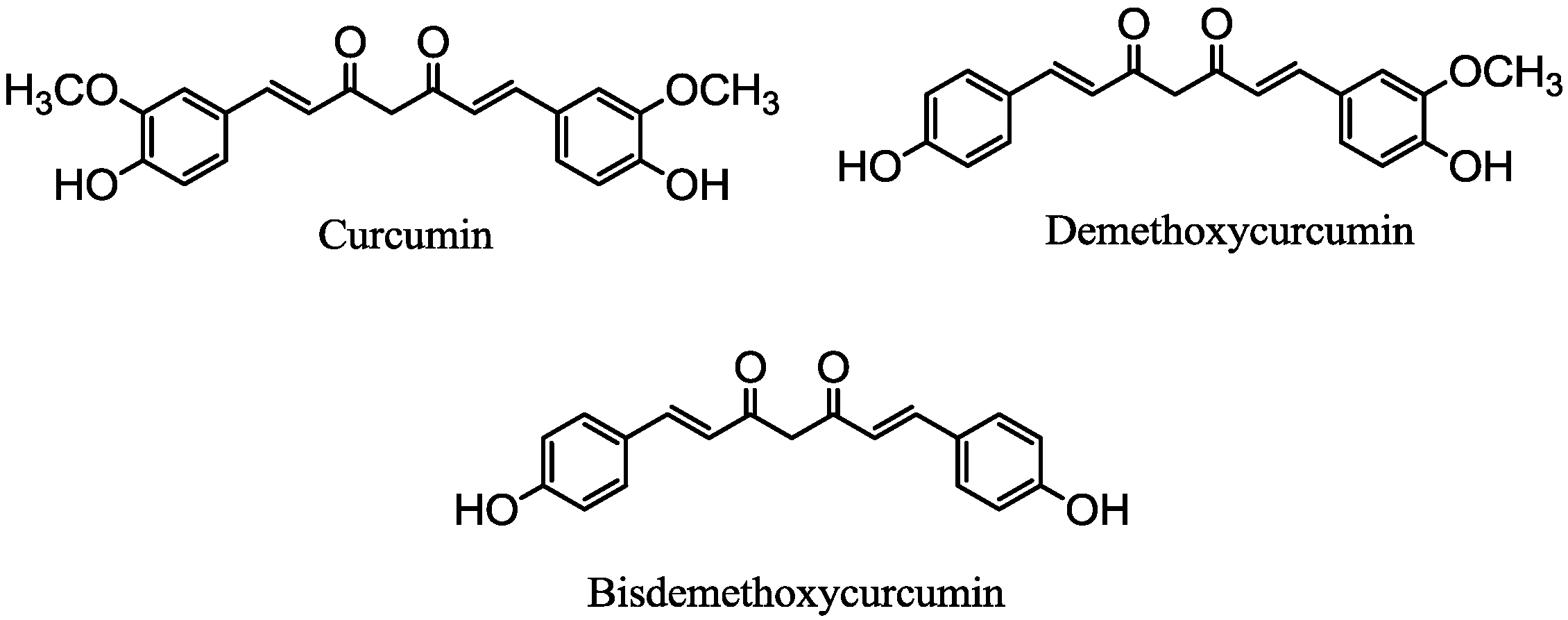
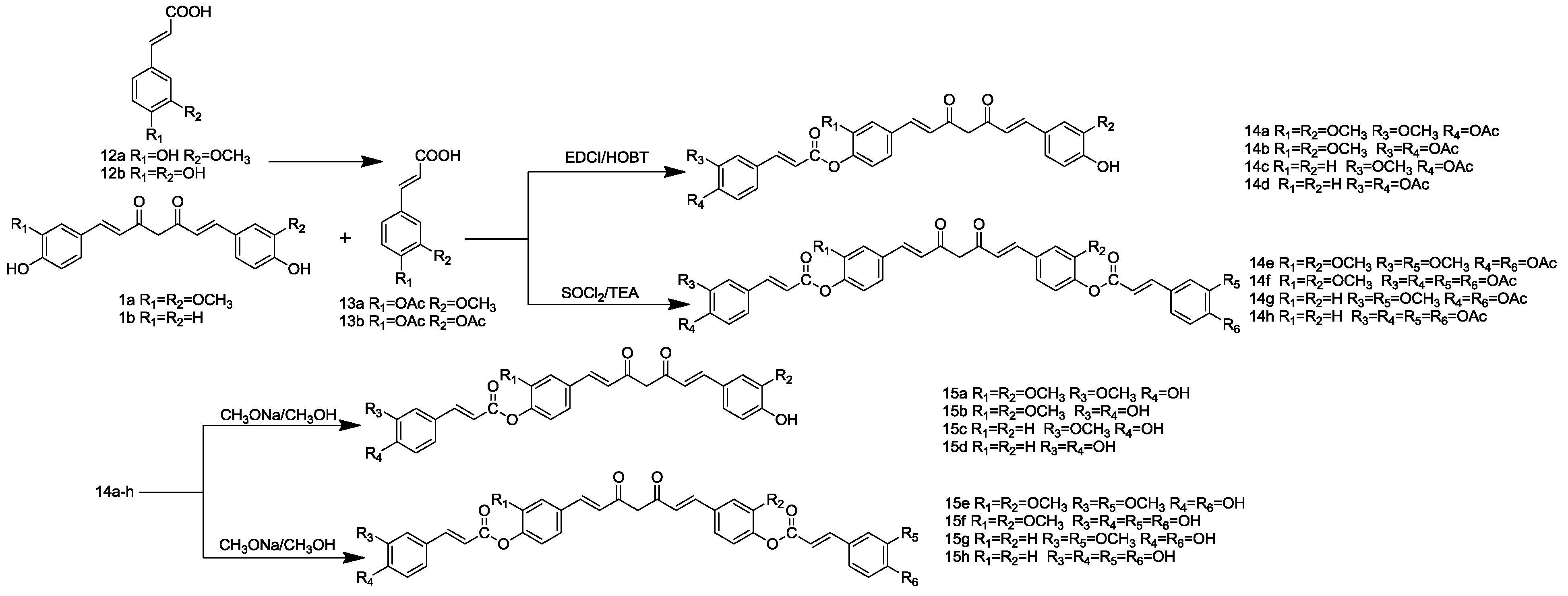
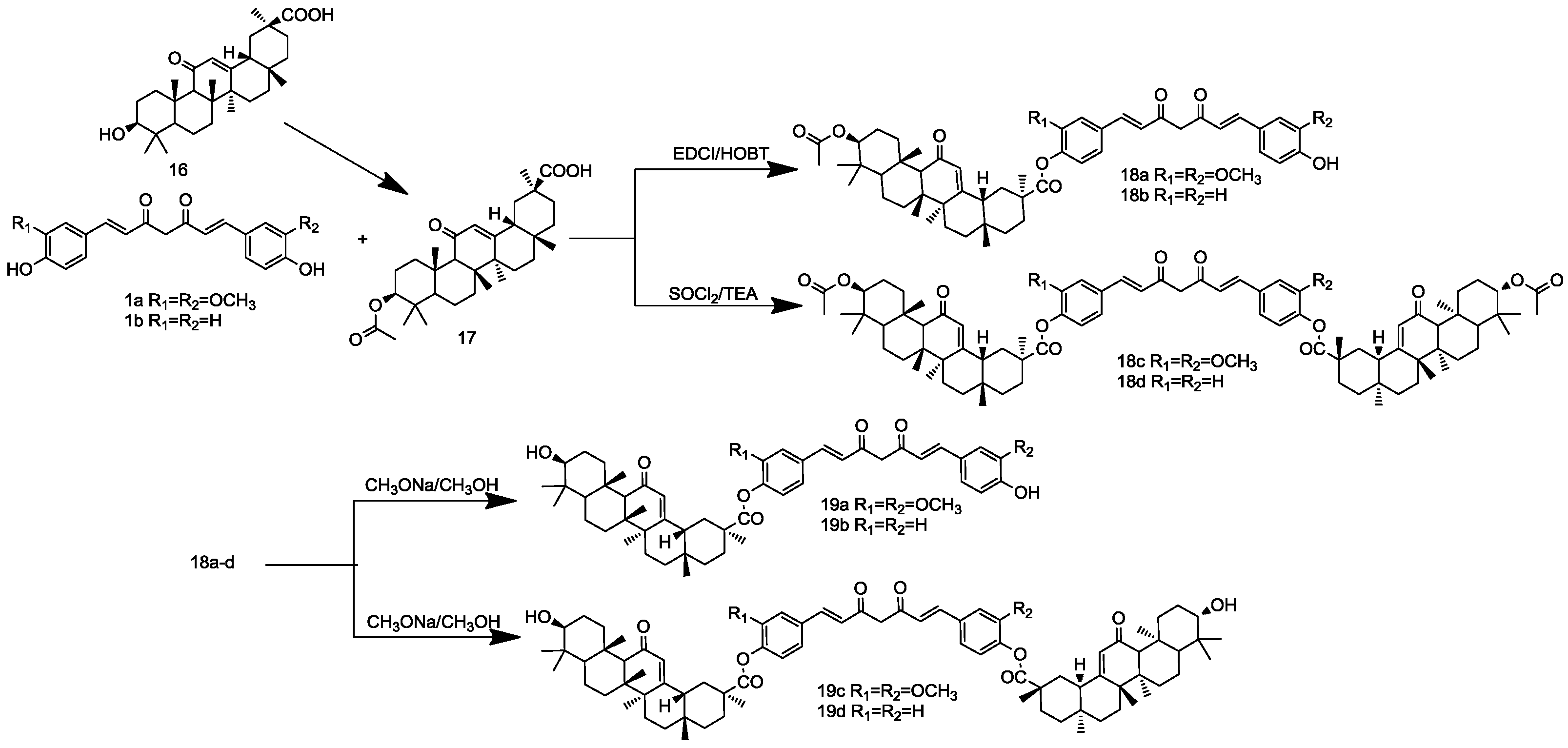
3.3. Procedure of the Preparation of Selenomethionine-Substituted Curcuminoids and Methionine-Substituted Curcuminoids
According to the procedure previously reported [
33], selenomethionine (8) was prepared. Methionine (2) was used as the starting material in a three-pot, seven-step procedure to obtain selenomethionine in 47% yield. In a 100-mL round-bottomed flask, selenomethionine or methionine was dissolved in water and NaHCO
3 (3 eq.) was added. A solution of (BOC)
2O (1.5 eq.) in dioxane was added to this mixture. The reaction mixture was stirred at 25 °C overnight. The reaction mixture was washed with ethyl acetate. The resulting aqueous layer was acidified to pH 2 with concentrated hydrochloric acid and extracted with ethyl acetate. The combined organic extracts were dried over Na
2SO
4, filtered and concentrated
in vacuo to give
9a or
9b as colorless gums.
3.3.1. General Procedure for the Synthesis of 10a–d
To a solution of 9a or 9b (10 mmol) in chloroform (50 mL) was added EDCI (12 mmol), HOBT (12 mmol) and DIEA (1 mL). After stirring at 0 °C for 1 h, a solution of CCM (10 mmol) or BCM (10 mmol) in chloroform (40 mL) was added dropwise to the reaction mixture, which was stirred overnight at room temperature. After completion of the reaction, as indicated by TLC, the mixture was washed with hydrochloric acid (1 M) and brine. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. Purification by column chromatography over polyamide gel (1:10, CH3OH/CCl4) gave 10a–d as yellow solids.
Curcumin-mono-N-(tert-butoxycarbonyl)-methionine (10a). Yield 48%. m.p. 165–166 °C. 1H-NMR (400 MHz, CDCl3): δ 7.63 (d, J = 5.6 Hz, 1H), 7.59 (d, J = 5.6 Hz, 1H), 7.21–7.07 (m, 4H), 7.06 (d, J = 1.8 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.55 (d, J = 15.8 Hz, 1H), 6.49 (d, J = 15.8 Hz, 1H), 5.83 (s, 1H), 5.21 (d, J = 8.0 Hz, 1H), 4.71 (s, 1H), 3.95 (s, 3H), 3.87 (s, 3H), 2.69 (t, J = 7.8 Hz, 2H), 2.34 (m, 2H), 2.15 (s, 3H), 1.47 (s, 9H). 13C-NMR (CDCl3): δ 198.9, 173.4, 155.2, 151.4, 149.5, 147.9, 142.8, 141.1, 130.6, 127.6, 122.2, 121.9, 116.8, 112.5, 110.9, 79.5, 56.5, 55.8, 51.9, 30.9, 29.7, 28.4. ESI-MS (m/z): (M−H)− = 598.31. Anal. Calcd. for C31H37NO9S: C, 62.09; H, 6.22; N, 2.34%. Found: C, 62.12; H, 6.24; N, 2.38%.
Curcumin-mono-N-(tert-butoxycarbonyl)-selenomethionine (10b). Yield 52%. m.p. 172–173 °C. 1H-NMR (400 MHz, CDCl3): δ 7.63 (d, J = 6.0 Hz, 1H), 7.59 (d, J = 6.0 Hz, 1H), 7.22–7.07 (m, 4H), 7.06 (d, J = 1.6 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.56 (d, J = 15.8 Hz, 1H), 6.50 (d, J = 15.8 Hz, 1H), 5.83 (s, 1H), 5.20 (d, J = 8.1 Hz, 1H), 4.71 (d, J = 5.0 Hz, 1H), 3.95 (s, 3H), 3.87 (s, 3H), 2.75–2.67 (m, 2H), 2.41–2.21 (m, 2H), 2.05 (s, 3H), 1.47 (s, 9H). 13C-NMR (CDCl3): δ 198.5, 172.4, 155.9, 152.3, 149.5, 148.6, 142.6, 142.5, 133.8, 131.4, 125.6, 124.3, 122.9, 121.9, 116.8, 111.9, 110.9, 56.1, 55.8, 51.9, 28.4, 25.1. ESI-MS (m/z): (M−H)− = 646.08. Anal. Calcd. for C31H37NO9Se: C, 57.58; H, 5.77; N, 2.17%. Found: C, 57.50; H, 5.71; N, 2.12%.
Bisdesmethoxycurcumin-mono-N-(tert-butoxycarbonyl)-methionine (10c). Yield 55%, m.p. 183–184 °C. 1H-NMR (400 MHz, CDCl3): δ 7.61 (d, J = 12.9 Hz, 2H), 7.54 (d, J = 8.3 Hz, 2H), 7.43 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 8.5 Hz, 2H), 6.85 (d, J = 7.8 Hz, 2H), 6.56 (d, J = 15.8 Hz, 1H), 6.48 (d, J = 15.8 Hz, 1H), 5.80 (s, 1H), 5.24 (d, J = 7.8 Hz, 1H), 4.67 (s, 1H), 2.66 (t, J = 8.1 Hz, 2H),2.25 (m, 2H) 2.14 (s, 3H), 1.48 (s, 9H). 13C-NMR (CDCl3): δ 198.5, 171.4, 158.8, 153.9, 150.2, 134.8, 131.9, 130.8, 130.2, 129.6, 121.6, 116.8, 79.2, 56.8, 51.9, 30.6, 28.6, 15.6. ESI-MS (m/z): (M−H)− = 538.33. Anal. Calcd. for C29H33NO7S: C, 64.54; H, 6.16; N, 2.60%. Found: C, 64.50; H, 6.11; N, 2.65%.
Bisdemethoxycurcumin-mono-N-(tert-butoxycarbonyl)-selenomethionine (10d). Yield 60%. m.p. 192–193 °C. 1H-NMR (400 MHz, CDCl3): δ 7.61 (d, J = 14.9 Hz, 2H), 7.54 (d, J = 8.3 Hz, 2H), 7.43 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 7.6 Hz, 2H), 6.85 (d, J = 5.5 Hz, 2H), 6.49 (d, J = 13.5 Hz, 2H), 5.80 (s, 1H), 5.18 (d, J = 8.2 Hz, 1H), 4.66 (s, 1H), 2.66 (t, 2H), 2.41–2.31 (m, 2H), 2.03 (s, 3H), 1.48 (s, 9H). 13C-NMR (CDCl3): δ 198.6, 170.5, 155.6, 150.4, 135.8, 131.6, 130.9, 130.4, 128.6, 122.5, 115.8, 79.4, 60.2, 51.8, 28.6, 25.1, 22.4, 9.5. ESI-MS (m/z): (M−H)− = 586.22. Anal. Calcd. for C29H33NO7Se: C, 59.38; H, 5.67; N, 2.39%. Found: C, 59.32; H, 5.61; N, 2.33.
3.3.2. General Procedure for the Synthesis of 10e–h
To a stirred solution of 9a or 9b (10 mmol) in anhydrous chloroform (50 mL) was added a solution of CCM or BCM (3 mmol) in chloroform (40 mL) dropwise. After 15 min, the reaction mixture was basified with triethylamine (1 mL) and stirred for 20 min, followed by the addition of DCC (3 mmol). The reaction mixture was stirred for 8 h and monitored by TLC. Upon completion of the reaction, 1, 3-dicyclohexylurea (DCU) was removed by filtration, and the filtrate was evaporated. The resulting residue was dissolved in chloroform (50 mL), washed consecutively with 1 M HCl (50 mL), 5% NaHCO3 solution (50 mL), brine (50 mL) and H2O (50 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. Purification by column chromatography over polyamide gel (1:10, CH3OH/CCl4) gave 10e–h as yellow solids.
Curcumin-di-N-(tert-butoxycarbonyl)-methionine (10e). Yield 50%. m.p. 187–188 °C. 1H-NMR (400 MHz, CDCl3): δ 7.64 (d, J = 15.8 Hz, 2H), 7.21–7.09 (m, 6H), 6.60 (d, J = 15.8 Hz, 2H), 5.89 (s, 1H), 5.25 (d, J = 7.7 Hz, 2H), 4.74 (d, J = 4.5 Hz, 2H), 3.89 (s, 6H), 2.71 (t, J = 7.6 Hz, 4H), 2.34 (m, 4H), 2.18 (s, 6H), 1.49 (s, 18H). 13C-NMR (CDCl3): δ 198.5, 170.3, 155.6, 151.2, 142.5, 141.3, 131.8, 130.6, 124.4, 121.9, 110.9, 79.6, 56.7, 55.9, 51.3, 30.2, 29.4, 28.2, 15.1. ESI-MS (m/z): (M−H)− = 829.33. Anal. Calcd. for: C41H54N2O12S2: C, 59.26; H, 6.55; N, 3.37%. Found: C, 59.21; H, 6.51; N, 3.32%.
Curcumin-di-N-(tert-butoxycarbonyl)-selenomethionine (10f). Yield 58%. m.p. 200–201 °C. 1H-NMR (400 MHz, CDCl3): δ 7.62 (d, J = 15.4 Hz, 2H), 7.24–6.81 (m, 6H), 6.58 (d, J = 14.6 Hz, 2H), 5.87 (s, 1H), 5.19 (d, J = 5.7 Hz, 2H), 4.71 (s, 2H), 3.87 (s, 6H), 2.70 (t, 4H), 2.29 (m, 4H), 2.05 (s, 6H), 1.47 (s, 18H). 13C-NMR (CDCl3): δ 198.9, 170.7, 155.9, 151.8, 143.2, 141.1, 131.8, 124.4, 121.9, 110.9, 60.1, 55.8, 51.9, 28.4, 25.1, 9.4. ESI-MS (m/z): (M−H)− = 926.08. Anal. Calcd. for: C41H54N2O12Se2: C, 51.58; H, 5.70; N, 3.17%. Found: C, 51.51; H, 5.73; N, 3.12%.
Bisdesmethoxycurcumin-di-N-(tert-butoxycarbonyl)-methionine (10g). Yield 65%. m.p. 207–208 °C. 1H-NMR (400 MHz, CDCl3): δ 7.64 (d, J = 15.8 Hz, 2H), 7.58 (d, J = 8.4 Hz, 4H), 7.16 (d, J = 8.4 Hz, 4H), 6.59 (d, J = 15.8 Hz, 2H), 5.84 (s, 1H), 5.20 (d, J = 6.2 Hz, 2H), 4.66 (s, 2H), 2.66 (t, 4H), 2.31 (m, 2H), 2.15 (s, 6H), 2.10(m, 2H), 1.47 (s, 18H). 13C-NMR (CDCl3): δ198.9, 170.2, 159.8, 150.7, 135.2, 130.6, 129.4, 121.7, 79.7, 56.9, 52.3, 31.2, 30.4, 28.6, 15.5. ESI-MS (m/z): (M−H)− = 769.5. Anal. Calcd. for: C39H50N2O10S2: C, 60.76; H, 6.54; N, 3.63%. Found: C, 60.71; H, 6.50; N, 3.67%.
Bisdesmethoxycurcumin-di-N-(tert-butoxycarbonyl)-selenomethionine (10h). Yield 62%. m.p. 214–215 °C. 1H-NMR (400 MHz, CDCl3): δ 7.65 (d, J = 15.8 Hz, 2H), 7.58 (d, J = 8.6 Hz, 4H), 7.16 (d, J = 8.6 Hz, 4H), 6.59 (d, J = 15.8 Hz, 2H), 5.84 (s, 1H), 5.15 (d, J = 7.0 Hz, 2H), 4.65 (s, 2H), 2.67 (t, J = 7.4 Hz, 4H), 2.44–2.29 (m, 4H), 2.34 (d, J = 18.5 Hz, 2H), 2.27–2.08 (m, 4H), 2.04 (s, 6H), 1.47 (s, 18H). 13C-NMR (CDCl3): δ 198.8, 170.2, 156.2, 150.7, 135.2, 130.5, 129.7, 122.2, 79.5, 60.3, 52.4, 28.7, 25.2, 22.3, 9.3. ESI-MS (m/z): (M−H)− = 863.3. Anal. Calcd. for C39H50N2O10Se2: C, 54.17; H, 5.83; N, 3.24%. Found: C, 54.11; H, 5.89; N, 3.20%.
3.3.3. General Procedure for the Synthesis of Compounds 11a–h
A solution of HCl/EA (4 mL, 4 M) in a 25-mL round-bottom flask equipped with a magnetic stir-bar was cooled by an ice-water bath under nitrogen. Compound (10a–h, 1 mmol) was added in one portion with stirring. The ice-bath was removed, and the mixture was stirred at 25 °C. After 30 min, TLC indicated that the reaction was completed. The product separated as a precipitate, as it was insoluble in EA.
Curcumin-mono-methionine (11a). Yield 60%. m.p. 60–61 °C. 1H-NMR (400 MHz, DMSO): δ 7.63 (d, J = 7.6 Hz, 1H), 7.59 (d, J = 7.9 Hz, 1H), 7.40–7.24 (m, 4H), 7.18 (d, J = 8.2 Hz, 1H), 7.00 (d, J = 16.0 Hz, 1H), 6.85 (d, J = 8.2 Hz, 1H), 6.81 (d, J = 15.9 Hz, 1H), 6.15 (s, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 2.78–2.71 (m, 2H), 2.28–2.23 (m, 2H), 2.12 (s, 3H). 13C-NMR (CDCl3): δ 198.7, 168.2, 151.7, 149.2, 147.5, 142.9, 141.4, 131.9, 130.5, 124.7, 122.9, 121.8, 117.2, 56.4, 52.3, 33.9, 29.7, 15.5. ESI-MS (m/z): (M−H)− = 498.17. Anal. Calcd. for C26H29NO7S: C, 62.51; H, 5.85; N, 2.80%. Found: C, 62.58; H, 5.80; N, 2.83%.
Curcumin-mono-selenomethionine (11b). Yield 65%. m.p. 69–70 °C. 1H-NMR (400 MHz, DMSO): δ 7.63 (d, J = 7.7 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.41–7.23 (m, 4H), 7.18 (d, J = 8.3 Hz, 1H), 7.01 (d, J = 16.0 Hz, 1H), 6.85 (d, J = 8.2 Hz, 1H), 6.81 (d, J = 15.8 Hz, 1H), 6.15 (s, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 2.78–2.69 (m, 2H), 2.33 (m, 2H), 2.02 (s, 3H). 13C-NMR (CDCl3): δ198.5, 167.8, 151.4, 149.2, 147.5, 142.9, 141.2, 131.7, 130.2, 123.1, 122.2, 120.8, 116.2, 55.8, 51.8, 32.8, 25.7, 9.4. ESI-MS (m/z): (M−H)− = 546.1. ESI-MS (m/z): (M−H)− = 546.1. Anal. Calcd. for C26H29NO7Se: C, 62.51; H, 5.85; N, 2.80%. Found: C, 62.58; H, 5.80; N, 2.83%.
Bisdesmethoxycurcumin-mono-methionine (11c). Yield 62%. m.p. 73–74 °C. 1H-NMR (400 MHz, DMSO): δ 7.84 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 8.7 Hz, 2H), 6.94 (d, J = 16.0 Hz, 1H), 6.85 (d, J = 8.6 Hz, 2H), 6.74 (d, J = 15.9 Hz, 1H), 6.14 (s, 1H), 4.40 (s, 2H), 2.69 (m, 2H), 2.37–2.21 (m, 2H), 2.11 (s, 3H). 13C-NMR (CDCl3): δ 198.9, 168.0, 157.4, 150.6, 135.1, 130.7, 130.5, 121.1, 115.2, 52.2, 34.1, 30.7, 15.4. ESI-MS (m/z): (M−H)− = 438.17. Anal. Calcd. for C24H25NO5S: C, 65.58; H, 5.73; N, 3.19%. Found: C, 65.53; H, 5.76; N, 3.21%.
Bisdesmethoxycurcumin-mono-selenomethionine (11d). Yield 65%. m.p. 77–78 °C. 1H-NMR (400 MHz, DMSO): δ 7.84 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.6 Hz, 2H), 6.95 (d, J = 15.9 Hz, 1H), 6.85 (d, J = 8.5 Hz, 2H), 6.74 (d, J = 15.9 Hz, 1H), 6.14 (s, 1H), 4.40 (s, 1H), 2.75 (t, J = 8.8 Hz, 2H), 2.38–2.28 (m, 2H), 2.01 (s, 3H). 13C-NMR (CDCl3): δ198.7, 168.5, 157.6, 150.8, 136.3, 130.8, 130.4, 121.3, 115.2, 55.5, 51.9, 34.1, 25.7, 9.4. ESI-MS (m/z): (M−H)− = 486.08. Anal. Calcd. for C24H25NO5Se: C, 59.26; H, 5.18; N, 2.88%. Found: C, 59.23; H, 5.16; N, 2.92%.
Curcumin-di-methionine (11e). Yield 70%. m.p. 82–83 °C. 1H-NMR (400 MHz, DMSO): δ 7.68 (d, J = 16.0 Hz, 2H), 7.60 (s, 2H), 7.41 (d, J = 7.9 Hz, 2H), 7.06 (d, J = 15.9 Hz, 2H), 6.24 (s, 1H), 4.44 (s, 2H), 3.87 (s, 6H), 2.82–2.69 (m, 4H), 2.32–2.24 (m, 4H), 2.12 (s, 6H). 13C-NMR (CDCl3): δ 197.7, 168.2, 151.6, 142.8, 141.3, 132.3, 130.6, 122.4, 120.3, 111.2, 56.5, 52.3, 33.9, 30.3, 15.7. ESI-MS (m/z): (M−H)− = 629.25. Anal. Calcd. for C31H38N2O8S2: C, 59.03; H, 6.07; N, 4.44%. Found: C, 59.07; H, 6.11; N, 4.45%.
Curcumin-di-selenomethionine (11f). Yield: 75%. m.p. 88–89 °C. 1H-NMR (400 MHz, DMSO): δ 7.68 (d, J = 15.9 Hz, 2H), 7.60 (s, 2H), 7.40 (d, J = 7.8 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H), 7.06 (d, J = 16.0 Hz, 2H), 6.23 (s, 1H), 4.44 (s, 2H), 3.87 (s, 6H), 2.80–2.69 (m, 4H), 2.35–2.26 (m, 4H), 2.02 (s, 6H). 13C-NMR (CDCl3): δ 198.2, 168.1, 151.1, 143.1, 141.2, 131.8, 130.5, 124.4, 121.3, 110.2, 56.5, 52.3, 25.3, 9.2. ESI-MS (m/z): (M–H)− = 725.17. Anal. Calcd. for C31H38N2O8Se: C, 51.39; H, 5.29; N, 3.87%. Found: C, 51.37; H, 5.25; N, 3.84%.
Bisdesmethoxycurcumin-di-methionine (11g). Yield 77%. M.p. 92–93 °C. 1H-NMR (400 MHz, DMSO): δ 7.86 (d, J = 8.5 Hz, 4H), 7.67 (d, J = 16.0 Hz, 2H), 7.35 (d, J = 8.5 Hz, 4H), 6.97 (d, J = 15.7 Hz, 2H), 6.18 (s, 1H), 4.40 (s, 2H), 2.78–2.69 (m, 4H), 2.27 (d, 4H), 2.11 (s, 6H). 13C-NMR (CDCl3): δ 198.8, 168.3, 150.5, 134.8, 130.8, 121.7, 121.8, 52.5, 33.8, 29.3, 15.4. ESI-MS (m/z): (M−H)− = 570.25. Anal. Calcd. for C29H34N2O6S2: C, 61.03; H, 6.00; N, 4.91%. Found: C, 61.11; H, 6.07; N, 4.92%.
Bisdesmethoxycurcumin-di-selenomethionine (11h). Yield 70%. m.p. 97–98 °C. 1H-NMR (400 MHz, DMSO): δ 7.86 (d, J = 8.6 Hz, 4H), 7.68 (d, J = 11.5 Hz, 2H), 7.35 (d, J = 8.6 Hz, 4H), 6.99 (d, J = 16.0 Hz, 2H), 6.23 (s, 1H), 4.40 (t, J = 6.2 Hz, 2H), 2.81–2.68 (m, 4H), 2.41–2.24 (m, 4H), 2.01 (s, 6H). 13C-NMR (CDCl3): δ 198.8, 168.2, 150.7, 135.0, 130.9, 129.8, 121.7, 55.8, 52.2, 25.3, 9.4. ESI-MS (m/z): (M−H)− = 665.00. Anal. Calcd. for C29H34N2O6Se2: C, 52.42; H, 5.16; N, 4.20%. Found: C, 52.49; H, 5.14; N, 4.23%.
3.4. General Method for the Synthesis of Curcuminoid-Caffeic Acid and Curcuminoid-Ferulic Acid Conjugates
3.4.1. General Procedure for the Synthesis of 13a–b
12a or 12b (1 mmol) was heated under reflux with Ac2O (20 mmol) for 5 h. Then, acetic acid solution was added to the still hot solution. The product, crystallized upon cooling, was filtered off and washed with cold H2O and Et2O and dried in vacuo to give 13a or 13b as a white solid.
Ferulic acid acetate (13a). Yield 80%. 1H-NMR (400 MHz, CDCl3): δ 7.60 (d, J = 24 Hz, 1H), 7.01 (m, 3H). 6.30 (d, J = 24 Hz, 1H), 3.90 (s, 3H), 2.33(s, 3H). ESI-MS (m/z): (M−H)− = 236.08. Anal. Calcd. for C12H12O5: C, 61.01; H, 5.12; O, 33.87%. Found: C, 61.06; H, 5.16; O, 33.82%.
Caffeic acid acetate (13b). Yield 85%. 1H-NMR (400MHz, CDCl3): δ 7.47 (d, J = 15.8 Hz, 1H), 7.33 (m, 3H), 6.33(d, J = 16.0 Hz, 1H), 2.30(s, 3H), 2.28(s, 3H). ESI-MS (m/z): (M−H)− = 263.52. Anal. Calcd. for C13H12O6: C, 59.09; H, 4.58; O, 36.33%. Found: C, 59.12; H, 4.52; O, 36.30%.
3.4.2. General Procedure for the Synthesis of 9a–d
To a solution of 13a or 13b (1 mmol) in chloroform (50 mL), EDCI (1.2 mmol), HOBT (1.2 mmol) and DIEA (1 mL) were added. After stirring at 0 °C for 1 h, a chloroform solution (40 mL) of CCM or BCM (1 mmol) was added dropwise to the mixtures, which was allowed to be stirred overnight at room temperature. After completion of the reaction, as indicated by TLC, the mixture was washed with hydrochloric acid (1 M) and then with brine. The organic layer was dried with anhydrous Na2SO4 and evaporated to dryness under vacuum. Purification by dry column flash chromatography on silica gel-G (CHCl2:MeOH = 1:10) gave 14a–d as a yellow solid.
Curcumin-mono-ferulic acid acetate (14a). Yield 57%. 183–184 °C. 1HNMR (400 MHz, CDCl3): δ 7.60 (d, J = 20.2 Hz, 1H),7.45 (d, J = 15.8 Hz, 1H), 7.10 (d, J = 2.5Hz, 1H), 7.05-6.92 (m, 6H), 7.01 (m, 3H), 6.92–6.72 (m, 2H), 6.30 (d, J = 20.0 Hz,1H), 6.20(d, J = 16.0 Hz, 1H), 5.50 (s, 1H), 3.90 (s, 3H), 3.91 ( s, 3H), 3.89 (s, 3H,), 3.87 (s, 3H), 2.32(s, 3H). 13C-NMR (CDCl3): δ 198.6, 169.0, 164.3, 151.2, 149.5, 147.6, 142.7, 137.5, 130.6, 128.3, 123.2, 122.5, 121.2, 116.8, 115.0, 111.2, 110.9, 55.9, 52.1, 20.1. ESI-MS (m/z): (M−H)− = 553.5. Anal. Calcd. for C33H30O10: C, 67.57; H, 5.15; O, 27.28%. Found: C, 67.50; H, 5.18; O, 27.30%.
Curcumin-mono-caffeic acid acetate (14b). Yield 60%. m.p. 179–180 °C. 1H-NMR (400 MHz, CDCl3): δ 7.65 (d, J = 6.0 Hz, 1H), 7.55 (d, J = 6.0 Hz, 1H), δ7.45 (d, J = 15.8 Hz, 1H), 7.33 (m, 3H), 7.21–7.09 (m, 4H), 7.06 (d, J = 1.8 Hz, 1H), 6.94 (d, J = 8.2 Hz, 1H), 6.58 (d, J = 15.8 Hz, 1H), 6.50 (d, J = 15.8 Hz, 1H), 6.33 (d, J = 16.0 Hz, 1H), 5.83 (s, 1H), 3.90 (s, 3H), 3.88 (s, 3H), 2.30 (s, 3H), 2.28 (s, 3H). 13C-NMR (CDCl3): δ 198.6, 169.2, 164.8, 151.7, 150.0, 148.1, 142.9, 137.8, 130.6, 128.2, 123.9, 122.8, 121.6, 117.2, 115.6, 111.8, 110.6, 55.9, 52.1, 20.5. ESI-MS (m/z): (M−H)− = 613.18. Anal. Calcd. for C34H30O11: C, 66.44; H, 4.92; O, 28.64%. Found: C, 66.40; H, 4.88; O, 28.60%.
Bisdesmethoxycurcumin-mono-ferulic acid acetate (14c). Yield 55%. m.p. 197–198 °C. 1H-NMR (400 MHz, CDCl3): δ 7.67 (d, J = 15.5 Hz, 2H), 7.62 (d, J = 20.4 Hz, 1H), 7.57 (d, J = 8.6 Hz, 2H), 7.46 (d, J = 8.6 Hz, 2H), 7.12 (d, J = 7.6 Hz, 2H), 7.01 (m, 3H), 6.85 (d, J = 7.5 Hz, 2H), 6.49 (d, J = 13.5 Hz, 2H), 6.33 (d, J = 20.6 Hz, 1H), 5.80 (s, 1H), 3.90 (s, 3H), 2.31(s, 3H). 13C-NMR (CDCl3): δ 198.7, 169.0, 164.2, 157.6, 151.8, 150.6, 148.1, 141.3, 135.3, 132.0, 130.6, 124.8, 121.7, 115.8, 110.5, 110.5, 55.9, 52.2, 20.4. ESI-MS (m/z): (M−H)− = 525.56. Anal. Calcd. for C31H26O8: C, 70.71; H, 4.98; O, 24.31%. Found: C, 70.65; H, 5.02; O, 24.36%.
Bisdesmethoxycurcumin-mono-caffeic acid acetate (14d). Yield 55%. m.p. 193–194 °C. 1H-NMR (400 MHz, CDCl3): δ 7.61 (d, J = 12.9 Hz, 2H), 7.54 (d, J = 8.3 Hz, 2H), δ7.49 (d, J = 15.8 Hz, 1H), 7.43 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 8.5 Hz, 2H), 7.36 (m, 3H), 6.85 (d, J = 7.8 Hz, 2H), 6.56 (d, J = 15.8 Hz, 1H), 6.48 (d, J = 15.8 Hz, 1H), 6.38(d, J = 16.0 Hz, 1H), 5.80 (s, 1H), 2.32(s, 3H), 2.30(s, 3H). 13C-NMR (CDCl3): δ 198.7, 168.8, 164.2, 157.5, 150.7, 147.8, 142.6, 135.1, 130.6, 130.2, 126.3, 122.9, 121.5, 115.8, 115.2, 53.3, 20.7. ESI-MS (m/z): (M−H)− = 553.10. Anal. Calcd. for C32H26O9: C, 69.31; H, 4.73; O, 25.97%. Found: C, 69.35; H, 4.76; O, 25.94%.
3.4.3. General Procedure for the Synthesis of 14e–h
To a stirred, ice-cooled, solution of 13a or 13b (20 mL, 10 mmol), a solution of CCM or BCM (20 mmol) in dry CHCl3 (30 mL) was added dropwise, and the resulting solution was stirred for 10 min at 0 °C. Then, TEA (10 mmol) was added to the solution and stirred at room temperature for 10 h. After completion of the reaction, as indicated by TLC, the mixture was washed with sodium bicarbonate (1 M) and then with brine. The organic layer was dried with anhydrous Na2SO4 and evaporated to dryness under vacuum. Purification by chromatography on silica (CHCl3:MeOH = 20:1) gave 14e–h as a yellow solid.
Curcumin-di-ferulic acid acetate (14e). Yield 60%. m.p. 206–207 °C. 1HNMR (400 MHz, CDCl3): δ 7.69 (d, J = 15.8 Hz, 2H), 7.64 (d, J = 20.8 Hz, 1H), 7.31–7.13 (m, 6H), 7.09 (m, 6H), 6.65 (d, J = 15.8 Hz, 2H), 5.89 (s, 1H), 6.30 (d, J = 20.0 Hz, 2H), 3.90 (s, 6H), 2.33(s, 6H), 2.31(s, 6H). 13C-NMR (CDCl3): δ198.9, 169.0, 164.3, 151.5, 147.8, 142.5, 141.2, 137.4, 131.6, 130.4, 124.5, 121.9, 115.5, 110.8, 55.9, 51.9, 20.2. ESI-MS (m/z): (M−H)− = 803.45. Anal. Calcd. for C45H40O14: C, 67.16; H, 5.01; O, 27.83%. Found: C, 67.20; H, 5.07; O, 27.80%.
Curcumin-di-caffeic acid acetate (14f). Yield 57%. m.p. 201–202 °C. 1HNMR (400 MHz, CDCl3): 1H-NMR (400 MHz, CDCl3) δ 7.66 (d, J = 16.2 Hz, 2H), δ7.47 (d, J = 15.8 Hz, 2H), 7.36–7.25 (m, 6H), 7.21–7.10 (m, 6H), 6.60 (d, J = 16.0 Hz, 2H), 6.33(d, J = 16.0 Hz, 2H), 3.89 (s, 6H), 2.30 (s, 6H), 2.28 (s, 6H). 13C-NMR (CDCl3): δ 198.9, 169, 164.3, 151.5, 147.9, 143.2, 142.6, 142.2, 137.4, 131.5, 131.0, 130.6, 126.8, 124.6, 123.8, 122.8, 121.2, 115.1, 110.7, 55.8, 51.8, 20.3. ESI-MS (m/z): (M−H)− = 859.25. Anal. Calcd. for C47H40O16: C, 65.58; H, 4.68; O, 29.74%. Found: C, 65.50; H, 4.62; O, 29.70%.
Bisdesmethoxycurcumin-di-ferulic acid acetate (14g). Yield 55%. m.p. 217–218 °C. 1H-NMR (400 MHz, CDCl3): δ 7.68 (d, J = 15.8 Hz, 2H), 7.63 (d, J = 21.0 Hz, 2H), 7.60 (d, J = 8.5 Hz, 4H), 7.21 (d, J = 8.6 Hz, 4H), 7.05 (m, 6H), 6.62 (d, J = 15.8 Hz, 2H), 6.30 (d, J = 20.8 Hz, 1H), 5.86 (s, 1H), 3.91 (s, 6H), 2.33(s, 6H). 13C-NMR (CDCl3): δ 198.9, 169.3, 164.3, 151.5, 150.5, 147.9, 141.2, 134.8, 131.6, 130.7, 130.4, 129.6, 129.6, 124.4, 121.9, 121.5, 121.5, 115.5, 110.9, 55.8, 51.9, 20.4. ESI-MS (m/z): (M−H)− = 743.21. Anal. Calcd. for C43H36O12: C, 69.35; H, 4.87; O, 25.78%. Found: C, 69.40; H, 4.80; O, 25.82%.
Bisdesmethoxycurcumin-di-caffeic acid acetate (14h). Yield 65%. m.p. 213–214 °C. 1HNMR (400 MHz, CDCl3): δ 7.68 (d, J = 15.8 Hz, 2H), 7.59 (d, J = 8.5 Hz, 4H), 7.50(d, J = 16.0 Hz, 2H), 7.35–7.25 (m, 6H), 7.20 (d, J = 8.5 Hz, 4H), 6.61 (d, J = 16.2 Hz, 2H), 6.30 (d, J = 16.2 Hz, 2H), 2.34 (s, 6H), 2.32 (s, 6H). 13C-NMR (CDCl3): δ 198.9, 169.3, 164.3, 150.5, 147.9, 143.2, 142.5, 134.9, 131.2, 130.7, 130.4, 129.6, 126.4, 123.8, 122.9, 121.5, 121.5, 115.5, 51.9, 20.3. ESI-MS (m/z): (M−H)− = 799.20. Anal. Calcd. for C45H36O14: C, 67.50; H, 4.53; O, 27.97%. Found: C, 67.55; H, 4.50; O, 27.92%.
3.4.4. General Procedure for the Synthesis of 15a–h
A solution of 14a–h (1 mmol) and CH3ONa (10 mmol) in MeOH (10 mL) was stirred at room temperature overnight. The solvent was evaporated and the residue diluted with water and HCl (1 M) and extracted with CHCl3. The organic layers were dried, and the solvent was evaporated in vacuo. The residue was purified by flash chromatography on silica (CHCl3:MeOH = 10:1) to give 15a–h as a yellow powder.
Curcumin-mono-ferulic acid (15a): Yield 65%. m.p 143–144 °C. 1H-NMR (400 MHz, CDCl3): δ 7.66 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.57 (d, J = 19.5 Hz, 1H), 7.38–7.26 (m, 4H), 7.19 (d, J = 8.5 Hz, 1H), 7.03 (m, 3H), 7.07 (d, J = 16.0 Hz, 1H), 6.85 (d, J = 8.2 Hz, 1H), 6.81 (d, J = 15.8 Hz, 1H), 6.36 (d, J = 20.0 Hz, 1H), 3.92 (s, 3H), 3.87 (s, 3H). 13C-NMR (CDCl3): δ 198.8, 164.5, 151.2, 149.1, 147.5, 138.2, 132.2, 132.2, 130.4, 126.8, 123.8, 121.6, 116.8, 115.8, 112.2, 56.4, 52.6. ESI-MS (m/z): (M−H)− = 543.10. Anal. Calcd. for C31H28O9: C, 68.37; H, 5.18; O, 26.44%. Found: C, 68.40; H, 5.20; O, 26.50%.
Curcumin-mono-caffeic acid (15b): Yield 60%. m.p. 135–136 °C. 1H-NMR (400MHz, CDCl3): δ 7.66 (d, J = 7.5 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 15.8 Hz, 1H), 7.38–7.26 (m, 4H), 7.35 (m, 3H), 7.20(d, J = 8.0 Hz, 1H), 7.04 (d, J = 16.0 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.85 (d, J = 15.9 Hz, 1H), 6.38 (d, J = 16.0 Hz, 1H), 3.90 (s, 3H). 13C-NMR (CDCl3): δ 198.9, 164.3, 150.8, 147.9, 146.5, 145.9, 142.8, 139.2, 132.2, 131.2, 128.4, 125.8, 123.5, 122.6, 116.8, 115.5, 115.0, 114.2, 51.6. ESI-MS (m/z): (M−H)− = 529.12. Anal. Calcd. for C30H26O9: C, 67.92; H, 4.94; O, 27.14%. Found: C, 67.90; H, 4.90; O, 27.10%.
Bisdesmethoxycurcumin-mono-ferulic acid (15c). Yield 65%. m.p. 155–156 °C. 1H-NMR (400 MHz, CDCl3): δ 7.88 (d, J = 8.5 Hz, 2H), 7.63(d, J = 8.6Hz, 2H), 7.60 (d, J = 20.0 Hz, 1H), 7.38 (d, J = 8.6 Hz, 2H), 7.01 (m, 3H), 6.98 (d, J = 15.9 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 6.78 (d, J = 16.0 Hz, 1H), 6.30 (d, J = 20.6 Hz, 1H). 13C-NMR (CDCl3): δ198.5, 164.3, 157.3, 150.5, 149.4, 147.3, 130.8, 130.5, 121.5, 115.9, 111.6, 56.3, 52.5. ESI-MS (m/z): (M−H)− = 483.19. Anal. Calcd. for C29H24O7: C, 71.89; H, 4.99; O, 23.12%. Found: C, 71.80; H, 4.90; O, 23.14%.
Bisdesmethoxycurcumin-mono-caffeic acid (15d). Yield 65%. m.p 148–149 °C. 1H-NMR (400 MHz, CDCl3): δ 7.86 (d, J = 8.5 Hz, 2H), 7.62 (d, J = 8.5 Hz, 2H), 7.48 (d, J = 15.8 Hz, 1H), 7.38 (d, J = 8.5 Hz, 2H), 7.35-7.28 (m, 3H), 6.94 (d, J = 16.0 Hz, 1H), 6.85 (d, J = 8.6 Hz, 2H), 6.74 (d, J = 15.9 Hz, 1H), 6.36 (d, J = 16.0 Hz, 1H). 13C-NMR (CDCl3): δ 198.9, 164.3, 157.9, 150.4, 147.98, 146.3, 145.8, 134.6, 130.4, 123.5, 121.3, 117.5, 115.9, 51.6. ESI-MS (m/z): (M−H)− = 469.39. Anal. Calcd. for C28H22O7: C, 71.48; H, 4.71; O, 23.81%. Found: C, 71.40; H, 4.80; O, 23.94%.
Curcumin-di-ferulic acid (15e). Yield 60%. m.p. 166–167 °C. 1H-NMR (400 MHz, CDCl3): δ 7.66 (d, J = 15.9 Hz, 2H), 7.60 (s, 2H), 7.56 (d, J = 23.8 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.2 Hz, 2H), 7.05 (m, 6H), 7.01 (d, J = 16.0 Hz, 2H), 6.30 (d, J = 23.8 Hz, 2H), 3.90 (s, 6H), 3.88 (s, 3H), 3.87 (s, 3H). 13C-NMR (CDCl3): δ 198.9, 164.5, 151.5, 149.2, 147.8, 142.5, 137.5, 131.6, 127.6, 124.4, 122.5, 121.4, 116.9, 109.6, 55.6, 52.1. ESI-MS (m/z): (M−H)− = 719.20. Anal. Calcd. for C39H32O12: C, 68.33; H, 5.03; O, 26.64%. Found: C, 68.40; H, 5.00; O, 26.70%.
Curcumin-di-caffeic acid (15f). Yield 62%. m.p. 162–163 °C. 1H-NMR (400 MHz, CDCl3): δ 7.69 (d, J = 16.0 Hz, 2H), 7.49 (d, J = 15.8 Hz, 2H), 7.60 (d, J = 8.5 Hz, 2H), 7.46 (d, J = 7.9 Hz, 2H), 7.38 (m, 6H), 6.38 (d, J = 16.0 Hz, 2H), 7.09 (d, J = 15.9 Hz, 2H), 3.90 (s, 6H). 13C-NMR (CDCl3): δ 198.9, 164.2, 151.7, 148.4, 146.4, 143.3, 137.9, 132.4, 130.6, 128.5, 124.6, 123.2, 121.9, 115.3, 110.5, 55.5, 51.5. ESI-MS (m/z): (M−H)− = 743.25. Anal. Calcd. for C39H32O12: C, 67.63; H, 4.66; O, 27.72%. Found: C, 67.70; H, 4.70; O, 28.10%.
Bisdesmethoxycurcumin-di-ferulic acid (15g). Yield 57%. m.p. 191-192 °C. 1H-NMR (400 MHz, CDCl3): δ 7.88 (d, J = 8.6 Hz, 4H), 7.70 (d, J = 11.5 Hz, 2H), 7.60 (d, J = 20.0 Hz, 2H), 7.38 (d, J = 8.5 Hz, 4H), 7.25–7.13 (m, 6H), 6.96 (d, J = 16.0 Hz, 2H), 6.36 (d, J = 20.2 Hz, 1H), 3.90 (s, 6H). 13C-NMR (CDCl3): 198.9, 168.5, 148.7, 147.5, 134.6, 130.2, 129.5, 127.6, 123.3, 121.5, 116.8, 115.9, 149.2, 56.6, 52.2. ESI-MS (m/z): (M−H)− = 659.10. Anal. Calcd. for C39H32O10: C, 70.90; H, 4.88; O, 24.22%. Found: C, 70.85; H, 4.90; O, 24.30%.
Bisdesmethoxycurcumin-di-caffeic acid (15h). Yield 55%. m.p. 181–182 °C. 1H-NMR (400 MHz, CDCl3): δ 7.85 (d, J = 8.6 Hz, 4H), 7.69 (d, J = 16.0 Hz, 2H), 7.49 (d, J = 16.2 Hz, 2H), 7.35 (d, J = 8.6 Hz, 4H), 7.30-7.18 (m, 6H), 6.97 (d, J = 15.7 Hz, 2H), 6.37 (d, J = 16.4 Hz, 2H). 13C-NMR (CDCl3): 198.9, 150.4, 146.5, 145.5, 139.5, 130.3, 129.8, 128.4, 123.5, 121.5, 117.5, 115.3, 51.5. ESI-MS (m/z): (M−H)− = 631.10. Anal. Calcd. for C37H28O10: C, 70.25; H, 4.46; O, 25.29%. Found: C, 70.30; H, 4.50; O, 25.30%.
3.5. General Procedure for the Synthesis of Curcuminoid-Glycyrrhetinic Acid Conjugates
Glycyrrhetinic acid (16, 1 mmol) was heated under reflux with Ac2O (50 mmol) for 2 h. Then, acetic acid solution was added to the still hot solution. The product, crystallized upon cooling, was filtered off and washed with cold H2O and Et2O and dried in vacuo to give 17 as a white solid.
Glycyrrhetinic acid acetate (17). Yield 79%. m.p. 317–318 °C. 1H-NMR (400 MHz, CDCl3): δ 5.76 (s, 1H), 4.55 (dd, J = 11.8, 5.4 Hz, 1H), 2.85 (d, J = 13.9 Hz, 1H), 2.35 (s, 1H), 2.17 (d, J = 13.1 Hz, 1H), 2.07(s, 3H), 2.09–1.57 (m, 12H), 1.45–1.01 (m, 7H), 1.35 (s, 3H), 1.25(s, 3H), 1.22(s, 3H), 1.15 (s, 3H), 0.88 (s, 6H), 0.84 (s, 3H). ESI-MS (m/z): (M−H)− = 511.65. Anal. Calcd. for C32H48O5: C, 74.96; H, 9.44; O, 15.60%. Found: C, 74.95; H, 9.50; O, 15.55%.
3.5.1. General Procedure for the Synthesis of Compounds 18a–b
To the solution of 17 (1 mmol) in chloroform (50 mL), EDCI (1.2 mmol), HOBT (1.2 mmol) and DIEA (1 mL) were added. After stirring at 0 °C for 1 h, a chloroform solution (40 mL) of CCM (1 mmol) or BCM (1 mmol) was added dropwise to the mixtures, which was allowed to be stirred overnight at room temperature. After completion of the reaction, as indicated by TLC, the mixture was washed with hydrochloric acid (1 M) and then with brine. The organic layer was dried with anhydrous Na2SO4 and evaporated to dryness under vacuum. Purification by dry column flash chromatography on silica gel-G (EA:PE = 1:5) gave 18a–b as a yellow solid.
Curcumin-mono-glycyrrhetinic acid acetate (18a). Yield 55%. m.p. 225–226 °C. 1H-NMR (400 MHz, CDCl3): δ 7.63 (d, J = 5.6 Hz, 1H), 7.59 (d, J = 5.6 Hz, 1H), 7.25–7.07 (m, 4H), 7.09 (d, J = 2.0 Hz, 1H), 6.96 (d, J = 8.4 Hz, 1H), 6.59 (d, J = 16.2 Hz, 1H), 6.49 (d, J = 15.8 Hz, 1H), 5.79 (s, 1H), 4.55 (dd, J = 12.0, 5.8 Hz, 1H), 3.90(s, 3H), 3.88(s, 3H), 2.85 (d, J = 14.4 Hz, 1H), 2.38(s, 1H), 2.23(s, 3H), 2.18 (d, J = 13.1 Hz, 1H), 2.09–1.57 (m, 12H), 1.46–1.03 (m,7H), 1.35 (s, 3H), 1.27(s, 3H), 1.24(s, 3H), 1.17 (s, 3H), 0.89 (s, 6H), 0.85 (s, 3H). 13C-NMR (CDCl3): δ 200.9, 176.3, 170.6, 170.2, 151.5, 149.1, 147.9, 142.8, 141.5, 131.8, 130.6, 128.3, 127.8, 124.4, 122.5, 121.6, 116.8, 111.9, 110.9, 80.6, 61.3, 56.1, 55.9, 51.8, 48.2, 45.6, 43.6, 41.9, 41.1, 37.6, 37.1, 36.6, 35.7, 32.5, 32.1, 30.5, 28.3, 26.9, 26.7, 26.2, 25.7, 23.8, 23.6, 21.5, 18.4, 17.2, 16.8. ESI-MS (m/z): (M−H)− = 861.40. Anal. Calcd. for C53H66O10: C, 73.75; H, 7.71; O, 18.54%. Found: C, 73.78; H, 7.80; O, 18.59%.
Bisdesmethoxycurcumin-mono-glycyrrhetinic acid acetate (18b). Yield 55%. m.p. 232–233 °C. 1H-NMR (400 MHz, CDCl3): δ 7.69 (d, J = 15.9 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 8.6 Hz, 2H), 7.17 (d, J = 8.6 Hz, 2H), 6.89 (d, J = 7.8 Hz, 2H), 6.57 (d, J = 15.8 Hz, 1H), 6.45(d, J = 15.8 Hz, 1H), δ 5.79 (s, 1H), 4.58 (dd, J = 12.2, 6.0 Hz, 1H), 2.87 (d, J = 14.2 Hz, 1H), 2.36 (s, 1H), 2.25(s, 3H), 2.19 (d, J = 13.8 Hz, 1H), 2.09–1.59 (m, 12H), 1.48–1.04 (m, 7H), 1.37 (s, 3H), 1.26 (s, 3H), 1.23(s, 3H), 1.17 (s, 3H), 0.88 (s, 6H), 0.86 (s, 3H). 13C-NMR (CDCl3): δ 200.8, 176.3, 170.6, 170.2, 151.5, 149.1, 147.9, 142.8, 141.5, 131.8, 130.6, 128.3, 127.5, 124.3, 122.2, 121.4, 116.7, 111.6, 110.5, 80.3, 61.2, 55.5, 51.5, 48.1, 45.3, 43.2, 41.6, 41.0, 37.4, 37.0, 36.4, 35.5, 32.3, 32.0, 30.4, 28.1, 26.6, 26.5, 26.1, 26.0, 23.1, 23.3, 21.6, 18.4, 17.3, 16.9. ESI-MS (m/z): (M−H)− = 819.40. Anal. Calcd. for C51H64O9: C, 74.61; H, 7.86; O, 17.54%. Found: C, 74.68; H, 17.60; O, 17.59%.
3.5.2. General Procedure for the Synthesis of Compounds 18c–d
A stirred suspension of 17 (10 mmol) in acetyl chloride was heated up to 50 °C for 1 h resulting in a clear solution. Excess acetyl chloride was removed under vacuum and the residue triturated using diethyl ether. The residue was filtered and dried in vacuo to get glycyrrhetinic acid chloride. To a stirred, ice-cooled, solution of glycyrrhetinic acid chloride (20 mL, 10 mmol), a solution of CCM or BCM (20 mmol) in dry CHCl3 (30 mL) was added dropwise, and the resulting solution was stirred for 10 min at 0 °C. Then, TEA (10 mmol) was added to the solution and stirred at room temperature for 10 h. After completion of the reaction, as indicated by TLC, the mixture was washed with sodium bicarbonate (1M) and then with brine. The organic layer was dried with anhydrous Na2SO4 and evaporated to dryness under vacuum. Purification by chromatography on silica (CHCl3:MeOH = 20:1) gave 18c–d as a yellow solid.
Curcumin-di-glycyrrhetinic acid acetate (18c). Yield 65%. m.p. 238–239 °C. 1H-NMR (400 MHz, CDCl3) δ 7.66 (d, J = 15.8 Hz, 2H), 7.21–7.13 (m, 6H), 6.65 (d, J = 15.8 Hz, 2H), 5.80 (s, 2H), 4.55 (dd, J = 12.2, 5.6 Hz, 2H), 2.85 (d, J = 14.8 Hz, 2H), 2.38 (s, 2H), 2.25(s, 6H), 2.15 (d, J = 15.6 Hz, 2H), 2.08–1.67 (m, 24H), 1.52–1.10 (m, 14H), 1.33 (s, 6H), 1.28(s, 6H), 1.26 (s, 6H), 1.15 (s, 3H), 0.88 (s, 12H), 0.84 (s, 6H). 13C-NMR (CDCl3): δ 200.8, 198.9, 176.3, 171.6, 170.2, 151.7, 143.9, 141.3, 131.9, 130.6, 128.3, 125.4, 124.7, 121.9, 121.9, 110.9, 80.6, 61.3, 55.8, 54.8, 51.9, 48.2, 44.6, 43.6, 41.4, 41.1, 37.6, 37.1, 36.3, 35.8, 32.5, 32.1, 30.5, 28.5, 26.9, 26.7, 26.4, 25.7, 24.2, 23.7, 21.3, 18.7, 17.3, 16.8. ESI-MS (m/z): (M−H)− = 1,355.80. Anal. Calcd. for C85H112O14: C, 75.19; H, 8.31; O, 16.50%. Found: C, 75.20; H, 8.37; O, 16.59%.
Bisdesmethoxycurcumin-di-glycyrrhetinic acid acetate (18d). Yield 62%. m.p. 245–246 °C. 1H-NMR (400 MHz, CDCl3): δ 7.69 (d, J = 16.4 Hz, 2H), 7.59 (d, J = 12.0 Hz, 4H), 7.18 (d, J =12.2 Hz, 4H), 6.63 (d, J = 15.8 Hz, 2H), 4.70 (dd, J = 16.2, 8.2 Hz, 2H), 2.86 (d, J = 14.8 Hz, 2H), 2.38 (s, 2H), 2.28(s, 6H), 2.17 (d, J = 15.6 Hz, 2H), 2.11–1.78 (m, 20H), 1.58–1.19 (m, 14H), 1.37 (s, 6H), 1.30 (s, 6H), 1.29 (s, 6H), 1.19 (s, 3H), 0.89 (s, 12H), 0.87 (s, 6H). 13C-NMR (CDCl3): δ 200.8, 198.9, 175.3, 170.6, 169.2, 168.7, 142.9, 140.3, 130.7, 130.2, 127.3, 124.4, 122.7, 121.9, 120.4, 111.5, 80.3, 60.2, 52.8, 50.9, 47.2, 44.2, 43.1, 41.0, 40.1, 36.6, 36.1, 35.3, 34.8, 32.5, 32.1, 30.5, 28.5, 26.5, 26.0, 25.7, 24.2, 23.4, 21.1, 18.2, 17.0, 16.8. ESI-MS (m/z): (M−H)− = 1,355.80. Anal. Calcd. for C83H108O12: C, 76.82; H, 8.39; O, 14.79%. Found: C, 76.80; H, 8.30; O, 14.69%.
3.5.3. General Procedure for the Synthesis of Compounds 19a–d
A solution of 19a–d (1 mmol) and CH3ONa (10 mmol) in MeOH (10 mL) was stirred at room temperature overnight. The solvent was evaporated and the residue diluted with water and HCl (1 M) and extracted with CHCl3. The organic layers were dried, and the solvent was evaporated in vacuo. The residue was purified by flash chromatography on silica (CHCl3:MeOH = 20:1) to give 19a–d as a yellow powder.
Curcumin-mono-glycyrrhetinic acid (19a). Yield 62%. 198–199 °C. 1H-NMR (400 MHz, CDCl3): δ 7.69 (d, J = 8.0 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.45–7.28 (m, 4H), 7.17 (d, J = 8.0 Hz, 1H), 7.04 (d, J = 16.0 Hz, 1H), 6.89 (d, J = 8.4 Hz, 1H), 6.84 (d, J = 16.2 Hz, 1H), 5.76 (s, 1H), 4.59 (dd, J = 12.8, 5.4 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 2.85 (d, J = 14.0 Hz, 1H), 2.38 (s, 2H), 2.19 (d, J = 14.0 Hz, 1H), 2.09(s, 3H), 2.15–1.76 (m, 12H), 1.47–1.15 (m, 7H), 1.38 (s, 3H), 1.28(s, 3H), 1.25(s, 3H), 1.17 (s, 3H), 0.88 (s, 6H), 0.84 (s, 3H). ESI-MS (m/z): (M−H)− = 819.40. Anal. Calcd. for C51H64O9: C, 76.82; H, 8.39; O, 14.79%. 13C-NMR (CDCl3): δ 200.8, 198.9, 176.3, 170.6, 151.5, 149.1, 147.9, 142.8, 141.1, 131.8, 130.4, 128.3, 127.6, 124.4, 122.9, 121.9, 116.8, 111.9, 110.9, 78.6, 61.3, 56.1, 55.8, 54.6, 51.9, 48.2, 44.6, 43.6, 41.4, 41.1, 38.9, 36.7, 35.6, 33.2, 32.5, 30.6, 28.5, 27.4, 26.9, 26.5, 26.0, 25.4, 23.2, 18.8, 17.6, 17.0. Found: C, 76.80; H, 8.30; O, 14.69%. Found: C, 76.80; H, 8.31; O, 14.71%.
Bisdesmethoxycurcumin-mono-glycyrrhetinic acid (19b). Yield 58%. 208–209 °C. 1H-NMR (400 MHz, CDCl3): δ 7.88 (d, J = 8.5 Hz, 2H), 7.63 (d, J = 8.6 Hz, 2H), 7.39 (d, J = 8.5 Hz, 2H), 6.99 (d, J = 16.0 Hz, 1H), 6.89 (d, J = 8.6 Hz, 2H), 6.78 (d, J = 16.2Hz, 1H), 2.88 (d, J = 14.0 Hz, 1H), 2.38 (s, 1H), 2.19 (d, J = 13.9 Hz, 1H), 2.11(s, 3H), 2.11–1.69 (m, 12H), 1.40–1.19 (m, 7H), 1.39 (s, 3H), 1.28 (s, 3H), 1.24 (s, 3H), 1.17 (s, 3H), 0.87 (s, 6H), 0.85 (s, 3H). 13C-NMR (CDCl3): δ 201.2, 198.8, 177.3, 171.6, 152.5, 149.3, 147.6, 142.4, 141.3, 131.5, 130.6, 128.5, 126.6, 125.2, 121.9, 121.2, 117.8, 111.9, 110.5, 78.6, 61.3, 55.8, 54.6, 51.9, 48.2, 44.6, 43.6, 42.4, 41.5, 38.5, 36.5, 35.6, 33.6, 32.2, 30.3, 28.5, 27.9, 26.8, 26.4, 26.2, 25.5, 23.3, 18.9, 17.9, 17.4. ESI-MS (m/z): (M−H)− = 759.40. Anal. Calcd for: C, 77.34; H, 7.95; O, 14.72%. Found: C, 77.40; H, 8.01; O, 14.68%.
Curcumin-di-glycyrrhetinic acid (19c). Yield 66%. 217–219 °C. 1H-NMR (400 MHz, CDCl3): δ 7.84 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.6 Hz, 2H), 7.38 (d, J = 8.7 Hz, 2H), 6.98 (d, J = 16.2 Hz, 1H), 6.89 (d, J = 8.5 Hz, 2H), 6.78 (d, J = 16.0Hz, 1H), 3.90 (s, 6H), 2.89 (d, J = 16.0 Hz, 2H), 2.38 (s, 2H), 2.20 (d, J = 16.0 Hz, 2H), 2.07 (s, 3H), 2.09–1.57 (m, 24H), 1.55–1.19 (m, 7H), 1.35 (s, 6H), 1.28 (s, 6H), 1.24 (s, 6H), 1.19 (s, 6H), 0.89 (s, 12H), 0.86 (s, 6H). 13C-NMR (CDCl3): δ 200.8, 198.5, 177.3, 171.6, 153.5, 150.1, 148.9, 143.8, 141.9, 132.2, 131.4, 129.3, 128.2, 125.4, 123.6, 122.3, 117.8, 113.9, 111.5, 79.5, 61.8, 57.2, 56.9, 55.6, 52.9, 49.2, 45.6, 44.1, 42.0, 41.2, 38.9, 36.9, 35.6, 33.2, 31.5, 30.6, 28.8, 27.3, 26.9, 26.5, 26.2, 25.8, 23.2, 19.8, 18.6, 17.6. ESI-MS (m/z): (M−H)− = 1,243.70. Anal. Calcd. for C79H104O12: C, 76.17; H, 8.42; O, 15.41%. Found: C, 76.10; H, 8.47; O, 15.46%.
Bisdesmethoxycurcumin-di-glycyrrhetinic acid (19d). Yield 66%. 225–226 °C. 1H-NMR (400 MHz, CDCl3): δ 7.84 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.6 Hz, 2H), 6.95 (d, J = 16.4 Hz, 1H), 6.85 (d, J = 8.5 Hz, 2H), 6.79 (d, J = 16.2 Hz, 1H), 2.88 (d, J = 13.9 Hz, 2H), 2.47 (s, 2H), 2.17 (d, J = 14.0 Hz, 2H), 2.14–1.77 (m, 24H), 1.55–1.14 (m, 14H), 1.37 (s, 6H), 1.28(s, 6H), 1.24(s, 6H), 1.18 (s, 6H), 0.88 (s, 12H), 0.85 (s, 6H). 13C-NMR (CDCl3): δ 202.2, 199.2, 179.3, 172.6, 153.6, 149.6, 147.6, 142.4, 141.3, 131.5, 130.6, 128.5, 126.6, 125.2, 121.9, 121.2, 117.8, 111.9, 110.5, 78.6, 61.3, 56.3, 54.8, 51.6, 48.5, 44.2, 43.8, 42.6, 41.6, 38.8, 36.2, 35.2, 33.2, 32.0, 30.5, 28.2, 27.9, 26.5, 26.0, 25.9, 25.5, 23.6, 18.5, 17.4, 17.1. ESI-MS (m/z): (M−H)− = 1,211.75. Anal. Calcd. for C79H104O10: C, 78.18; H, 8.64; O, 13.18%. Found: C, 78.10; H, 8.67; O, 13.12%.