Studies Regarding As(V) Adsorption from Underground Water by Fe-XAD8-DEHPA Impregnated Resin. Equilibrium Sorption and Fixed-Bed Column Tests
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Adsorbent Preparation Process
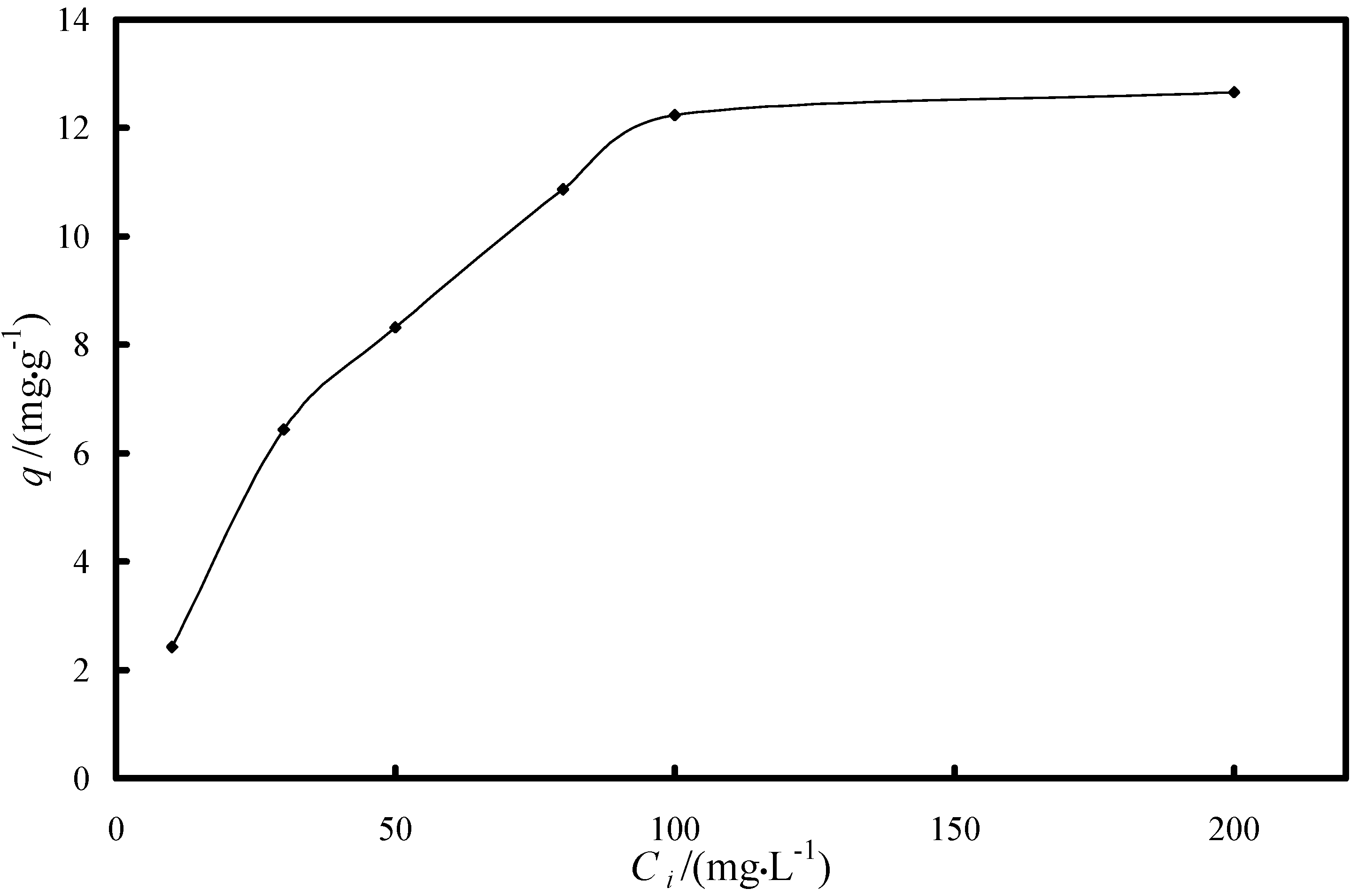
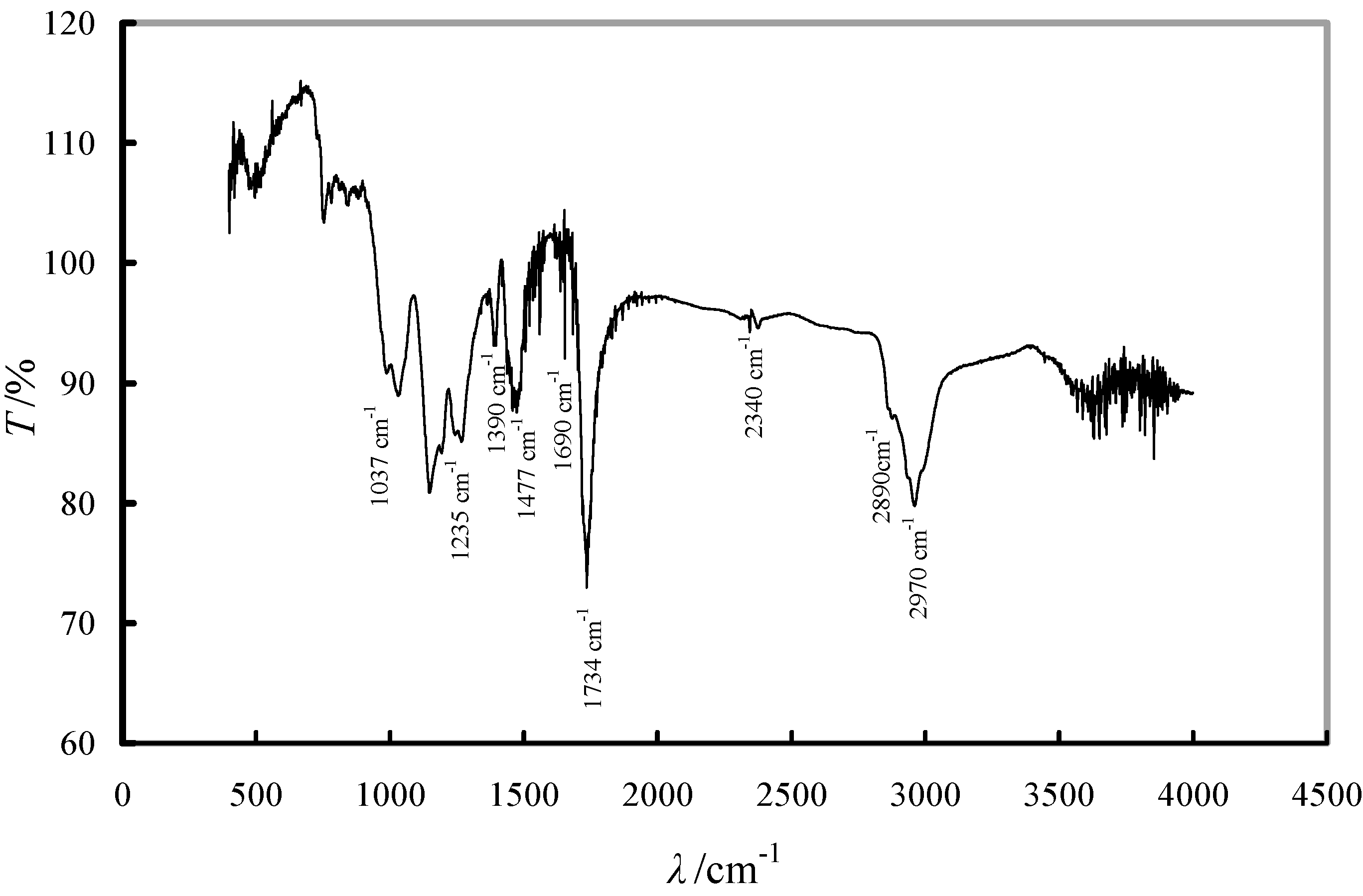
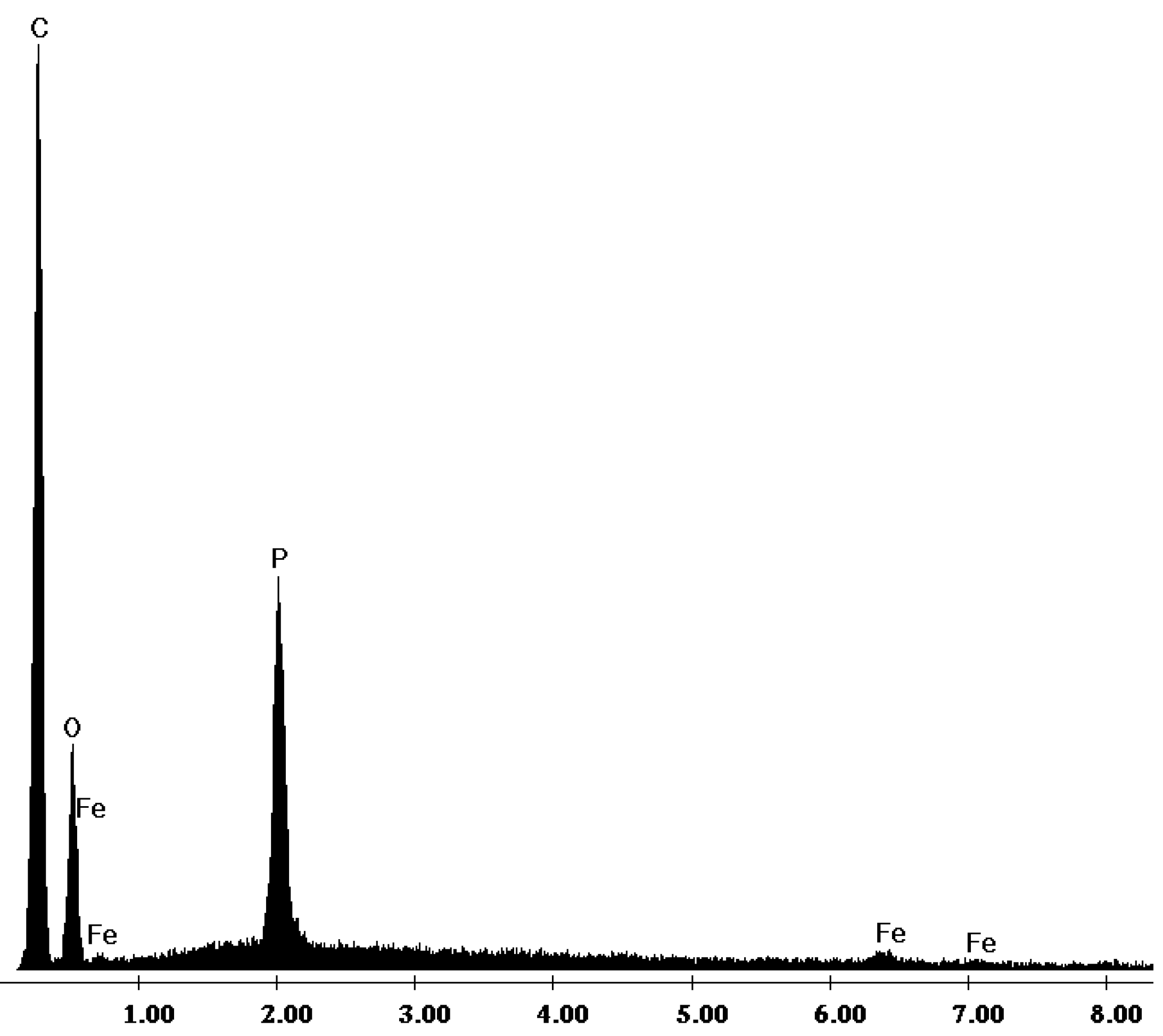
2.2. Sorption Equilibrium Tests
2.2.1. Study of the Kinetics of Adsorption
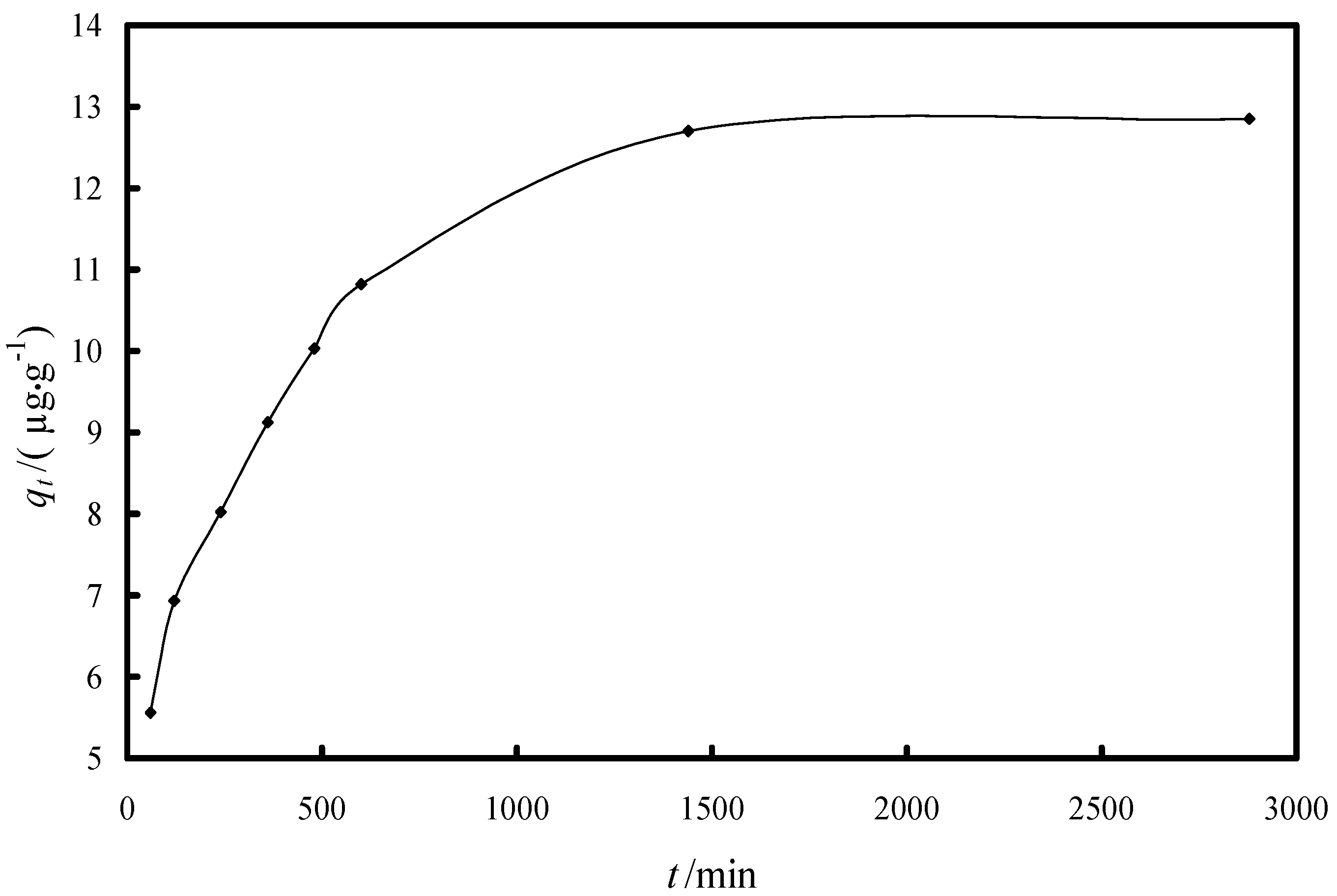
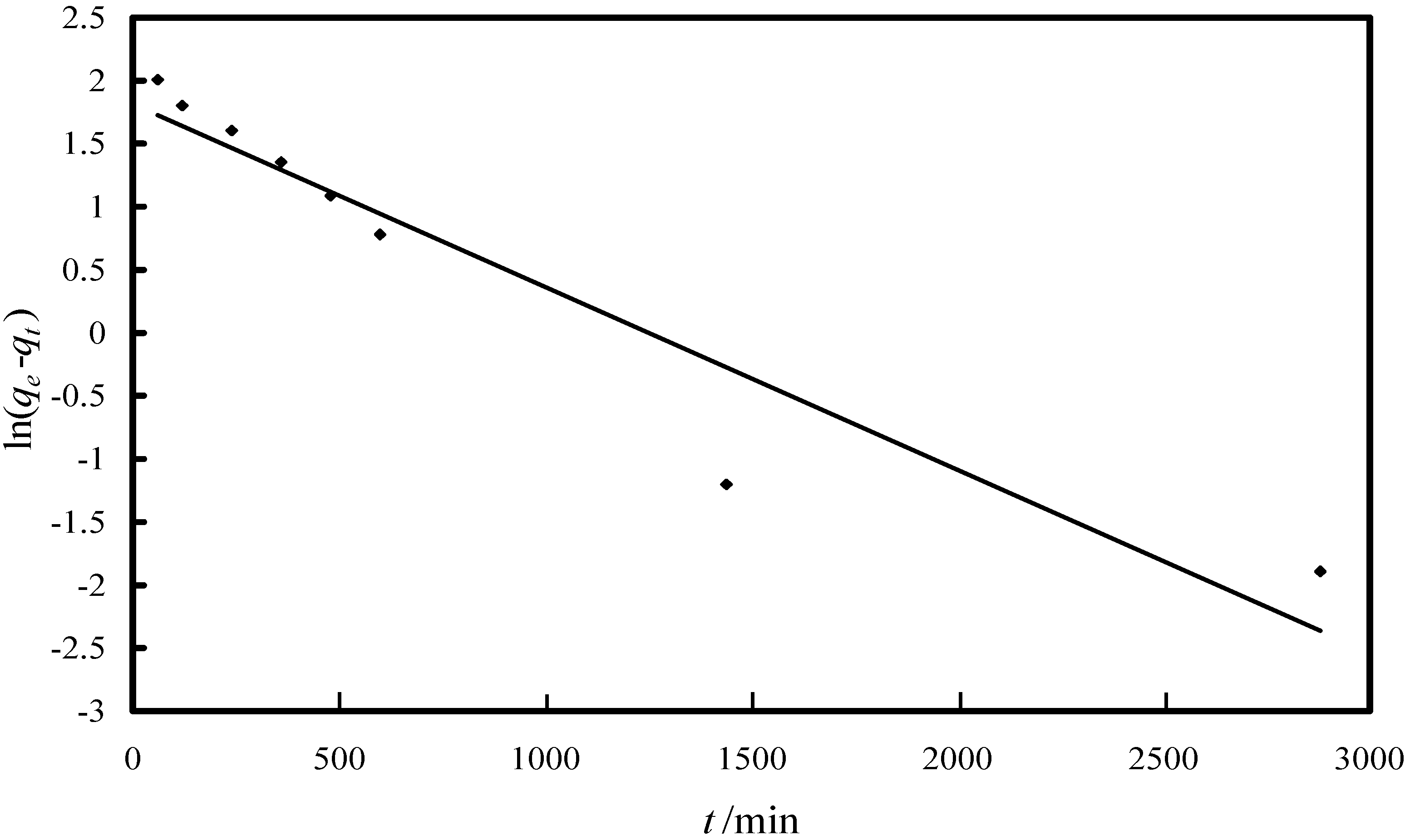

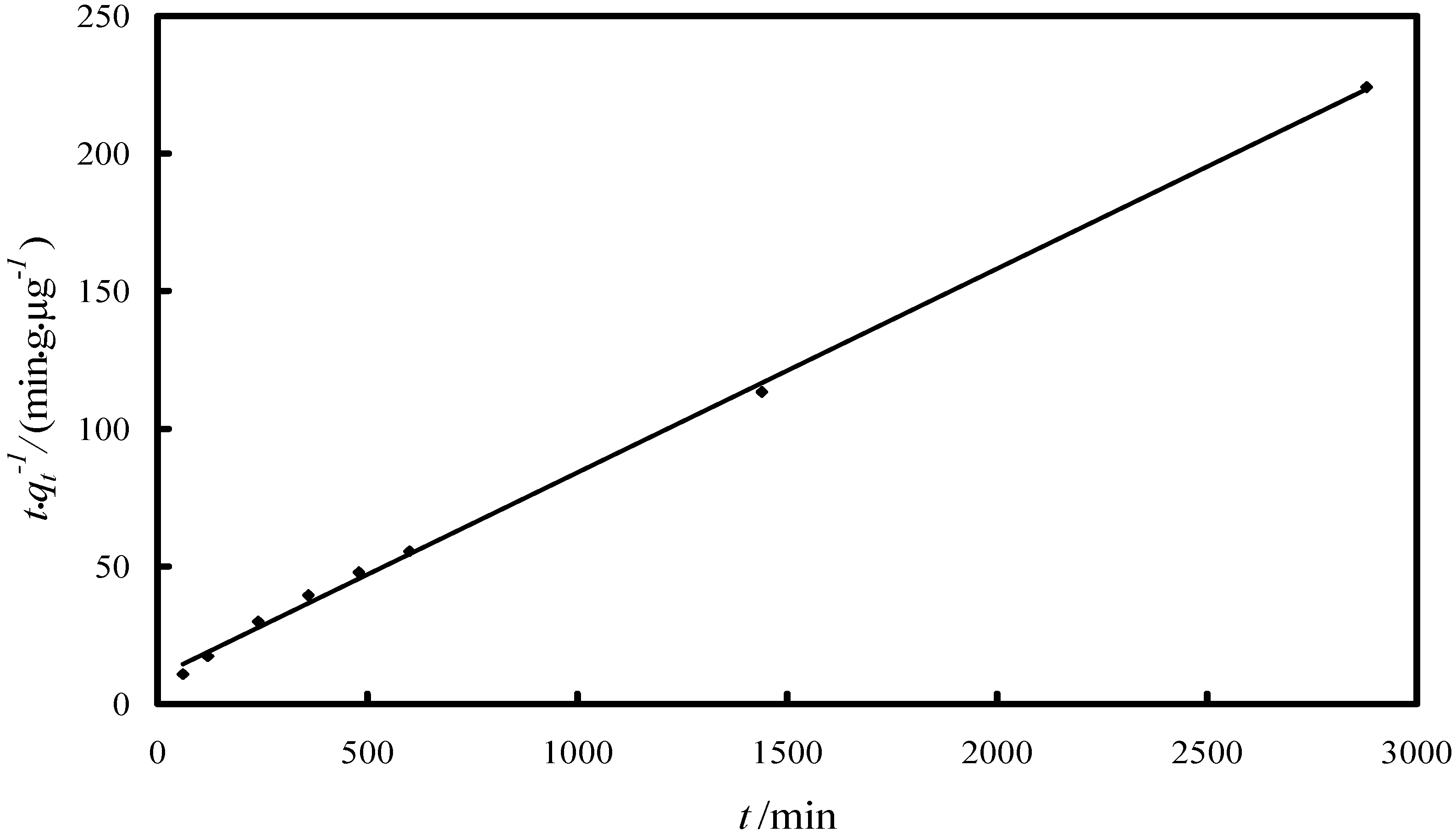

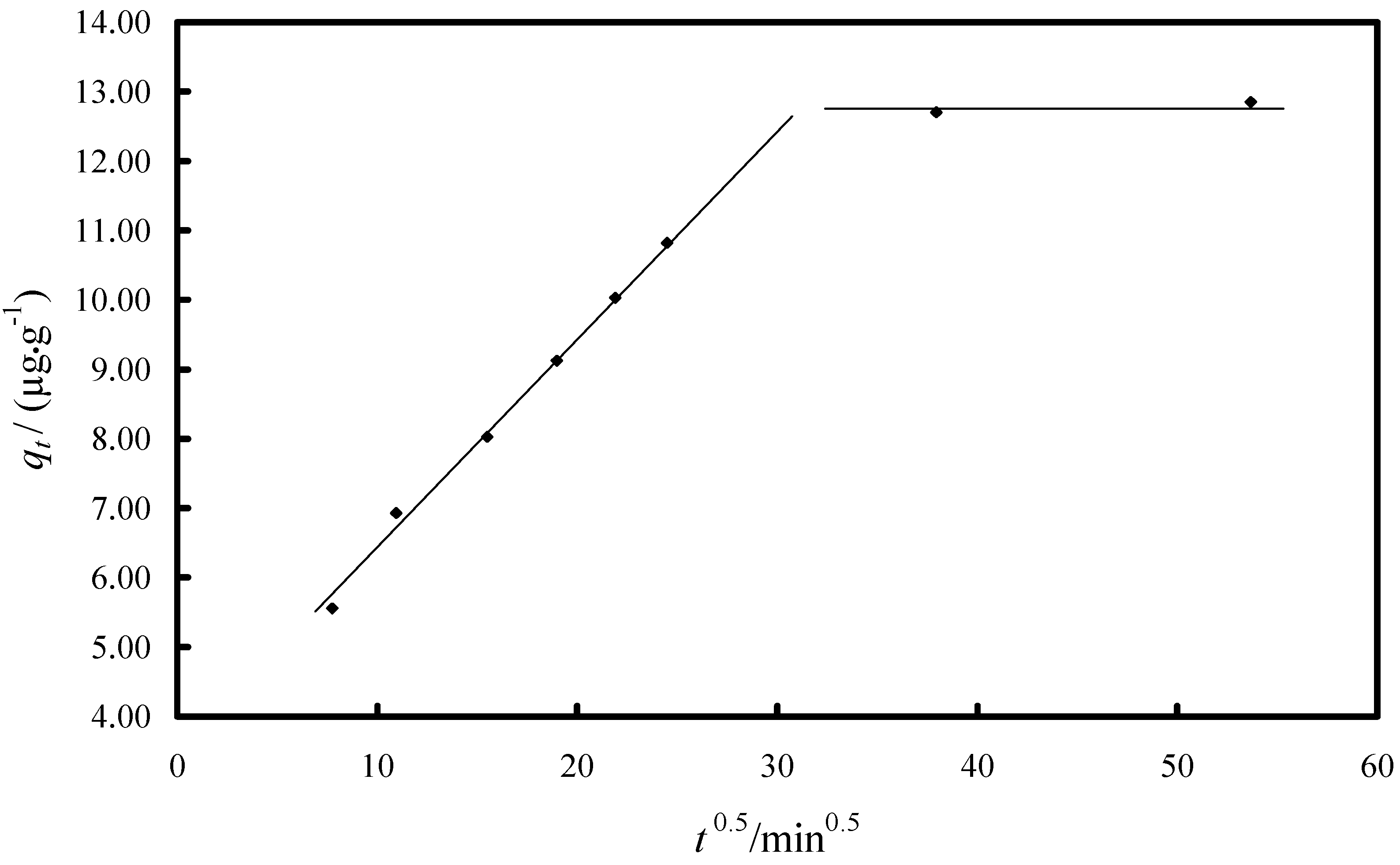
| Model/Parameter | Value |
|---|---|
| Pseudo-first-order model | |
| k1/min−1 | 0.0014 |
| R2 | 0.9158 |
| qe (experimental)/(µg·g−1) | 13 |
| qe (kinetic plot)/(µg·g−1) | 6.13 |
| Pseudo-second-order model | |
| k2/(g·µg−1·min−1) | 5.45 × 10−4 |
| R2 | 0.9957 |
| qe (experimental)/(µg·g−1) | 13 |
| qe (kinetic plot)/(µg·g−1) | 13.5 |
| Intra-particle diffusion model | |
| kdif/(µg·g−1·min−1/2) | 0.3049 |
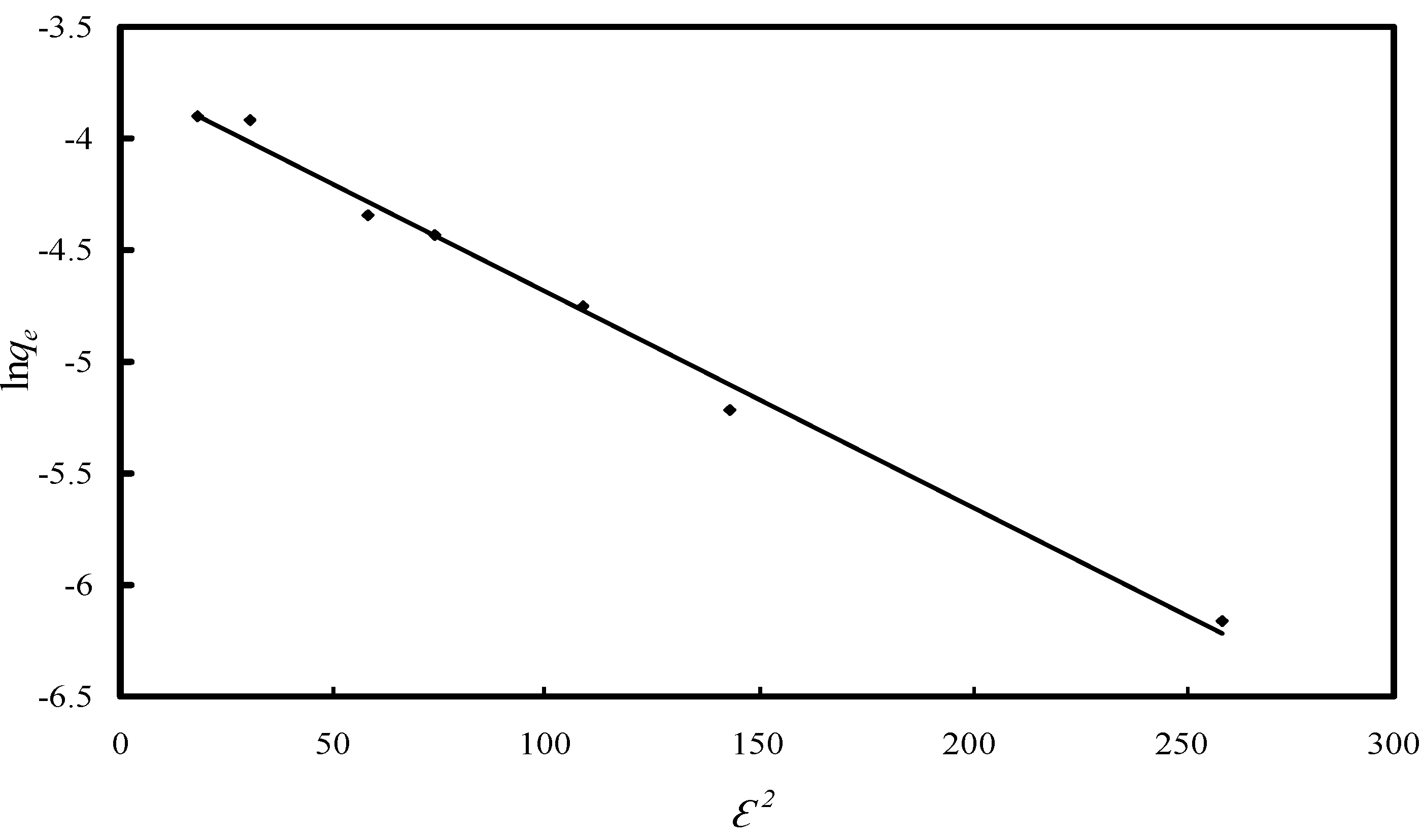
2.2.2. Study of the Adsorption Isotherm


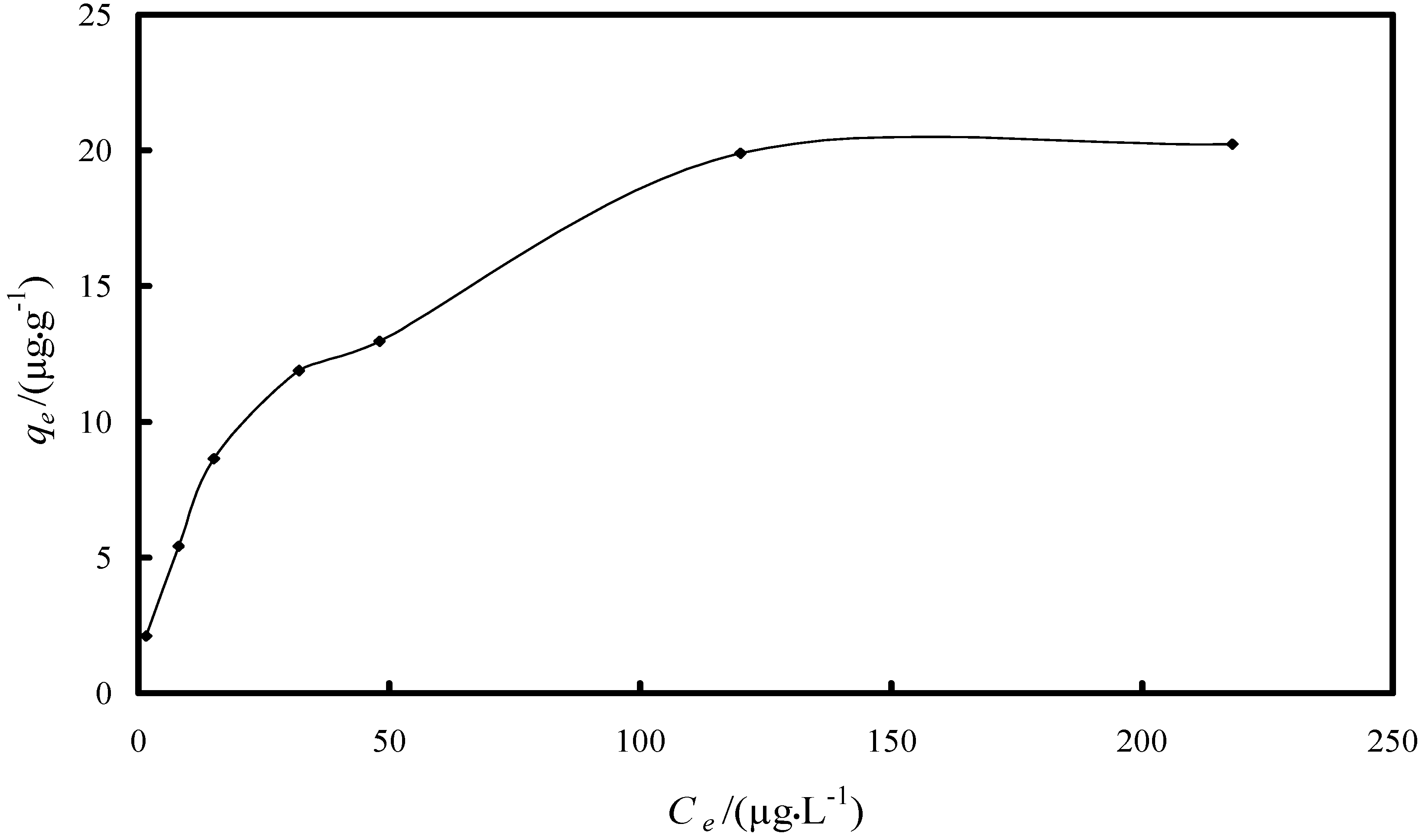
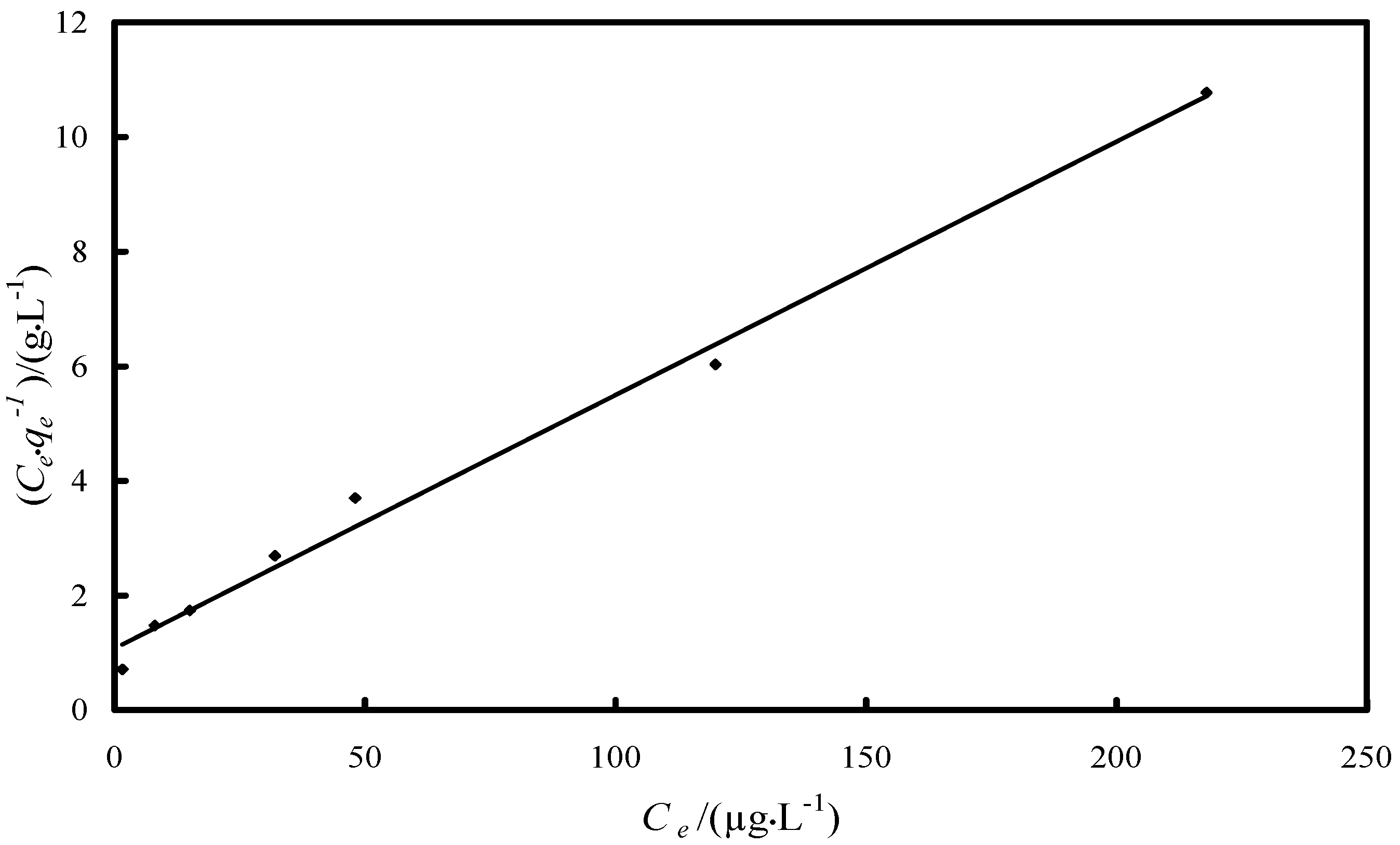
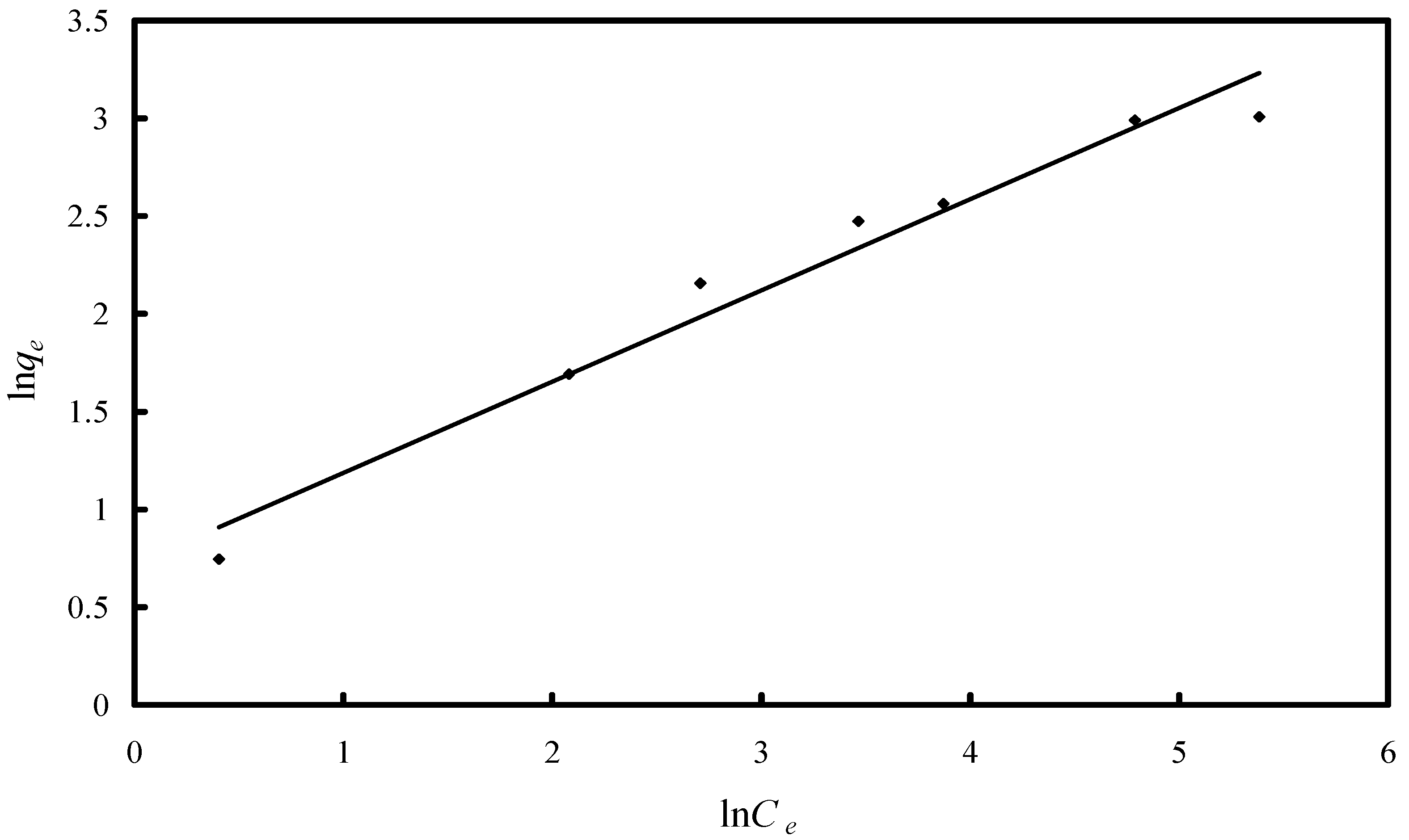
| Isotherm/Parameter | Value |
|---|---|
| Langmuir isotherm | |
| KL/(L·µg−1) | 0.041 |
| qm/(µg·g−1) | 22.6 |
| R2 | 0.9918 |
| Freundlich isotherm | |
| KF/(µg·g−1) | 2.055 |
| 1/n | 0.4664 |
| R2 | 0.9667 |
| D-R isotherm | |
| k/(mol2·kJ−2) | 0.0096 |
| qm/(µg·g−1) | 24.16 |
| R2 | 0.9921 |
| Adsorbent | qm, µg·g−1 | References |
|---|---|---|
| Fe-XAD8-DEHPA | 22.6 | Present work |
| Fe-XAD7-DEHPA | 17.6 | [18] |
| Fe-IR-120(Na)-DEHPA | 21.8 | [41] |
| Alginate bead (doped and coated with iron ions | 14 | [42] |
| Iron oxide coated sand | 8 | [43] |
| Iron oxide coated sand-2 | 18 | [44] |




2.3. Fixed-Bed Column Study


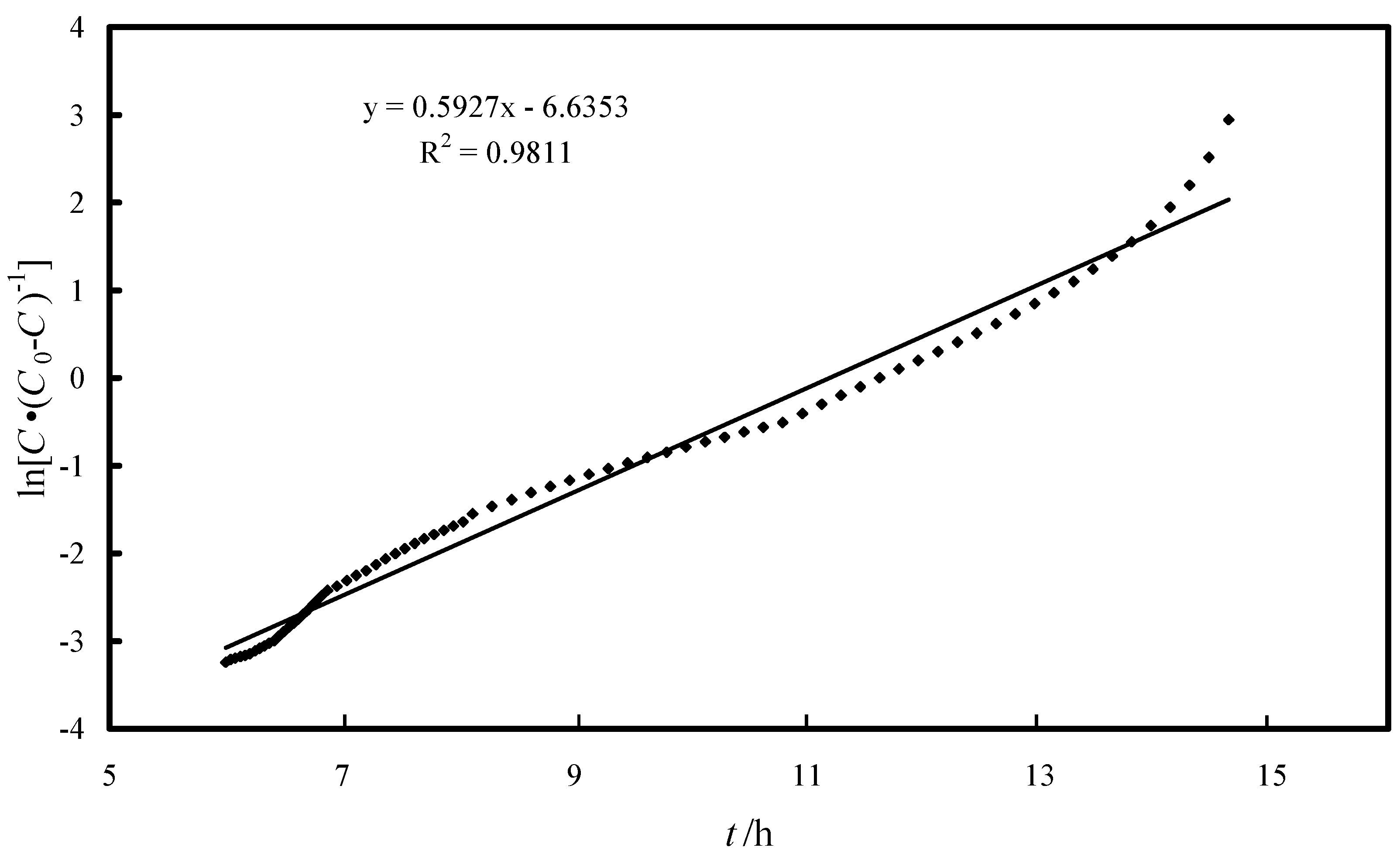
2.4. Quality Parameters of the Effluent Water
2.5. Desorption Study
3. Experimental Section
3.1. Adsorbent Preparation
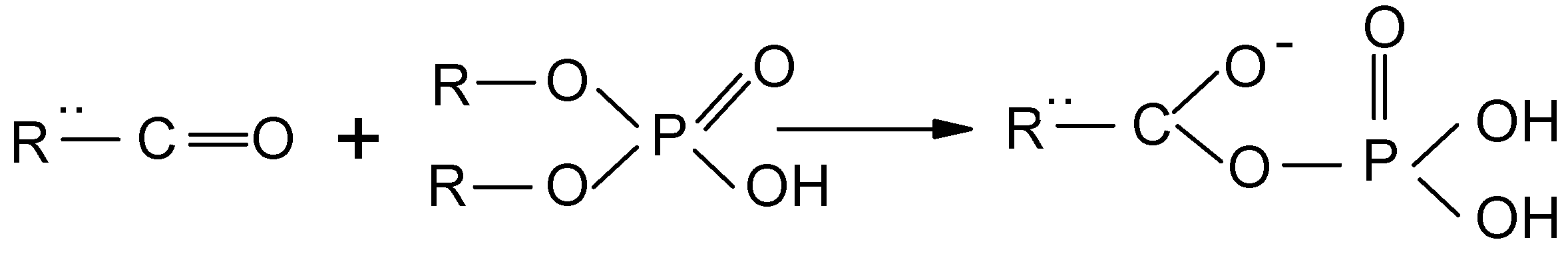
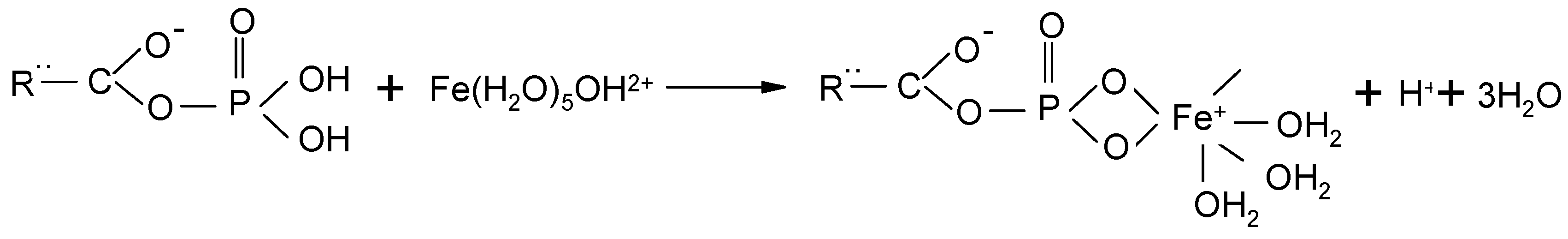
3.2. Batch Experiments for Arsenic Removal
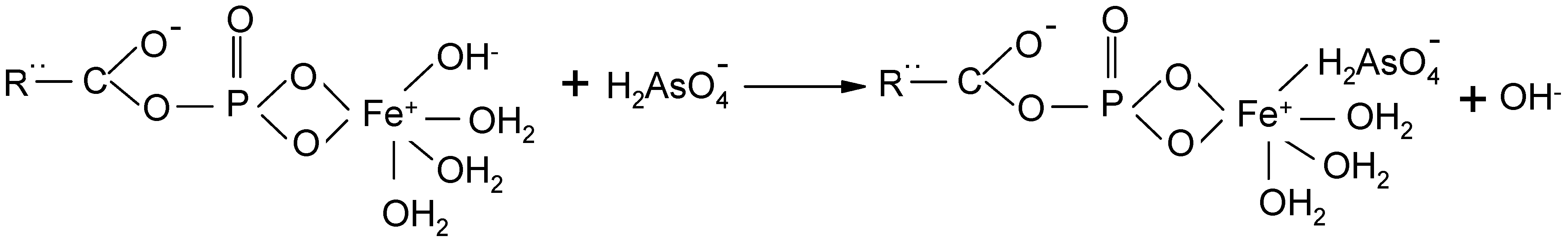

3.3. Fixed-Bed Column Study for Arsenic Removal
3.4. Desorption Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cotruvo, J.; Fawell, J.K.; Giddings, M.; Jackson, P.; Magara, Y.; Festo Ngowy, A.V.; Ohanian, E. Arsenic in Drinking Water; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Ramesh, A.; Hasegawa, H.; Maki, T.; Ueda, K. Adsorption of inorganic and organic arsenic from aqueous solutions by polymeric Al/Fe modified montmorillonite. Sep. Purif. Technol. 2007, 56, 90–100. [Google Scholar]
- Guo, H.; Stuben, D.; Berner, Z. Arsenic removal from water using natural iron mineral-quartz sand columns. Sci. Total Environ. 2007, 377, 142–151. [Google Scholar]
- Kundu, S.; Gupta, A.K. Adsorptive removal of As(III) from aqueous solution using iron oxide coated cement (IOCC): Evaluation of kinetic, equilibrium and thermodynamic models. Sep. Purif. Technol. 2006, 51, 165–172. [Google Scholar]
- Borah, D.; Satokawa, S.; Kato, S.; Kojima, T. Surface-modified carbon black for As(V) removal. J. Colloid Interface Sci. 2008, 319, 53–62. [Google Scholar]
- Borah, D.; Satokawa, S.; Kato, S.; Kojima, T. Sorption of As(V) from aqueous solution using acid modified carbon black. J. Hazard. Mater. 2009, 162, 1269–1277. [Google Scholar]
- Negrea, A.; Lupa, L.; Ciopec, M.; Lazau, R.; Muntean, C.; Negrea, P. Adsorption of As(III) ions onto iron-containing waste sludge. Adsorpt. Sci. Technol. 2010, 28, 467–484. [Google Scholar]
- Goswami, R.; Deb, P.; Thakur, R.; Sarma, K.P.; Bsumalick, A. Removal of As(III) from aqueous solution using functionalized ultrafine iron oxide nanoparticles. Sep. Sci. Technol. 2011, 46, 1017–1022. [Google Scholar]
- Gupta, K.; Ghosh, U.C. Arsenic removal using hydrous nanostructure iron(III)-titanium(IV) binary mixed oxide from aqueous solution. J. Hazard. Mater. 2009, 161, 884–892. [Google Scholar]
- Hlavay, J.; Polyak, K. Determination of surface properties of iron hydroxide coated alumina adsorbent prepared from drinking water. J. Colloid Interface Sci. 2005, 284, 71–77. [Google Scholar]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar]
- Oke, I.A.; Olarinoye, N.O.; Adewusi, S.R.A. Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell. Adsorption 2008, 14, 73–83. [Google Scholar]
- Juang, R.S. Preparation, properties and sorption behaviour of Impregnated resins containing acidic organophosphorus extractants. Proc. Natl. Sci. Counc. ROC 1999, 23, 353–364. [Google Scholar]
- Shao, W.; Li, X.; Cao, Q.; Luo, F.; Li, J.; Du, Y. Adsorption of arsenate and arsenite anions from aqueous medium by using metal(III)-loaded Amberlite resins. Hydrometallurgy 2008, 91, 138–143. [Google Scholar]
- Zhu, X.; Jyo, A. Removal of arsenic(V) by zirconium(IV)-loaded phosphoric acid chelating resin. Sep. Sci. Technol. 2001, 36, 3175–3189. [Google Scholar]
- Saha, B.; Gill, R.J.; Bailey, D.G.; Kabay, N.; Arda, M. Sorption of Cr(VI) from aqueous solution by Amberlite XAD-7 resin impregnated with ALiquat 336. React. Funct. Polym. 2004, 60, 223–244. [Google Scholar]
- Belkhouche, N.E.; Didi, M.A. Extraction of Bi(III) from nitrate medium by D2EHPA impregnated onto Amberlite XAD-1180. Hydrometallurgy 2010, 103, 60–67. [Google Scholar]
- Negrea, A.; Ciopec, M.; Lupa, L.; Davidescu, C.M.; Popa, A.; Ilia, G.; Negrea, P. Removal of As(V) by Fe(III) loaded XAD7 impregnated resin containing di(2-ethylhexyl) phosphoric acid (DEHPA): Equilibrium, Kinetic and Thermodynamic modelling studies. J. Chem. Eng. Data 2011, 56, 3830–3838. [Google Scholar]
- Ciopec, M.; Negrea, A.; Davidescu, C.M.; Negrea, P.; Muntean, C.; Popa, A. Use of Di-(2-Ethylhexyl)-Phosphoric Acid (DEHPA) Impregnated XAD-8 Copolymer Resin for the Separation of Metal Ions from Water. Chem. Bull. 2010, 55, 127–131. [Google Scholar]
- Suzuki, T.M.; Bomani, J.O.; Matsunga, H.; Yokoyama, T. Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 2000, 43, 165–172. [Google Scholar]
- Haron, M.J.; Shiah, L.L.; Wan Yunus, W.M.Z. Sorption of arsenic *V( by titanium oxide loaded poly(hydroxamic acid) resin. Malays. J. Anal. Sci. 2006, 10, 261–268. [Google Scholar]
- Benamor, M.; Bouariche, Z.; Belaid, T.; Draa, M.T. Kinetic studies on cadmium ions by Amberlite XAD7 impregnated resin containing di(2-ethylhexyl) phosphoric acid as extractant. Sep. Purif. Technol. 2008, 59, 74–84. [Google Scholar]
- Mendoza, R.N.; Medina, I.S.; Vera, A.; Rodriguez, M.A. Study of the sorption of Cr(III) with XAD-2 resin impregnated with di-(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272). Solvent Extr. Ion Exch. 2000, 18, 319–343. [Google Scholar]
- Muraviev, D.; Ghantous, L.; Valiente, M. Stabilization of solvent impregnated resin capacities by different techniques. React. Funct. Polym. 1998, 38, 259–268. [Google Scholar]
- Mukherjee, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Das, B.; Nayak, B.; Lodh, D.; Rahman, M.M.; Chakraborti, D. Arsenic contamination in groundwater: A global perspective with emphasis on the Asian scenario. J. Health Popul. Nutr. 2006, 24, 142–163. [Google Scholar]
- Negrea, A.; Muntean, C.; Ciopec, M.; Lupa, L.; Negrea, P. Removal of arsenic from underground water to obtain drinking water. Chem. Bull. 2009, 54, 82–84. [Google Scholar]
- Awual, M.R.; Shenashen, M.A.; Yaita, T.; Shiwaku, H.; Jyo, A. Efficient arsenic(V) removal from water by ligand exchange fibrous adsorbent. Water Res. 2012, 46, 5541–5550. [Google Scholar]
- Awual, M.R.; El-Safty, S.A.; Jyo, A. Removal of trace arsenic (V) and phosphate from water by a highly selective ligand exchange adsorbent. J. Environ. Sci. 2011, 23, 1947–1954. [Google Scholar]
- Awual, M.R.; Urata, S.; Jyo, A.; Tamada, M.; Katakai, A. Arsenate removal from water by a weak-base anion exchange fibrous adsorbent. Water Res. 2008, 42, 689–696. [Google Scholar]
- Awual, M.R.; Hossain, A.; Shenashen, M.A.; Yaita, T.; Suzuki, S.; Jyo, A. Evaluating of arsenic(V) removal from water by weak-base anion exchange adsorbent. Environ. Sci. Pollut. Res. 2013, 20, 421–430. [Google Scholar]
- Awual, M.R.; Jyo, A. Rapid column-mode removal of arsenate from water by crosslinked poly(allylamine) resin. Water Res. 2009, 43, 1229–1236. [Google Scholar]
- Chanda, M.; O’Driscoll, K.F.; Rempel, G.L. Ligand exchange sorption of arsenate and arsenite anions by chelating resins in ferric ion form: II. Iminodiacetic chelating resin chelex 100. React. Polym. 1988, 8, 85–95. [Google Scholar]
- Greenleaf, J.E.; Lin, J.C.; Sengupta, A.K. Two novel applications of ion exchange fibers: Arsenic removal and chemical-free softening of hard water. Environ. Prog. 2006, 25, 300–311. [Google Scholar]
- German, M.; Seingheng, H.; SenGupta, A.K. Mitigating arsenic crisis in the developing world: Role of robust, reusable and selective hybrid anion exchange (HAIX). Sci. Total Environ. 2014. [Google Scholar]
- Etmannski, T.R.; Darton, R.C. A methodology for the sustainability assessment of arsenic mitigation technology for drinking water. Sci. Total Environ. 2014, 488, 509–515. [Google Scholar]
- Streat, M.; Hellgardt, K.; Newton, N.L.R. Hydrous ferric oxide as an adsorbent in water treatment: Part 2. Adsorption studies. Process Saf. Environ. 2008, 86, 11–20. [Google Scholar]
- An, B.; Steinwinder, T.R.; Zhao, D. Selective removal of arsenate from drinking water using a polymeric ligand exchanger. Water Res. 2005, 39, 4993–5004. [Google Scholar]
- Chen, Y.N.; Chai, L.Y.; Shu, Y.D. Study of arsenic (V) adsorption on bone char from aqueous solution. J. Hazard. Mater. 2008, 160, 168–172. [Google Scholar]
- Weber, W.J.; Moris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar]
- Mendoza-Barron, J.; Jacobo-Azuara, A.; Levya-Ramos, R.; Berber-Mendoza, S.; Guerrero-Coronado, R.M.; Fuentes-Rubio, L.; Martinez-Rosales, J.M. Adsorption of arsenic (V) from a water solution onto a surfactant-modified zeolite. Adsorption 2011, 17, 489–496. [Google Scholar]
- Ciopec, M.; Negrea, A.; Lupa, L.; Davidescu, C.; Negrea, P.; Sfarloaga, P. Performance evaluation of the Fe-IR-120(Na)-DEHPA impregnated resin in the removal process of As(V) from aqueous solution. J. Mater. Sci. Eng. B 2011, 1, 421–432. [Google Scholar]
- Zouboulis, A.J.; Kalsoyiannis, I.A. Arsenic removal using iron oxide, loaded aliginate beads. Ind. Eng. Chem. Res. 2002, 41, 6149–6155. [Google Scholar]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S.; Tanjore, S. Organic arsenic removal from drinking water. Urban Water 2002, 4, 415–421. [Google Scholar]
- Viraghavan, T.; Thirunavukkarasu, O.S.; Subramanian, K.S. Removal of arsenic in drkingin water by iron oxide coated sand and ferrihydrite—batch studies. Water Qual. Res. J. Can. 2001, 36, 55–70. [Google Scholar]
- Maji, S.K.; Pal, A.; Pal, T. Arsenic removal from real-life ground water by adsorption on laterite soil. J. Hazard. Mater. 2008, 151, 811–820. [Google Scholar]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of anionic surfactant form wastewater by alumina: A case study. Colloid Surf. A: Physicochem. Eng. Asp. 2005, 254, 165–171. [Google Scholar]
- Noubactep, C.; Care, S.; Blatkeu, B.D.; Nanseu-Njiki, C.P. Enhancing the sustainability of household Fe0/sand filters by using bimetallics and MnO2. CLEAN Soil Air Water 2012, 40, 100–109. [Google Scholar]
- Dominguez-Ramos, A.; Chavan, K.; Garcia, V.; Jimeno, G.; Albo, J.; Marathe, K.V.; Yadav, G.D.; Irabien, A. Arsenic removal from natural waters by adsorption or ion exchange: An environmental sustainability assessement. Ind. Eng. Chem. Res. 2014. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar]
- Rau, I.; Gonzalo, A.; Valiente, M. Arsenic (V) adsorption by immobilized iron mediation. Modeling of the adsorption process and influence of interfering anions. React. Funct. Polym. 2003, 54, 85–94. [Google Scholar]
- Ohe, K.; Tagai, Y.; Nakamura, S.; Oshima, T.; Baba, Y. Adsorption behaviour of arsenic(III) and arsenic(V) using magnetite. J. Chem. Eng. Jpn. 2005, 38, 671–676. [Google Scholar]
- Sample Availability: Samples of the compounds Fe-XAD8-DEHPA are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciopec, M.; Negrea, A.; Lupa, L.; Davidescu, C.M.; Negrea, P. Studies Regarding As(V) Adsorption from Underground Water by Fe-XAD8-DEHPA Impregnated Resin. Equilibrium Sorption and Fixed-Bed Column Tests. Molecules 2014, 19, 16082-16101. https://doi.org/10.3390/molecules191016082
Ciopec M, Negrea A, Lupa L, Davidescu CM, Negrea P. Studies Regarding As(V) Adsorption from Underground Water by Fe-XAD8-DEHPA Impregnated Resin. Equilibrium Sorption and Fixed-Bed Column Tests. Molecules. 2014; 19(10):16082-16101. https://doi.org/10.3390/molecules191016082
Chicago/Turabian StyleCiopec, Mihaela, Adina Negrea, Lavinia Lupa, Corneliu M. Davidescu, and Petru Negrea. 2014. "Studies Regarding As(V) Adsorption from Underground Water by Fe-XAD8-DEHPA Impregnated Resin. Equilibrium Sorption and Fixed-Bed Column Tests" Molecules 19, no. 10: 16082-16101. https://doi.org/10.3390/molecules191016082





