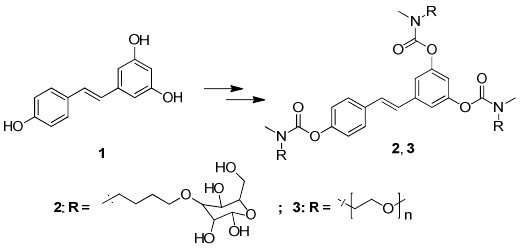
New Water-Soluble Carbamate Ester Derivatives of Resveratrol
Abstract
:1. Introduction

2. Results and Discussion
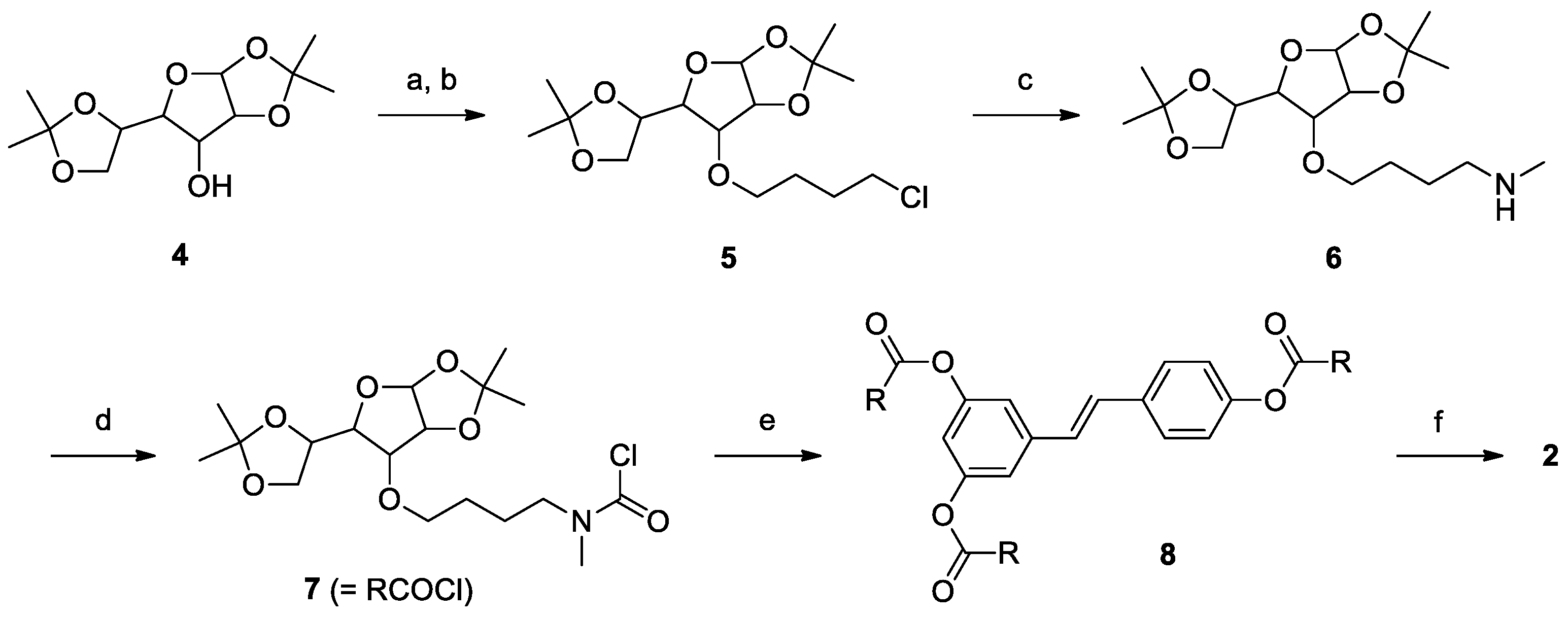
2.1. Synthesis

2.2. Solubility
2.3. Permeation across Rat Intestine
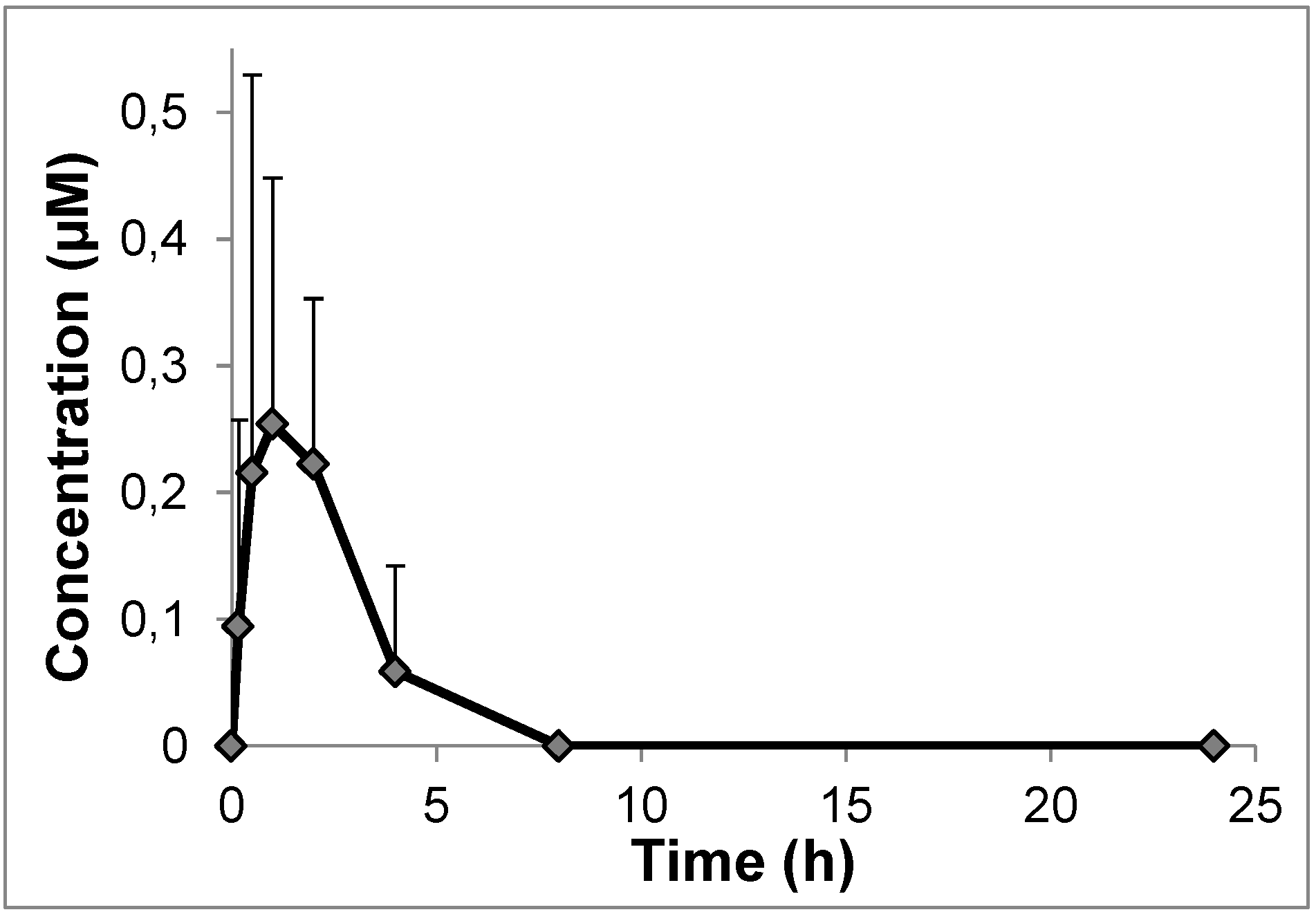
2.4. In Vivo Pharmacokinetics
3. Experimental Section
3.1. Materials, Reagents and Standard Procedures
3.2. Synthesis
3.3. Permeation Studies with Rat Intestinal Segments (Ex Vivo)
3.4. Pharmacokinetic Studies
3.5 HPLC-UV Analysis
3.6 Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Holme, A.L. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Sign. 2009, 11, 2851–2897. [Google Scholar] [CrossRef]
- Biasutto, L.; Szabo, I.; Zoratti, M. Mitochondrial effects of plant-made compounds. Antioxid. Redox Sign. 2011, 15, 3039–3059. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Zoratti, M. Resveratrol and health: the starting point. ChemBioChem 2012, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. Resveratrol and calcium signaling: Molecular mechanisms and clinical relevance. Molecules 2014, 19, 7327–7340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pallas, M.; Porquet, D.; Vicente, A.; Sanfeliu, C. Resveratrol: New avenues for a natural compound in neuroprotection. Curr. Pharm. Des. 2013, 19, 6726–6731. [Google Scholar] [CrossRef] [PubMed]
- Widlund, A.L.; Baur, J.A.; Vang, O. mTOR: More targets of resveratrol? Expert Rev. Mol. Med. 2013, 15. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Liu, X.; Cho, W.C. Genetic and epigenetic studies for determining molecular targets of natural product anticancer agents. Curr. Cancer Drug Targets 2013, 13, 506–518. [Google Scholar] [PubMed]
- Um, J.H.; Park, S.J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Vallejo, F.; Pallares, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mohsen, M.; Bayele, H.; Kuhnle, G.; Gibson, G.; Debnam, E.; Srai, S.K.; Rice-Evans, C.; Spencer, J.P. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br. J. Nutr. 2006, 96, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Maijo, M.; Planas, J.M. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J. Pharm. Biomed. Anal. 2010, 51, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Spina, M.L.; Mattarei, A.; Paradisi, C.; Zoratti, M.; Biasutto, L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol. Nutr. Food Res. 2014. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Planas, J.M.; Alfaras, I.; Colom, H.; Juan, M.E. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch. Biochem. Biophys. 2012, 527, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kaldas, M.I.; Walle, U.K.; Walle, T. Resveratrol transport and metabolism by human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2003, 55, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shin, Y.G.; Chow, A.; Li, Y.; Kosmeder, J.W.; Lee, Y.S.; Hirschelman, W.H.; Pezzuto, J.M.; Mehta, R.G.; van Breemen, R.B. Human, rat, and mouse metabolism of resveratrol. Pharm. Res. 2002, 19, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Maier-Salamon, A.; Bohmdorfer, M.; Thalhammer, T.; Szekeres, T.; Jaeger, W. Hepatic glucuronidation of resveratrol: Interspecies comparison of enzyme kinetic profiles in human, mouse, rat, and dog. Drug Metab. Pharmacokinet. 2011, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Menet, M.C.; Marchal, J.; Dal-Pan, A.; Taghi, M.; Nivet-Antoine, V.; Dargere, D.; Laprevote, O.; Beaudeux, J.L.; Aujard, F.; Epelbaum, J.; et al. Resveratrol metabolism in a non-human primate, the grey mouse lemur (Microcebus murinus), using ultra-high-performance liquid chromatography-quadrupole time of flight. PLoS One 2014, 9, e91932. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Garcia, A.M.; Meyskens, F.L., Jr. Differences in the glucuronidation of resveratrol and pterostilbene: Altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2014, 29, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Miksits, M.; Wlcek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jager, W. Antitumor activity of resveratrol and its sulfated metabolites against human breast cancer cells. Planta Med. 2009, 75, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Calamini, B.; Ratia, K.; Malkowski, M.G.; Cuendet, M.; Pezzuto, J.M.; Santarsiero, B.D.; Mesecar, A.D. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem. J. 2010, 429, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, J.; Park, E.J.; Kondratyuk, T.P.; Marler, L.; Pezzuto, J.M.; van Breemen, R.B.; Mo, S.; Li, Y.; Cushman, M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J. Med. Chem. 2010, 53, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Churruca, I.; Eseberri, I.; Andres-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Calani, L.; Fietta, E.; Brighenti, F.; Crozier, A.; Meda, C.; Maggi, A.; Ottonello, S.; Del Rio, D. Anti-estrogenic activity of a human resveratrol metabolite. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Schueller, K.; Schaefer, L.M.; Pignitter, M.; Esefelder, L.; Somoza, V. Resveratrol and its metabolites inhibit pro-inflammatory effects of lipopolysaccharides in U-937 macrophages in plasma-representative concentrations. Food Funct. 2014, 5, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Calani, L.; Bocchi, L.; Delucchi, F.; Savi, M.; Ray, S.; Brighenti, F.; Stilli, D.; Del Rio, D. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.; Iwuchukwu, O.F.; Canney, D.J.; Zimmerman, C.L.; Nagar, S. In vivo-formed versus preformed metabolite kinetics of trans-resveratrol-3-sulfate and trans-resveratrol-3-glucuronide. Drug Metab. Dispos. 2012, 40, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, C.; Britton, R.G.; Patel, K.R.; Brown, K. Resveratrol-sulfates provide an intracellular reservoir for generation of parent resveratrol, which induces autophagy in cancer cells. Autophagy 2014, 10, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Youssef, S.; Bhattacharya, S.; Kenealey, J.; Polans, A.S.; van Ginkel, P.R. Resveratrol: Challenges in translation to the clinic—a critical discussion. Clin. Cancer Res. 2010, 16, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Moyano-Mendez, J.R.; Fabbrocini, G.; de Stefano, D.; Mazzella, C.; Mayol, L.; Scognamiglio, I.; Carnuccio, R.; Ayala, F.; la Rotonda, M.I.; de Rosa, G. Enhanced antioxidant effect of trans-resveratrol: Potential of binary systems with polyethylene glycol and cyclodextrin. Drug Dev. Ind. Pharm. 2014, 40, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kao, L.; Lin, C.C. Comparison of the antioxidant and transmembrane permeative activities of the different Polygonum cuspidatum extracts in phospholipid-based microemulsions. J. Agric. Food Chem. 2011, 59, 9135–9141. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsi, F. Evaluation of the stability and antioxidant activity of nanoencapsulated resveratrol during in vitro digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Chen, J.K.; Liao, M.H.; Lo, H.M.; Fang, J.Y. Development and evaluation of emulsion-liposome blends for resveratrol delivery. J. Nanosci. Nanotechnol. 2006, 6, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Kobierski, S.; Ofori-Kwakye, K.; Muller, R.H.; Keck, C.M. Resveratrol nanosuspensions: Interaction of preservatives with nanocrystal production. Pharmazie 2011, 66, 942–947. [Google Scholar] [PubMed]
- Stammet, M.; Kwon, G.S.; Rao, D.A. Drug loading in Pluronic(R) micelles made by solvent casting and equilibrium methods using resveratrol as a model drug. J. Control. Release 2010, 148, e50–e51. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ji, C.; Xu, H.; Li, X.; Ding, H.; Ye, M.; Zhu, Z.; Ding, D.; Jiang, X.; Ding, X.; et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int. J. Pharm. 2009, 375, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kristl, J.; Teskac, K.; Caddeo, C.; Abramovic, Z.; Sentjurc, M. Improvements of cellular stress response on resveratrol in liposomes. Eur. J. Pharm. Biopharm. 2009, 73, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomedicine 2013, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, F.; Bernardi, A.; Frozza, R.L.; Terroso, T.; Zanotto-Filho, A.; Jandrey, E.H.; Moreira, J.C.; Salbego, C.G.; Edelweiss, M.I.; Pohlmann, A.R.; et al. Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J. Biomed. Nanotechnol. 2013, 9, 516–526. [Google Scholar] [PubMed]
- Cadena, P.G.; Pereira, M.A.; Cordeiro, R.B.; Cavalcanti, I.M.; Barros Neto, B.; Pimentel, M.C.C.B.; José Luiz Lima Filho; Silva, V.L.; Santos-Magalhaes, N.S. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochim. Biophys. Acta 2013, 1828, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sih, J.; Bansal, S.S.; Filippini, S.; Ferrati, S.; Raghuwansi, K.; Zabre, E.; Nicolov, E.; Fine, D.; Ferrari, M.; Palapattu, G.; et al. Characterization of nanochannel delivery membrane systems for the sustained release of resveratrol and atorvastatin: New perspectives on promoting heart health. Anal. Bioanal. Chem. 2013, 405, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, X.; Lu, X.; Jiang, C.; Hu, Y.; Li, Q.; You, Y.; Fu, Z. Enhanced growth inhibition effect of resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf. B Biointerfaces 2009, 72, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Bernardi, A.; Paese, K.; Hoppe, J.B.; da Silva, T.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J. Biomed. Nanotechnol. 2010, 6, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomedicine 2012, 7, 1841–1850. [Google Scholar]
- Sanna, V.; Roggio, A.M.; Siliani, S.; Piccinini, M.; Marceddu, S.; Mariani, A.; Sechi, M. Development of novel cationic chitosan-and anionic alginate-coated poly(d,l-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int. J. Nanomedicine 2012, 7, 5501–5516. [Google Scholar]
- Lu, X.; Xu, H.; Sun, B.; Zhu, Z.; Zheng, D.; Li, X. Enhanced neuroprotective effects of resveratrol delivered by nanoparticles on hydrogen peroxide-induced oxidative stress in rat cortical cell culture. Mol. Pharm. 2013, 10, 2045–2053. [Google Scholar] [CrossRef]
- Lee, C.W.; Yen, F.L.; Huang, H.W.; Wu, T.H.; Ko, H.H.; Tzeng, W.S.; Lin, C.C. Resveratrol nanoparticle system improves dissolution properties and enhances the hepatoprotective effect of resveratrol through antioxidant and anti-inflammatory pathways. J. Agric. Food Chem. 2012, 60, 4662–4671. [Google Scholar] [PubMed]
- Tsai, C.H.; Vivero-Escoto, J.L.; Slowing, I.I.; Fang, I.J.; Trewyn, B.G.; Lin, V.S. Surfactant-assisted controlled release of hydrophobic drugs using anionic surfactant templated mesoporous silica nanoparticles. Biomaterials 2011, 32, 6234–6244. [Google Scholar] [PubMed]
- Bu, L.; Gan, L.C.; Guo, X.Q.; Chen, F.Z.; Song, Q.; Qi, Z.; Gou, X.J.; Hou, S.X.; Yao, Q. Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma. Int. J. Pharm. 2013, 452, 355–362. [Google Scholar]
- Guo, L.; Peng, Y.; Yao, J.; Sui, L.; Gu, A.; Wang, J. Anticancer activity and molecular mechanism of resveratrol-bovine serum albumin nanoparticles on subcutaneously implanted human primary ovarian carcinoma cells in nude mice. Cancer Biother. Radiopharm. 2010, 25, 471–477. [Google Scholar] [CrossRef]
- Biasutto, L.; Zoratti, M. Prodrugs of quercetin and resveratrol: A strategy under development. Current drug metabolism 2014, 15, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Sassi, N.; Azzolini, M.; Romio, M.; Paradisi, C.; Zoratti, M. Improving the efficacy of plant polyphenols. Anticancer Agents Med. Chem. 2014. [Google Scholar] [CrossRef]
- Nagamura, S.; Kanda, Y.; Kobayashi, E.; Gomi, K.; Saito, H. Synthesis and antitumor activity of duocarmycin derivatives. Chem. Pharm. Bull. (Tokyo) 1995, 43, 1530–1535. [Google Scholar] [CrossRef]
- Senter, P.D.; Beam, K.S.; Mixan, B.; Wahl, A.F. Identification and activities of human carboxylesterases for the activation of CPT-11, a clinically approved anticancer drug. Bioconjug. Chem. 2001, 12, 1074–1080. [Google Scholar] [CrossRef]
- Savolainen, J.; Leppanen, J.; Forsberg, M.; Taipale, H.; Nevalainen, T.; Huuskonen, J.; Gynther, J.; Mannisto, P.T.; Jarvinen, T. Synthesis and in vitro/in vivo evaluation of novel oral N-alkyl- and N,N-dialkyl-carbamate esters of entacapone. Life Sci. 2000, 67, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Thorberg, S.O.; Berg, S.; Lundstrom, J.; Pettersson, B.; Wijkstrom, A.; Sanchez, D.; Lindberg, P.; Nilsson, J.L. Carbamate ester derivatives as potential prodrugs of the presynaptic dopamine autoreceptor agonist (−)-3-(3-hydroxyphenyl)-N-propylpiperidine. J. Med. Chem. 1987, 30, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [PubMed]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of important biotech molecules: Delivery aspects. Expert Opin. Drug Del. 2008, 5, 371–383. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lin, H.S.; Ho, P.C.; Ng, K.Y. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Cottart, C.H.; Rhayem, Y.; Nivet-Antoine, V.; Djelidi, R.; Collin, F.; Beaudeux, J.L.; Bonnefont-Rousselot, D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta 2009, 634, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host-Guest interaction study of resveratrol with natural and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Mattarei, A.; Azzolini, M.; Carraro, M.; Sassi, N.; Zoratti, M.; Paradisi, C.; Biasutto, L. Acetal derivatives as prodrugs of resveratrol. Mol. Pharm. 2013, 10, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; Mattarei, A.; Beltramello, S.; Caliceti, P.; Salmaso, S.; Bernkop-Schnurch, A.; Garbisa, S.; Zoratti, M.; Paradisi, C. Absorption and metabolism of resveratrol carboxyesters and methanesulfonate by explanted rat intestinal segments. Cell. Physiol. Biochem. 2009, 24, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Gouyon, F.; Caillaud, L.; Carriere, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J. Physiol. 2003, 552, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, P.A.; Richardson, M.; Affleck, J.; Kellett, G.L. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem. J. 2000, 350, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I. Oral drug delivery utilizing intestinal OATP transporters. Adv. Drug Deliv. Rev. 2012, 64, 508–514. [Google Scholar] [CrossRef]
- Tamai, I.; Nakanishi, T. OATP transporter-mediated drug absorption and interaction. Curr. Opin. Pharmacol. 2013, 13, 859–863. [Google Scholar] [PubMed]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: Their roles in hepatic clearance and intestinal absorption. Biopharm. Drug Dispos. 2013, 34, 45–78. [Google Scholar] [CrossRef] [PubMed]
- Riha, J.; Brenner, S.; Bohmdorfer, M.; Giessrigl, B.; Pignitter, M.; Schueller, K.; Thalhammer, T.; Stieger, B.; Somoza, V.; Szekeres, T.; et al. Resveratrol and its major sulfated conjugates are substrates of organic anion transporting polypeptides (OATPs): Impact on growth of ZR-75–1 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 1830–1842. [Google Scholar] [CrossRef] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. OCTN cation transporters in health and disease: Role as drug targets and assay development. J. Biomol. Screen. 2013, 18, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm. Drug Dispos. 2013, 34, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Vacondio, F.; Silva, C.; Mor, M.; Testa, B. Qualitative structure-metabolism relationships in the hydrolysis of carbamates. Drug Metab. Rev. 2010, 42, 551–589. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; Garbisa, S.; Zoratti, M.; Paradisi, C. Determination of quercetin and resveratrol in whole blood—implications for bioavailability studies. Molecules 2010, 15, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2 and 3 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattarei, A.; Carraro, M.; Azzolini, M.; Paradisi, C.; Zoratti, M.; Biasutto, L. New Water-Soluble Carbamate Ester Derivatives of Resveratrol. Molecules 2014, 19, 15900-15917. https://doi.org/10.3390/molecules191015900
Mattarei A, Carraro M, Azzolini M, Paradisi C, Zoratti M, Biasutto L. New Water-Soluble Carbamate Ester Derivatives of Resveratrol. Molecules. 2014; 19(10):15900-15917. https://doi.org/10.3390/molecules191015900
Chicago/Turabian StyleMattarei, Andrea, Massimo Carraro, Michele Azzolini, Cristina Paradisi, Mario Zoratti, and Lucia Biasutto. 2014. "New Water-Soluble Carbamate Ester Derivatives of Resveratrol" Molecules 19, no. 10: 15900-15917. https://doi.org/10.3390/molecules191015900