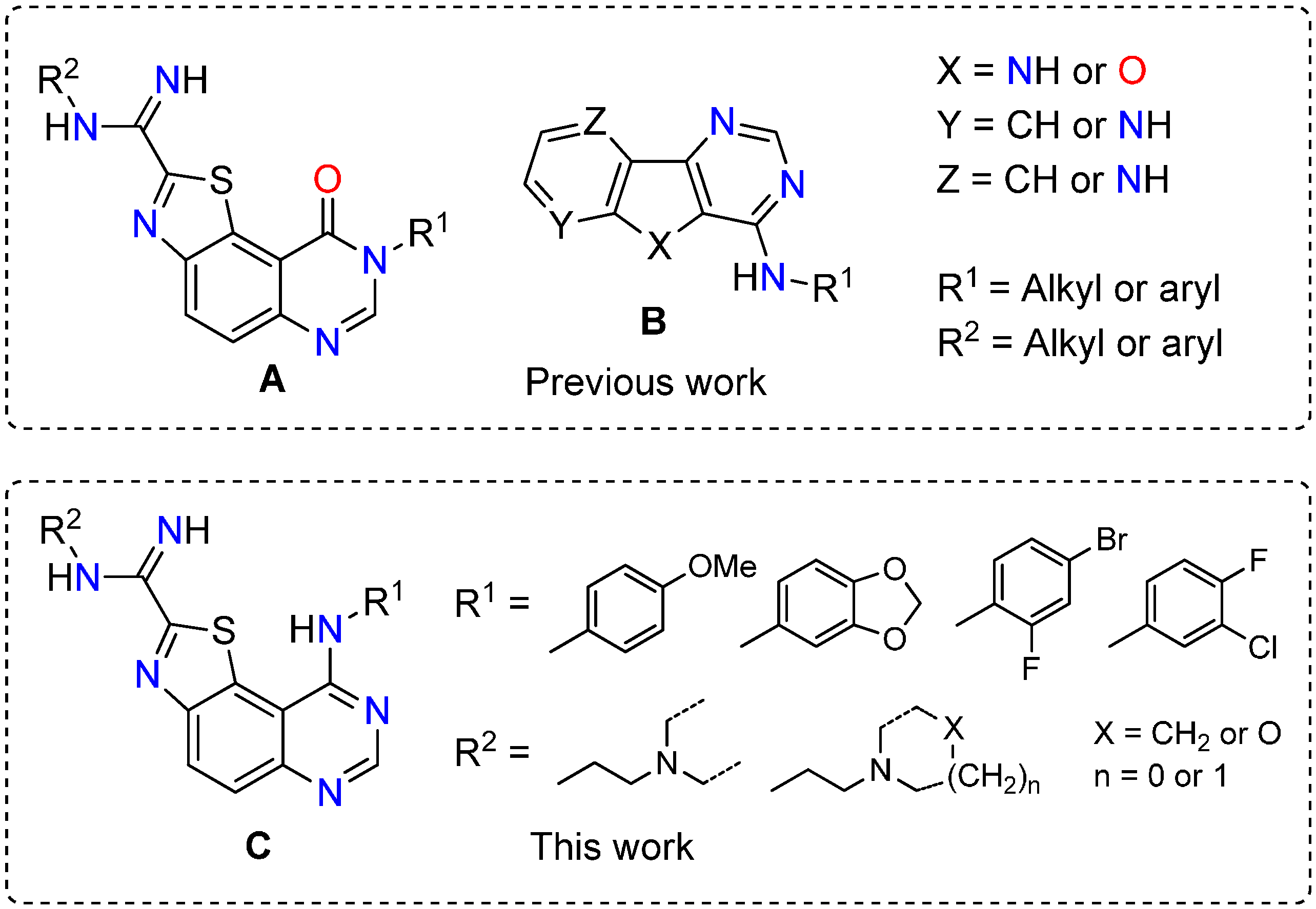
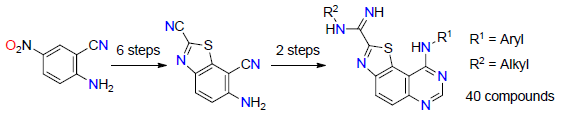
3.2. Synthesis
3.2.1. Synthesis of Formamidine 2 and Amines 3a, 3c and 3d
N'-(2-Cyano-4-nitrophenyl)-
N,
N-dimethylformamidine (
2),
N-(4-methoxyphenyl)-6-nitro-quinazolin-4-amine (
3a),
N-(4-bromo-2-fluorophenyl)-6-nitroquinazolin-4-amine (
3c) and
N-(3-chloro-4-fluorophenyl)-6-nitroquinazolin-4-amine (
3d) were prepared and characterized following the general procedure described in [
6].
N-(Benzo[d][1,3]dioxol-5-yl)-6-nitroquinazolin-4-amine (3b). A mixture of N'-(2-cyano-4-nitrophenyl)-N,N-dimethylformamidine (2, 1.0 g, 4.58 mmol) and 3,4-(methylenedioxy)aniline (0.63 g, 4.58 mmol) in acetic acid (5 mL) was heated at 118 °C under microwaves (600 W). On completion (followed by TLC or GC-MS), the reaction was cooled to ambient temperature. The separated solid was filtered and washed with diethyl ether to obtain the expected compound 3b (1.08 g, 76%) as a brown solid; mp > 260 °C. IR (cm−1) νmax 1581, 1512, 1490, 1321, 1245, 1222, 1188, 1031, 921, 896, 846, 807; 1H-NMR (DMSO-d6) δ 10.3 (s, 1H, NH), 9.57 (d, 1H, J = 2.1 Hz), 8.64 (s, 1H), 8.52 (dd, 1H, J1 = 2.1 Hz, J2 = 9.0 Hz), 7.88 (d, 1H, J = 9.0 Hz), 7.44 (s, 1H), 7.18 (m, 1H), 6.96 (d, 1H, J = 9.0 Hz), 5.75 (s, 2H); 13C-NMR (DMSO-d6) δ 158.6, 157.6, 152.9, 146.8, 144.2, 144.1, 132.3, 129.2, 126.2, 120.5, 116.1, 114.1, 107.6, 105.0, 101.2; HRMS calcd for C15H11N4O4 [M+H]+ 311.0780, found 311.0794.
3.2.2. Synthesis of Diamines 4b, 4c and 4d
General procedure: a stirred mixture of 3b, 3c and 3d (1 mmol), ammonium formate (5 mmol) and a catalytic amount of 10% palladium charcoal in 20 mL of ethanol was heated at reflux (600 W) for 30 min. The catalyst was removed by filtration through Celite and washed with ethanol. The resulting filtrate was evaporated under reduced pressure to give 4b, 4c and 4d.
N4-(Benzo[d][1,3]dioxol-5-yl)quinazoline-4,6-diamine (4b). Prepared from 36 (quant. yield); brown solid; mp = 112–114 °C. IR (KBr) νmax/cm−1 3339, 2976, 1619, 1572, 1530, 1499, 1448, 1384, 1343, 1255, 1190, 1095, 1045, 932, 882, 823; 1H-NMR (DMSO-d6) δ 8.20 (s, 1H), 7.51 (dd, 2H, J1 = 2.0 Hz, J2 = 9.0 Hz), 7.32 (d, 1H, J = 2.0 Hz), 7.23–7.18 (m, 2H), 6.91 (d, 1H, J = 9.0 Hz), 6.00 (s, 2H); 13C-NMR (DMSO-d6) δ 163.7, 156.3, 149.9, 147.3, 146.9, 143.2, 142.1, 134.0, 128.4, 123.6, 116.6, 115.2, 107.8, 104.6, 101.1; HRMS calcd for C15H13N4O2 [M+H]+: 281.1039, found 281.1033.
N4-(4-Bromo-2-fluorophenyl)quinazoline-4,6-diamine (4c). Prepared from 3c (yield: 93%); brown solid; mp = 216–218 °C. IR (cm−1) νmax 3245, 3067, 1923, 1614, 1561, 1533, 1493, 1424, 1390, 1335, 1237, 1206, 1070, 887, 846, 825; 19F-NMR (DMSO-d6) δ −115.6; 1H-NMR (DMSO-d6) δ 8.53 (s, 1H), 7.72 (d, 1H, J = 9.9 Hz), 7.62 (d, 1H, J = 8.7 Hz), 7.52 (m, 2H), 7.40–7.36 (m, 2H); 13C-NMR (DMSO-d6) δ 158.8, 158.4, 155.1, 149.6, 146.6, 131.2, 130.1, 128.0, 127.9, 126.0, 124.7, 124.5, 121.7, 120.0, 119.9, 119.8, 119.5, 115.1, 101.4; HRMS calcd for C14H11N4BrF [M+H]+: 334.0151, found 334.0158.
N4-(3-Chloro-4-fluorophenyl)quinazoline-4,6-diamine (4d). Prepared from 3d (99% yield); brown solid; mp = 244–246 °C. IR (cm−1) νmax 2501, 1633, 1614, 1568, 1503, 1432, 1373, 1266, 1214, 888, 871, 794; 19F-NMR (DMSO-d6) δ −123.9; 1H-NMR (DMSO-d6) δ 9.48 (s, 1H, NH), 8.36 (s, 1H), 8.21 (dd, 1H, J1 = 2.4 Hz, J2 = 6.9 Hz), 7.85–7.80 (m, 1H), 7.55 (d, 1H, J = 9.3 Hz), 7.41 (t, 1 H, J = 9.3 Hz); 7.30–7.24 (m, 2H); 13C-NMR (DMSO-d6) δ 155.7, 154.5, 151.3, 149.5, 147.5, 142.6, 137.4, 137.3, 128.7, 123.8, 122.8, 121.8, 121.7, 118.8, 188.6, 116.7, 116.6, 116.3, 100.9; HRMS calcd for C14H11N4ClF [M+H]+: 289.0656, found 289.0663.
3.2.3. Synthesis of Brominated Diamines 5a, 5c and 5d
General procedure: a solution of bromine (2.9 mmol) in dichloromethane (3 mL) was added dropwise, under an argon atmosphere, to a solution of amines 4a, 4c and 4d (2.9 mmol) in acetic acid (34 mL). After 3.5 h of stirring at room temperature, the solvent was removed in vacuo. Excess acetic acid was co-evaporated with heptane to afford the expected compounds 5a, 5c and 5d.
5-Bromo-N4-(4-methoxyphenyl)quinazoline-4,6-diamine (5a). Prepared from 4a (quant. Yield), beige solid; mp > 260 °C. IR (cm−1) νmax 3297, 3106, 3065, 2950, 2668, 1607, 1567, 1510, 1381, 1356, 1299, 1229, 1168, 1073, 1024, 820, 802; 1H-NMR (DMSO-d6) δ 10.54 (s, 1H, NH), 8.69 (s, 1H), 7.71–7.68 (m, 2H), 7.64 (d, 2H, J = 9.0 Hz), 7.03 (d, 2H, J = 9.0 Hz), 3.78 (s, 3H); 13C-NMR (DMSO-d6) δ 157.7, 157.1, 147.9, 145.9, 131.7, 129.3, 125.6, 125.2, 119.4, 114.1, 113.0, 96.3, 55.5; HRMS calcd for C15H14N4OBr [M+H]+: 345.0351, found 345.0367.
5-Bromo-N4-(4-bromo-2-fluorophenyl)quinazoline-4,6-diamine (5c). Prepared from 4c (quant. Yield), yellow solid; mp > 260 °C. IR (cm−1) νmax 3369, 3334, 3256, 1619, 1565, 1514, 1477, 1432, 1410, 1390, 1327, 1262, 1235, 1179, 1165, 1100, 1063, 954, 928, 875, 857, 828, 815; 19F-NMR (DMSO-d6) δ −119.3; 1H-NMR (DMSO-d6) δ 8.46 (s, 1H), 8.01 (t, 1H, J = 9.0 Hz), 7.69–7.61 (m, 2H), 7.53 (d, 1H, J = 9.0 Hz), 7.47 (m, 1H); 13C-NMR (DMSO-d6) δ 156.3, 155.9, 152.9, 147.0, 137.3, 127.5, 126.8, 124.9, 123.5, 118.9, 118.6, 117.5, 113.6, 94.9; HRMS calcd for C14H10N4Br2F [M+H]+: 410.9256, found 410.9243.
5-Bromo-N4-(3-chloro-4-fluorophenyl)quinazoline-4,6-diamine (5d). Prepared from 4d (quant. Yield), orange solid; mp > 260 °C. IR (cm−1) νmax 3464, 3319, 3286, 3185, 2736, 1705, 1626, 1566, 1519, 1495, 1425, 1384, 1360, 1329, 1302, 1267, 1214, 1159, 1136, 1054, 930, 872, 826; 19F-NMR (DMSO-d6) δ −118.8; 1H-NMR (DMSO-d6) δ 10.51 (s, 1H, NH), 8.75 (s, 1H), 8.04 (dd, 1H, J1 = 2.4 Hz, J2 = 6.9 Hz), 7.71–7.68 (m, 2H), 7.63 (d, 1H, J = 9.0 Hz), 7.55 (t, 1H, J = 9.0 Hz); 13C-NMR (DMSO-d6) δ 157.4, 156.8, 153.5, 148.1, 145.9, 133.8, 133.7, 132.5, 126.1, 125.6, 125.0, 124.9, 119.8, 119.5, 119.3, 117.2, 116.9, 113.3, 95.9; HRMS calcd for C14H10N4BrClF [M+H]+: 366.9761, found 366.9770.
3.2.4. Synthesis of Imino-1,2,3-dithiazoles 6a, 6c and 6d
General procedure: a suspension of the 5-bromoquinazoline 5a, 5c and 5d. (10 mmol), 4,5-dichloro-1,2,3-dithiazolium chloride 12 (22 mmol) in dichloromethane (50 mL) was stirred at room temperature under an argon atmosphere. After 3 h of stirring at room temperature, pyridine (44 mmol) was added and the mixture was stirred again for 1 h at room temperature. Then the resulting solution was concentrated under reduced pressure. The obtained crude residue was purified by flash chromatography (DCM–EtOAc, 8:2) to afford the expected compounds 6a, 6c and 6d.
(Z)-5-Bromo-N6-(4-chloro-5H-1,2,3-dithiazol-5-ylidene)-N4-(4-methoxyphenyl)quinazoline-4,6-diamine (6a). Prepared from 5a (yield: 39%), brown solid; mp > 260 °C. IR (cm−1) νmax 3239, 1607, 1567, 1504, 1440, 1377, 1303, 1249, 1172, 1027, 830; 1H-NMR (DMSO-d6) δ 10.93 (s, 1H, NH), 8.96 (s, 1H), 7.95–7.85 (m, 2H), 7.66 (d, 2H, J = 9.0 Hz), 7.07 (d, 2H, J = 9.0 Hz), 3.79 (s, 3H); HRMS calcd for C17H12N5OS2BrCl (M + H+): 479.9355, found 479.9362.
(Z)-5-Bromo-N4-(4-bromo-2-fluorophenyl)-N6-(4-chloro-5H-1,2,3-dithiazol-5-ylidene)quinazoline-4,6-diamine (6c). Prepared from 5c (yield: 30%), yellow solid; mp = 198–200 °C. IR (cm−1) νmax 3336, 3086, 1704, 1621, 1610, 1593, 1530, 1489, 1478, 1407, 1389, 1314, 1237, 1180, 1146, 1109, 945, 921, 859, 852, 835, 818, 810; 19F-NMR (DMSO-d6) δ −118.3; 1H-NMR (DMSO-d6) δ 9.78 (s, 1H, NH), 8.57 (s, 1H), 7.98 (m, 1H), 7.91 (d, 1H, J = 9.0 Hz), 7.76 (d, 1H, J = 9.0 Hz), 7.70 (m, 1H), 7.49 (m, 1H); HRMS calcd for C16H8N5S2Br2ClF [M+H]+: 545.8260, found 585.8273.
(Z)-5-Bromo-N4-(3-chloro-4-fluorophenyl)-N6-(4-chloro-5H-1,2,3-dithiazol-5-ylidene)quinazoline-4,6-diamine (6d). Prepared from 5d (yield: 39%), orange solid; mp = 218–220 °C. IR (cm−1) νmax 3353, 2980, 1571, 1534, 1492, 1414, 1383, 1337, 1261, 1207, 1153, 1054, 960, 921, 869, 803; 19F-NMR (DMSO-d6) δ−118.1; 1H-NMR (DMSO-d6) δ 9.90 (s, 1H, NH), 8.62 (s, 1H), 8.08 (dd, 1H, J1 = 2.4 Hz, J2 = 6.9 Hz), 7.93 (d, 1H, J = 9.0 Hz), 7.77 (d, 1H, J = 9.0 Hz), 7.71–7.66 (m, 1H), 7.46 (t, 1H, J = 9.0 Hz); HRMS calcd for C16H8N5S2BrCl2F [M+H]+: 501.8765, found 501.8766.
3.2.5. Synthesis of 6-Aminobenzo[d]thiazole-2,7-dicarbonitrile (16) and Its Dimethylformimidamide Derivative (17)
tert-Butyl 2-cyano-4-nitrophenylcarbamate (11). To a solution of 2-amino-5-nitrobenzonitrile (1) (10.0 g, 61.3 mmol) in dichloromethane (100 mL) were added triethylamine (8.50 mL, 61.3 mmol), di-tert-butyl dicarbonate (26.8 g, 123 mmol), and 4-(dimethylamino)pyridine (7.50 g, 61.3 mmol). The solution was stirred for 4 h at room temperature under an argon atmosphere. The solvent was removed in vacuo and the crude residue was purified by flash chromatography (DCM-petroleum ether, 8:2) to afford the expected compound 11 (14.6 g, 91% yield) as a white solid; mp = 134–136 °C. IR (cm−1) νmax 3412, 3072, 3012, 2982, 2935, 2229, 1735, 1617, 1582, 1543, 1508, 1473, 1455, 1420, 1372, 1350, 1320, 1303, 1257, 1234, 1176, 1143, 1052, 1028, 923, 915, 889, 853; .1H-NMR (DMSO-d6) δ 10.04 (s, 1H, NH), 8.63 (d, 1H, J = 2.7 Hz), 8.44–8.40 (dd, 1H, J1 = 2.7 Hz, J2 = 9.3 Hz), 7.85 (d, 1H, J = 9.3 Hz), 1.49 (s, 9H); 13C-NMR (DMSO-d6) δ 153.1, 146.6, 142.6, 129.3, 128.8, 123.4, 115.2, 105.3, 81.2, 27.3; HRMS calcd for C12H12N3O4 [M+H]+: 262.0828, found 262.0831.
tert-Butyl 4-amino-2-cyanophenylcarbamate (12). A stirred mixture of 11 (10.0 g, 37.9 mmol), ammonium formate (189.5 mmol) and palladium charcoal (10%) in 300 mL of ethanol was heated at reflux under microwaves (600 W) for 30 min. The catalyst was removed by filtration through Celite and washed with ethanol. The resulting filtrate was evaporated under reduced pressure. Then, the residue was dissolved in EtOAc, washed with water, dried over MgSO4 and concentrated under reduced pressure to give the reduced compound 12 (8.4 g, 95% yield) as a pale yellow solid, mp = 126–128 °C. IR (cm−1) νmax 3476, 3431, 3365, 3398, 2988, 2934, 2222, 1697, 1628, 1587, 1521, 1443, 1429, 1392, 1367, 1324, 1294, 1274, 1250, 1230, 1161, 1053, 1028, 947, 902, 872, 849, 824; 1H-NMR (DMSO-d6) δ 8.82 (s, 1H, NH), 7.02 (d, 1H, J = 8.1 Hz), 6.81 (s, 1H), 6.78 (d, 1H, J = 2.7 Hz), 5.44 (s, 2H, NH2), 1.43 (s, 9H); 13C-NMR (DMSO-d6) δ 153.8, 146.7, 128.8, 127.9, 118.7, 117.5, 115.9, 109.8, 79.0, 28.0; HRMS calcd for C12H16N3O2 [M+H]+: 234.1243, found 234.1240.
tert-Butyl 4-amino-3-bromo-2-cyanophenylcarbamate (13). A solution of bromine (25.1 mmol) in dichloromethane (1.3 mL) was added dropwise, under an argon atmosphere, to a solution of amine 12 (6.5 g, 27.9 mmol) in acetic acid (325 mL). After 2.5 h of stirring at room temperature, the solvent was removed in vacuo. The excess of acetic acid was co-evaporated with heptane to afford the expected compound 13 (10.1 g, 99% yield) as a beige solid, mp = 163–165 °C. IR (cm−1) νmax 3327, 2826, 2605, 2566, 2236, 1955, 1716, 1610, 1561, 1496, 1481, 1398, 1369, 1280, 1238, 1193, 1153, 1059, 963, 906, 838; 1H-NMR (DMSO-d6) δ 9.05 (s, 1H, NH), 7.11 (d, 1H, J = 8.7 Hz), 7.05 (d, 1H, J = 8.7 Hz), 6.01 (s, 2H, NH2), 1.43 (s, 9H); 13C-NMR (DMSO-d6) δ 153.6, 144.1, 131.6, 126.9, 119.6, 116.1, 112.8, 107.8, 79.5, 28.1; HRMS calcd for C12H15N3O2Br [M+H]+: 312.0348, found 312.0354.
tert-Butyl-3-bromo-4-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)-2-cyanophenylcarbamate (14). A suspension of 13 (5.0 g, 16.0 mmol), 4,5-dichloro-1,2,3-dithiazolium chloride (7.34 g, 35.2 mmol) in dichloromethane (100 mL) was stirred at room temperature under argon atmosphere. After 3 h of stirring, pyridine (5.7 mL, 70.5 mmol) was added and the mixture was stirred again for 1 h at room temperature. The resulting solution was concentrated under reduced pressure. The obtained crude residue was purified by flash chromatography (DCM-petroleum ether, 5:5) to afford the expected compound 14 (3.3 g, 46% yield) as an orange solid, mp = 140–150 °C (dec). IR (cm−1) νmax 3366, 2977, 2935, 2227, 1714, 1597, 1570, 1560, 1508, 1392, 1369, 1270, 1238, 1156, 1057, 971, 859, 846, 809; 1H-NMR (DMSO-d6) δ 9.64 (s, 1H, NH), 7.60 (d, 1H, J = 8.7 Hz), 7.55 (d, 1H, J = 8.7 Hz), 1.48 (s, 9H); 13C-NMR (DMSO-d6) δ 163.5, 152.9, 147.4, 146.0, 140.0, 126.1, 123.6, 117.7, 115.3, 112.2, 80.4, 27.9; HRMS calcd for C14H13N4O2S2BrCl [M+H]+: 446.9352, found 446.9340.
6-Amino-2-bromo-3-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)benzonitrile (15). A mixture of carbamate 14 (3.3 g, 7.4 mmol) and acetic acid (100 mL) was heated at 118 °C under microwaves (800 W) for 2 h. After cooling, the resulting solution was concentrated under reduced pressure to give the desired compound 15 (2.8 g, quantitative yield) as an orange solid, mp = 188–198 °C (dec.). IR (cm−1) νmax/3421, 3340, 3231, 2220, 1701, 1647, 1596, 1575, 1473, 1405, 1291, 1251, 1192, 1137, 973, 869, 847, 804; 1H-NMR (DMSO-d6) δ 7.36 (d, 1H, J = 9.0 Hz), 6.91 (d, 1H, J = 9.0 Hz), 6.55 (s, 2H, NH2); 13C-NMR (DMSO-d6) δ 159.6, 151.7, 146.7, 137.8, 124.4, 119.0, 116.4, 115.7, 97.2; HRMS calcd for C9H5N4S2BrCl [M+H]+): 346.8828, found 346.8846.
6-Aminobenzo[d]thiazole-2,7-dicarbonitrile (16). A suspension of imine 15 (2.5 g, 7.2 mmol), copper(I) iodide (2.7 g, 14.4 mmol) in pyridine (50 mL) was heated at 130 °C under microwaves (400 W) for 20 min. After cooling, the mixture was dissolved in EtOAc and washed with a sodium thiosulfate solution. The organic layer was dried over MgSO4, and the solvent was removed in vacuo. The crude residue was purified by flash chromatography (DCM–EtOAc, 9:1) to afford the expected compound 16 (0.96 g, 67% yield) as a pale brown solid; mp = 248 °C. IR (KBr) νmax/cm−1 3433, 3350, 3250, 2225, 1653, 1593, 1487, 1451, 1415, 1330, 1290, 1206, 1161, 1128, 821; 1H-NMR (DMSO-d6) δ 8.11 (d, 1H, J = 9.3 Hz), 7.31 (s, 2H, NH2), 7.10 (d, 1H, J = 9.3 Hz); 13C-NMR (DMSO-d6) δ 154.6, 143.7, 141.6, 131.5, 130.9, 119.4, 116.9, 114.3, 83.6; HRMS calcd for C9H3N4S (M−H)−: 199.0078, found 199.0076.
(E)-N'-(2,7-Dicyanobenzo[d]thiazol-6-yl)-N,N-dimethylformimidamide (17). A suspension of 16 (0.47 g, 2.34 mmol) in dimethylformamide dimethyl acetal (6 mL) was heated at 70 °C under microwaves (600 W) during 2 min. After cooling, the brown precipitate formed was filtered, washed with Et2O and dried to afford the expected compound 17 (0.51 g, 86% yield) as a brown solid; mp = 185–187 °C. IR (cm−1) νmax 2932, 2901, 2224, 1622, 1566, 1500, 1450, 1410, 1387, 1368, 1272, 1229, 1173, 1099, 1058, 995, 964, 928, 874, 819; 1H-NMR (DMSO-d6) δ 8.33 (d, 2H, J = 9.3 Hz), 7.62 (d, 1H, J = 9.3 Hz), 3.15 (s, 3H), 3.07 (s, 3H); 13C-NMR (DMSO-d6) δ 157.3, 156.5, 145.9, 140.2, 133.0, 129.3, 120.3, 116.6, 113.3, 96.4, 34.3; HRMS calcd for C12H10N5S [M+H]+: 256.0657, found 256.0644.
3.2.6. Synthesis of Thiazolo[5,4-f]quinazoline-2-carbonitriles 7–10
A-General Procedure from 6a–d
A suspension of imine 6a, 6c and 6d (1 mmol), copper iodide (2 mmol) in pyridine (4 mL) was heated at 120 °C (600 W) for 20 min. After cooling, the mixture was dissolved in ethyl acetate, washed with sodium thiosulfate solution. The organic layer was dried over MgSO4, and the solvent was removed in vacuo. The crude residue was purified by flash chromatography (DCM–EtOAc, 8:2) to afford the expected compounds 7, 9 and 10.
B-General Procedure from 17
A mixture of (E)-N'-(2,7-dicyanobenzo[d]thiazol-6-yl)-N,N-dimethylformimidamide (17, 0.05 g, 0.19 mmol) and the appropriate amine (0.29 mmol, 1.5 eq) in acetic acid (2 mL) was heated under microwaves at 118 °C (600 W). On completion (followed by TLC), the reaction was cooled to ambient temperature. The solvent was removed in vacuo and the crude residue was purified by flash chromatography to afford the expected compounds 7–10.
9-(4-Methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (7). Orange solid (yield: (A) 24%; (B) 99%); mp > 260 °C. IR IR (cm−1) νmax 3346, 2977, 2361, 2227, 1644, 1609, 1581, 1503, 1460, 1377, 1354, 1303, 1239, 1164, 1129, 1051, 1032, 975, 829; 1H-NMR (DMSO-d6) δ 8.44 (s, 1H), 7.95 (d, 1H, J = 9.0 Hz), 7.70 (d, 1H, J = 9.0 Hz), 7.45 (d, 2H, J = 9.0 Hz), 6.99 (d, 2H, J = 9.0 Hz), 3.76 (s, 3H); HRMS calcd for C17H12N5OS [M+H]+: 334.0763, found 334.0758.
9-(Benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (8). Orange solid (yield: (B) 95%); mp > 260 °C. IR (cm−1) νmax 2894, 2226, 1734, 1706, 1645, 1609, 1581, 1555, 1499, 1471, 1377, 1354, 1304, 1264, 1236 KBr, 1211, 1188, 1162, 1125, 1086, 1037, 972, 938, 924, 859, 829, 809; 1H-NMR (DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 8.14 (m, 1H), 7.76 (m, 1H), 6.94 (d, 2H, J = 9.0 Hz), 6.72 (m, 1H), 6.02 (s, 2H); HRMS calcd for C17H9N5O2S [M+H]+: 348.0555, found 348.0566.
9-(4-Bromo-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (9). Brown solid (yield: (A) 30%; (B) 70%); mp > 260 °C. IR (cm−1) νmax 3325, 3053, 2230, 1649, 1614, 1582, 1556, 1499, 1462, 1380, 1351, 1250, 1154, 1132, 1052, 969, 904, 875, 817; 19F-NMR (DMSO-d6) δ −119.9; 1H-NMR (DMSO-d6) δ 8.55 (d, 1H, J = 9.0 Hz), 8.26 (d, 1H, J = 9.0 Hz), 7.78 (m, 1H), 7.55–7.52 (m, 1H), 7.38–7.25 (m, 2H), HRMS calcd for C16H8N5SBrF [M+H]+: 399.9668, found 399.9662.
9-(3-Chloro-4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (10). Yellow solid (yield: (A) 64%; (B) 77%); mp = 252 °C. IR (cm−1) νmax 3456, 3015, 2970, 2946, 2229, 1642, 1441, 1153, 1129, 1051, 968, 903, 817, 774, 695; 19F-NMR (DMSO-d6) δ −123.8; 1H-NMR (DMSO-d6) δ 8.43 (d, 1H, J = 9.0 Hz), 8.30 (s, 1H), 7.62 (m, 2H), 7.34 (m, 2H); HRMS calcd for C16H8N5SClF [M+H]+: 356.0156, found 356.0167.
3.2.7. Synthesis of Carboximidamides 7a–g, 8a–g, 9a–g and 10a–g
General procedure: A stirred mixture of carbonitrile 16 (1 mmol) and appropriate amine (1.2 mmol) in dry THF (7 mL) under argon was stirred overnight at room temperature. The solvent was removed in vacuo and the crude residue purified by flash chromatography to afford the expected carboximidamides.
9-(4-Methoxyphenylamino)-N-(2-morpholinoethyl)thiazolo[5,4-f]quinazoline-2-carboximidamide (7a). Prepared from carbonitrile 7 and N-aminoethylmorpholine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 41%); mp = 130–132 °C. IR (cm−1) νmax 3362, 3175, 2920, 2852, 1643, 1567, 1504, 1469, 1386, 1234, 1111, 1032, 964, 836; 1H-NMR (MeOD-d4) δ 8.37 (d, 1H, J = 9.0 Hz), 8.11 (s, 1H), 7.75 (d, 1H, J = 9.0 Hz), 7.26 (d, 2H, J = 9.0 Hz), 7.01 (d, 2H, J = 9.0 Hz), 3.82 (s, 3H), 3.73 (m, 4H), 3.48 (t, 2H, J = 7.0 Hz), 2.76 (t, 2H, J = 7.0 Hz), 2.62 (m, 4H); HRMS calcd for C23H26N7O2S [M+H]+: 464.1869, found 464.1874.
9-(4-Methoxyphenylamino)-N-(2-(piperidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboximidamide (7b). Prepared from carbonitrile 7 and N-aminoethylpiperidine. Flash chromatography eluent (DCM–MeOH, 3:7). Yellow solid (yield: 43%); mp = 132–134 °C. IR (cm−1) νmax 2933, 2852, 1640, 1613, 1572, 1507, 1376, 1349, 1302, 1283, 1237, 1155, 1124, 1035, 962, 833; 1H-NMR (MeOD-d4) δ 8.34 (d, 1H, J = 9.0 Hz), 8.08 (s, 1H), 7.73 (d, 1H, J = 9.0 Hz), 7.23 (d, 2H, J = 9.0 Hz), 6.99 (d, 2H, J = 9.0 Hz), 3.82 (s, 3H), 3.49 (t, 2H, J = 7.0 Hz), 2.73 (t, 2H, J = 7.0 Hz), 2.56 (m, 4H), 1.66 (m, 4H), 1.52 (m, 2H); HRMS calcd for C24H28N7OS [M+H]+: 462.2076, found 462.2098.
9-(4-Methoxyphenylamino)-N-(2-(pyrrolidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboximidamide (7c). Prepared from carbonitrile 7 and N-aminoethylpyrrolidine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 47%); mp = 134–136 °C. IR (cm−1) νmax 2957, 2798, 1643, 1615, 1573, 1504, 1379, 1341, 1284, 1237, 1147, 1086, 1033, 962, 880, 833; 1H-NMR (MeOD-d4) δ 8.34 (d, 1H, J = 9.0 Hz), 8.08 (s, 1H), 7.73 (d, 1H, J = 9.0 Hz), 7.23 (d, 2H, J = 9.0 Hz), 6.99 (d, 2H, J = 9.0 Hz), 3.82 (s, 3H), 3.49 (t, 2H, J = 7.0 Hz), 2.88 (t, 2H, J = 7.0 Hz), 2.67 (m, 4H), 1.85 (m, 4H); HRMS calcd for C23H26N7OS [M+H]+: 448.1920, found 448.1926.
N-(2-(Dimethylamino)ethyl)-9-(4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carboximidamide (7d). Prepared from carbonitrile 7 and 2-dimethylaminoethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 53%); mp = 154–156 °C. IR (cm−1) νmax 2945, 2827, 2773, 1643, 1614, 1572, 1505, 1464, 1379, 1341, 1237, 1177, 1033, 962, 832; 1H-NMR (MeOD-d4) δ 8.30 (d, 1H, J = 9.0 Hz), 8.06 (s, 1H), 7.69 (d, 1H, J = 9.0 Hz), 7.22 (d, 2H, J = 9.0 Hz), 6.97 (d, 2H, J = 9.0 Hz), 3.81 (s, 3H), 3.43 (t, 2H, J = 7.0 Hz), 2.70 (t, 2H, J = 7.0 Hz), 2.34 (s, 6H); HRMS calcd for C21H24N7OS [M+H]+: 422.1763, found 422.1766.
N-(2-(Diethylamino)ethyl)-9-(4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carboximidamide (7e). Prepared from carbonitrile 7 and diethylethylenediamine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 50%); mp = 103–105 °C. IR (cm−1) νmax 2967, 2832, 1641, 1614, 1572, 1507, 1378, 1341, 1285, 1237, 1178, 1086, 1034, 962, 834; 1H-NMR (MeOD-d4) δ 8.35 (d, 1H, J = 9.0 Hz), 8.08 (s, 1H), 7.74 (d, 1H, J = 9.0 Hz), 7.23 (d, 2H, J = 9.0 Hz), 7.01 (d, 2H, J = 9.0 Hz), 3.82 (s, 3H), 3.43 (t, 2H, J = 7.0 Hz), 2.86 (t, 2H, J = 7.0 Hz), 2.71 (q, 4H, J = 7.0 Hz), 1.11 (t, 6H, J = 7.0 Hz); HRMS calcd for C23H28N7OS [M+H]+: 450.2076, found 450.2058.
N-Benzyl-9-(4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carboximidamide (7f). Prepared from carbonitrile 7 and benzylamine. Flash chromatography eluent (DCM–EtOAc, 2:8). Yellow solid (yield: 28%); mp = 128–130 °C. IR (cm−1) νmax 3362, 3028, 2832, 1640, 1598, 1572, 1509, 1377, 1356, 1299, 1238, 1177, 1084, 1030, 962, 833; 1H-NMR (MeOD-d4) δ 8.34 (d, 1H, J = 9.0 Hz), 8.08 (s, 1H), 7.73 (d, 1H, J = 9.0 Hz), 7.35 (m, 4H), 7.26 (m, 3H), 6.99 (d, 2H, J = 9.0 Hz), 4.55 (s, 2H), 3.83 (s, 3H); HRMS calcd for C24H21N6OS [M+H]+: 441.1498, found 441.1507.
9-(4-Methoxyphenylamino)-N,N-dimethylthiazolo[5,4-f]quinazoline-2-carboximidamide (7g). Prepared from carbonitrile 7 and dimethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Pale yellow solid (yield: 67%); mp = 150–152 °C. IR (KBr) νmax/cm−1 3139, 2924, 1681, 1644, 1571, 1509, 1383, 1347, 1286, 1237, 1176, 1031, 966, 834; 1H-NMR (MeOD-d4) δ 8.15 (d, 1H, J = 9.0 Hz), 7.84 (s, 1H), 7.53 (d, 1H, J = 9.0 Hz), 7.08 (d, 2H, J = 9.0 Hz), 6.89 (d, 2H, J = 9.0 Hz), 3.77 (s, 3H), 3.13 (s, 6H); HRMS calcd for C19H19N6OS [M+H]+: 379.1341, found 379.1333.
9-(Benzo[d][1,3]dioxol-5-ylamino)-N-(2-morpholinoethyl)thiazolo[5,4-f]quinazoline-2-carboximidamide (8a). Prepared from carbonitrile 8 and N-aminoethylmorpholine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 41%); mp = 168–170 °C. IR (cm−1) νmax 3324, 2965, 2901, 2861, 2812, 1641, 1616, 1587, 1503, 1468, 1382, 1353, 1304, 1271, 1232, 1180, 1148, 1114, 1067, 1036, 969, 935, 839; 1H-NMR (MeOD-d4) δ 8.33 (d, 1H, J = 9.0 Hz), 7.96 (s, 1H), 7.71 (d, 1H, J = 9.0 Hz), 6.87 (d, 1H, J = 9.0 Hz), 6.74 (s, 1H), 6.64 (d, 1H, J = 9.0 Hz), 5.96 (s, 2H), 3.74 (m, 4H), 3.55 (t, 2H, J = 7.0 Hz), 2.75 (t, 2H, J = 7.0 Hz), 2.61 (m, 4H); HRMS calcd for C23H24N7O3S [M+H]+: 478.1661, found 478.1649.
9-(Benzo[d][1,3]dioxol-5-ylamino)-N-(2-(piperidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboximi-damide (8b). Prepared from carbonitrile 8 and N-aminoethylpiperidine. Flash chromatography eluent (DCM–MeOH, 3:7). Yellow solid (yield: 34%); mp = 170–172 °C. IR (cm−1) νmax 3350, 2936, 2773, 2482, 2061, 1641, 1613, 1585, 1503, 1462, 1374, 1337, 1304, 1229, 1176, 1141, 1121, 1037, 983, 962, 931, 855, 832; 1H-NMR (MeOD-d4) δ 8.33 (d, 1H, J = 9.0 Hz), 7.89 (s, 1H), 7.69 (d, 1H, J = 9.0 Hz), 6.86 (d, 1H, J = 9.0 Hz), 6.75 (s, 1H), 6.63 (d, 1H, J = 9.0 Hz), 5.99 (s, 2H), 3.46 (t, 2H, J = 7.0 Hz), 2.71 (t, 2H, J = 7.0 Hz), 2.57 (m, 4H), 1.64 (m, 4H), 1.50 (m, 2H); HRMS calcd for C24H26N7O2S [M+H]+: 476.1869, found 476.1883.
9-(Benzo[d][1,3]dioxol-5-ylamino)-N-(2-(pyrrolidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (8c). Prepared from carbonitrile 8 and N-aminoethylpyrrolidine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 48%); mp = 172–174 °C. IR (cm−1) νmax 2906, 2803, 1643, 1614, 1573, 1500, 1467, 1377, 1347, 1305, 1265, 1228, 1175, 1121, 1036, 966, 933, 833; 1H-NMR (MeOD-d4) δ 8.27 (d, 1H, J = 9.0 Hz), 7.90 (s, 1H), 7.65 (d, 1H, J = 9.0 Hz), 6.86 (d, 1H, J = 9.0 Hz), 6.75 (s, 1H), 6.63 (d, 1H, J = 9.0 Hz), 5.97 (s, 2H), 3.46 (t, 2H, J = 7.0 Hz), 2.85 (t, 2H, J = 7.0 Hz), 2.67 (m, 4H), 1.83 (m, 4H); HRMS calcd for C23H24N7O2S [M+H]+: 462.1712, found 462.1736.
9-(Benzo[d][1,3]dioxol-5-ylamino)-N-(2-(dimethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (8d). Prepared from carbonitrile 8 and 2-dimethylaminoethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 10%); mp > 260 °C. IR (cm−1) νmax 3391, 2923, 2852, 1643, 1615, 1538, 1498, 1467, 1383, 1346, 1302, 1263, 1229, 1178, 1118, 1035, 965, 933, 856, 835; 1H-NMR (MeOD-d4) δ 8.17 (d, 1H, J = 9.0 Hz), 7.90 (s, 1H), 7.56 (d, 1H, J = 9.0 Hz), 6.86 (d, 1H, J = 9.0 Hz), 6.75 (s, 1H), 6.63 (d, 1H, J = 9.0 Hz), 5.91 (s, 2H), 3.43 (t, 2H, J = 7.0 Hz), 2.69 (t, 2H, J = 7.0 Hz), 2.33 (s, 6H); HRMS calcd for C21H22N7O2S [M+H]+: 436.1556, found 436.1549.
9-(Benzo[d][1,3]dioxol-5-ylamino)-N-(2-(diethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (8e). Prepared from carbonitrile 8 and diethylethylenediamine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 44%); mp = 144–146 °C. IR (cm−1) νmax 3333, 2970, 2920, 2832, 1641, 1614, 1585, 1502, 1463, 1421, 1378, 1348, 1303, 1231, 1179, 1145, 1122, 1088, 1037, 965, 933, 858, 831; 1H-NMR (MeOD-d4) δ 8.23 (d, 1H, J = 9.0 Hz), 7.89 (s, 1H), 7.62 (d, 1H, J = 9.0 Hz), 6.86 (d, 1H, J = 9.0 Hz), 6.75 (s, 1H), 6.63 (d, 1H, J = 9.0 Hz), 5.94 (s, 2H), 3.42 (t, 2H, J = 7.0 Hz), 2.83 (t, 2H, J = 7.0 Hz), 2.68 (q, 4H, J = 7.0 Hz), 1.10 (t, 6H, J = 7.0 Hz); HRMS calcd for C23H26N7O2S [M+H]+: 464.1869, found 464.1896.
9-(Benzo[d][1,3]dioxol-5-ylamino)-N-benzylthiazolo[5,4-f]quinazoline-2-carboximidamide (8f). Prepared from carbonitrile 8 and benzylamine. Flash chromatography eluent (DCM–EtOAc, 2:8). Yellow solid (yield: 21%); mp > 260 °C. IR (cm−1) νmax 2886, 2447, 1641, 1597, 1497, 1466, 1413, 1384, 1353, 1307, 1266, 1229, 1178, 1122, 1105, 1036, 967, 934, 861, 833; 1H-NMR (MeOD-d4) δ 8.27 (d, 1H, J = 9.0 Hz), 7.90 (s, 1H), 7.65 (d, 1H, J = 9.0 Hz), 6.86 (m, 4H), 6.75 (m, 3H), 6.63 (d, 1H, J = 9.0 Hz), 5.97 (s, 2H), 4.58 (s, 2H); HRMS calcd for C24H19N6O2S [M+H]+: 455.1290, found 455.1287.
9-(4-Bromo-2-fluorophenylamino)-N-(2-morpholinoethyl)thiazolo[5,4-f]quinazoline-2-carboximid-amide (9a). Prepared from carbonitrile 9 and N-aminoethylmorpholine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 85%); mp = 190–192 °C. IR (cm−1) νmax 3374, 2975, 2361, 1645, 1585, 1381, 1265, 1227, 1090, 1053, 973, 916, 882, 825; 19F-NMR (DMSO-d6) δ −120.0; 1H-NMR (DMSO-d6) δ 8.52 (d, 1H, J = 9.0 Hz), 8.26 (m, 1H), 7.78 (m, 1H), 7.57 (m, 1H), 7.36 (m, 1H), 7.16 (m, 1H), 3.66 (t, 4H, J = 4.5 Hz), 3.48–3.42 (m, 2H), 2.69 (t, 2H, J = 6.0 Hz), 2.61 (m, 4H); HRMS calcd for C22H22N7OSBrF [M+H]+: 530.0774, found 530.0782.
9-(4-Bromo-2-fluorophenylamino)-N-(2-(piperidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (9b). Prepared from carbonitrile 9 and N-aminoethylpiperidine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 72%); mp = 142–144 °C. IR (cm−1) νmax 3340, 2975, 2930, 2361, 1615, 1561, 1476, 1380, 1348, 1262, 1197, 1153, 1114, 1052, 881, 821; 19F-NMR (CDCl3) δ −123.8; 1H-NMR (CDCl3) δ 8.25 (d, 1H, J = 9.0 Hz), 8.09 (s, 1H), 7.55 (d, 1H, J = 9.0 Hz), 7.50–7.45 (m, 1H), 7.29 (t, 1H, J = 9.0 Hz), 7.12–7.06 (m, 1H), 3.54 (m, 2H), 2.67 (t, 2H, J = 5.7 Hz), 2.54 (m, 4H), 1.66–1.60 (m, 4H), 1.49 (m, 2H); HRMS calcd for C23H24N7SBrF [M+H]+: 528.0981, found 528.0986.
9-(4-Bromo-2-fluorophenylamino)-N-(2-(pyrrolidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (9c). Prepared from carbonitrile 9 and N-aminoethylpyrrolidine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 68%); mp = 138–140 °C. IR (cm−1) νmax 2968, 2361, 1563, 1476, 1380, 1262, 1223, 1067, 965, 880, 822; 19F-NMR (MeOD-d4) δ −123.6; 1H-NMR (MeOD-d4) δ 8.29 (d, 1H, J = 9.0 Hz), 7.89 (s, 1H), 7.63 (d, 1H, J = 9.0 Hz), 7.35–7.29 (m, 2H), 7.09 (t, 1H, J = 9.0 Hz), 3.46 (t, 2H, J = 6.6 Hz), 2.87 (t, 2H, J = 6.6 Hz), 2.69 (m, 4H), 1.89 (m, 4H); HRMS calcd for C22H22N7SBrF [M+H]+: 514.0825, found 514.0825.
9-(4-Bromo-2-fluorophenylamino)-N-(2-(dimethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (9d). Prepared from carbonitrile 9 and 2-dimethylaminoethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 64%); mp = 128–130 °C. IR (cm−1) νmax 3274, 3058, 2940, 2861, 2822, 2773, 1618, 1560, 1478, 1380, 1341, 1262, 1197, 1155, 1112, 1038, 965, 881, 823; 19F-NMR (MeOD-d4 ) δ −122.3; 1H-NMR (MeOD-d4) δ 8.33 (d, 1H, J = 9.0 Hz), 7.92 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 7.41–7.36 (m, 2H), 7.15 (t, 1H, J = 9.0 Hz), 3.46 (t, 2H, J = 6.6 Hz), 2.76 (t, 2H, J = 6.6 Hz), 2.37 (s, 6H); HRMS calcd for C20H20N7SBrF [M+H]+: 488.0668, found 488.0688.
9-(4-Bromo-2-fluorophenylamino)-N-(2-(diethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (9e). Prepared from carbonitrile 9 and diethylethylenediamine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 86%); mp = 88–100 °C. IR (cm−1) νmax 2965, 2360, 1793, 1619, 1520, 1477, 1378, 1265, 1198, 1067, 966, 880, 822; 19F-NMR (MeOD-d4) δ −122.1; 1H-NMR (MeOD-d4) δ 8.30 (d, 1H, J = 9.0 Hz), 7.93 (s, 1H), 7.66 (d, 1H, J = 9.0 Hz), 7.38–7.33 (m, 2H), 7.13 (t, 1H, J = 9.0 Hz), 3.58 (t, 2H, J = 6.6 Hz), 2.82 (t, 2H, J = 6.6 Hz), 2.68 (m, 4H), 1.09 (m, 6H); HRMS calcd for C22H24N7SBrF [M+H]+: 516.0981, found 516.0988.
N-Benzyl-9-(4-bromo-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carboximidamide (9f). Prepared from carbonitrile 9 and benzylamine. Flash chromatography eluent (DCM–EtOAc, 2:8). Yellow solid (yield: 68%); mp = 130–132 °C. IR (cm−1) νmax 2964, 2903, 2360, 1815, 1614, 1477, 1379, 1262, 1153, 1113, 1069, 966, 880, 821; 19F-NMR (MeOD-d4) δ −122.2; 1H-NMR (MeOD-d4) δ 8.35 (d, 1H, J = 9.0 Hz), 7.92 (s, 1H), 7.69 (d, 1H, J = 9.0 Hz), 7.43 (m, 3H), 7.39–7.30 (m, 3H), 7.25 (m, 1H), 7.15 (t, 1H, J = 9.0 Hz), 4.53 (s, 2H); HRMS calcd for C23H17N6SBrF [M+H]+: 507.0403, found 507.0412.
9-(4-Bromo-2-fluorophenylamino)-N-(2-(dimethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (9g). Prepared from carbonitrile 9 and dimethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 40%); mp = 222–224 °C. IR (cm−1) νmax 3270, 3154, 3061, 2923, 1634, 1584, 1519, 1492, 1410, 1346, 1291, 1226, 1201, 1152, 1116, 1049, 964, 881, 863, 831; 19F-NMR (MeOD-d4) δ −121.6; 1H-NMR (MeOD-d4) δ 8.18 (d, 1H, J = 9.0 Hz), 7.87 (s, 1H), 7.53 (d, 1H, J = 9.0 Hz), 7.28–7.19 (m, 2H), 7.12 (t, 1H, J = 9.0 Hz), 3.11 (s, 6H); HRMS calcd for C18H15N6SBrF [M+H]+: 445.0246, found 445.0264.
9-(3-Chloro-4-fluorophenylamino)-N-(2-morpholinoethyl)thiazolo[5,4-f]quinazoline-2-carboximid-amide (10a). Prepared from carbonitrile 10 and N-aminoethylmorpholine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 71%); mp = 158–160 °C. IR (cm−1) νmax 2956, 2816, 1797, 1614, 1561, 1485, 1256, 1199, 1113, 1069, 966, 916, 816; 19F-NMR (DMSO-d6) δ −119.1; 1H-NMR (DMSO-d6) δ 8.87 (s, 1H, NH), 8.43 (d, 1H, J = 7.2 Hz), 8.03 (m, 1H), 7.90 (m, 1H), 7.52 (m, 1H), 7.39 (m, 1H), 7.18 (m, 1H), 3.78–3.75 (m, 4H), 3.52 (m, 2H), 2.76–2.72 (m, 2H), 2.60 (m, 4H); HRMS calcd for C22H22N7OSClF [M+H]+: 486.1279, found 486.1292.
9-(3-Chloro-4-fluorophenylamino)-N-(2-(piperidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (10b). Prepared from carbonitrile 10 and N-aminoethylpiperidine. Flash chromatography eluent (DCM–MeOH, 3:7). Orange solid (yield: 82%); mp = 146–148 °C. IR (cm−1) νmax 2928, 2361, 1572, 1483, 1380, 1255, 1201, 1121, 1086, 1051, 964, 879, 818; 19F-NMR (CDCl3) δ ‒123.8; 1H-NMR (CDCl3) δ 8.62 (s, 1H), 8.35 (d, 1H, J = 9.0 Hz), 7.90 (d, 1H, J = 9.0 Hz), 7.70 (m, 1H), 7.32 (m, 1H), 7.12 (t, 1H, J = 9.0 Hz), 3.50 (m, 2H), 2.63 (t, 2H, J = 5.7 Hz), 2.49 (m, 4H), 1.63–1.58 (m, 4H), 1.48 (m, 2H); HRMS calcd for C23H24N7SClF [M + H]+: 484.1486, found 484.1501.
9-(3-Chloro-4-fluorophenylamino)-N-(2-(pyrrolidin-1-yl)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (10c). Prepared from carbonitrile 10 and N-aminoethylpyrrolidine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 34%); mp = 166–168 °C. IR (cm−1) νmax 3381, 3146, 2965, 2803, 1641, 1617, 1562, 1486, 1383, 1341, 1253, 1200, 1127, 1050, 965, 876, 816; 19F-NMR (MeOD-d4) δ −125.6; 1H-NMR (MeOD-d4) δ 8.22 (d, 1H, J = 9.0 Hz), 7.95 (s, 1H), 7.59 (d, 1H, J = 9.0 Hz), 7.31 (m, 1H), 7.18 (t, 1H, J = 9.0 Hz), 7.15–7.12 (m, 1H), 3.45 (m, 2H), 2.85 (m, 2H), 2.67 (m, 4H), 1.83 (m, 4H); HRMS calcd for C22H22N7SClF [M+H]+: 470.1330, found 470.1340.
9-(3-Chloro-4-fluorophenylamino)-N-(2-(dimethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (10d). Prepared from carbonitrile 10 and 2-dimethylaminoethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Pale yellow solid (yield: 50%); mp = 172–174 °C. IR (cm−1) νmax 3224, 3038, 2950, 2861, 2824, 2773, 1618, 1560, 1488, 1386, 1323, 1254, 1195, 1127, 1086, 1052, 967, 816; 19F-NMR (MeOD-d4) δ −125.5; 1H-NMR (MeOD-d4) δ 8.34 (d, 1H, J = 9.0 Hz), 7.93 (s, 1H), 7.67 (d, 1H, J = 9.0 Hz), 7.29 (m, 1H), 7.18 (t, 1H, J = 9.0 Hz), 7.10–7.06 (m, 1H), 3.64 (t, 2H, J = 6.0 Hz), 3.27 (t, 2H, J = 6.0 Hz), 2.80 (s, 6H); HRMS calcd for C20H20N7SClF [M+H]+: 444.1173, found 444.1155.
9-(3-Chloro-4-fluorophenylamino)-N-(2-(diethylamino)ethyl)thiazolo[5,4-f]quinazoline-2-carboxi-midamide (10e). Prepared from carbonitrile 10 and diethylethylenediamine. Flash chromatography eluent (DCM–MeOH, 5:5). Orange solid (yield: 50%); mp = 140–142 °C. IR (cm−1) νmax 3295, 2969, 2812, 1671, 1618, 1560, 1489, 1386, 1346, 1254, 1196, 1127, 1052, 966, 817; 19F-NMR (MeOD-d4) δ −125.3; 1H-NMR (MeOD-d4) δ 8.29 (d, 1H, J = 9.0 Hz), 7.94 (s, 1H), 7.63 (d, 1H, J = 9.0 Hz), 7.29–7.20 (m, 2H), 7.09 (m, 1H), 3.60–3.56 (m, 2H), 2.90–2.83 (m, 2H), 2.77–2.74 (m, 4H), 1.17–1.06 (m, 6H); HRMS calcd for C22H24N7SClF [M+H]+: 472.1486, found 472.1502.
N-Benzyl-9-(3-chloro-4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carboximidamide (10f). Prepared from carbonitrile 10 and benzylamine. Flash chromatography eluent (DCM–EtOAc, 2:8). Yellow solid (yield: 69%); mp = 230–232 °C. IR (cm−1) νmax 3057, 1725, 1639, 1490, 1377, 1341, 1252, 1202, 1151, 1121, 1086, 1050, 965, 818; 19F-NMR (282 MHz, MeOD-d4) δ −125.3; 1H-NMR (300 MHz, MeOD-d4) δ 8.29 (d, 1H, J = 9.0 Hz), 7.94 (s, 1H), 7.63 (d, 1H, J = 9.0 Hz), 7.43 (m, 2H), 7.35–7.17 (m, 5H), 7.10 (m, 1H), 4.53 (s, 2H); HRMS calcd for C23H17N6SClF [M+H]+: 463.0908, found 463.0916.
9-(3-Chloro-4-fluorophenylamino)-N,N-dimethylthiazolo[5,4-f]quinazoline-2-carboximidamide (10g). Prepared from carbonitrile 10 and dimethylamine. Flash chromatography eluent (DCM–MeOH, 5:5). Yellow solid (yield: 43%); mp > 260 °C. IR (KBr) νmax/cm−1 3411, 3051, 1663, 1623, 1559, 1488, 1383, 1254, 1202, 1150, 1051, 966, 819; 19F-NMR (MeOD-d4) δ −125.9; 1H-NMR (MeOD-d4) δ 8.45 (d, 1H, J = 9.0 Hz), 7.98 (s, 1H), 7.75 (d, 1H, J = 9.0 Hz), 7.30–7.21 (m, 2H), 7.12 (t, 1H, J = 9.0 Hz), 3.39 (s, 6H); HRMS calcd for C18H15N6SClF [M+H]+: 401.0751, found 401.0742.
3.2.8. Synthesis of Amides 7h–10h
General procedure: a stirred mixture of carbonitriles 7–10 (0.13 mmol) and NaOH (2.5 N sol., 50 μL) in butanol (2.5 mL) was heated under microwaves at 117 °C (600 W) for 30 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM–EtOAc, 5:5) to afford amides 7h–10h.
9-(4-Methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carboxamide (7h). Orange solid (yield: 98%); mp = 213 °C. IR (cm−1) νmax 3409, 1691, 1638, 1600, 1572, 1509, 1431, 1380, 1349, 1325, 1301, 1237, 1177, 1123, 1085, 1032, 964, 835, 814; 1H-NMR (DMSO-d6) δ 8.37 (s, 1H), 8.24 (d, 1H, J = 9.0 Hz), 8.06 (s, 1H), 7.92 (s, 1H), 7.61 (d, 1H, J = 9.0 Hz), 7.31 (d, 2H, J = 9.0 Hz), 6.89 (d, 2H, J = 9.0 Hz), 3.74 (s, 3H); HRMS calcd for C17H14N5O2S [M+H]+: 352.0868, found 352.0879.
9-(Benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carboxamide (8h). Yellow solid (yield: 31%); mp > 260 °C. IR (KBr) νmax/cm−1 3291, 2915, 1648, 1576, 1532, 1497, 1476, 1376, 1351, 1323, 1272, 1191, 1104, 1035, 965, 923, 830, 815; 1H-NMR (DMSO-d6) δ 8.05–7.98 (m, 2H), 7.49 (d, 1H, J = 2 Hz), 7.37–7.31 (m, 1H), 6.96 (dd, 1H, J1 = 2 Hz, J2 = 9 Hz), 6.73 (dd, 1H, J1 = 2 Hz, J2 = 9 Hz), 5.88 (s, 2H); HRMS calcd for C17H12N5O3S [M+H]+: 366.0661, found 366.0658.
9-(4-Bromo-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carboxamide (9h). Yellow solid (yield: 71%); mp > 260 °C. IR (KBr) νmax/cm−1 1682, 1645, 1615, 1575, 1557, 1486, 1347, 1254, 1200, 1158, 1118, 1074, 993, 967, 941, 865, 819; 19F-NMR (DMSO-d6) δ −120.5; 1H-NMR (DMSO-d6) δ 8.47 (s, 1H), 8.41 (d, 1H, J = 9.0 Hz), 8.39 (s, 1H), 8.14 (s, 1H), 8.03 (d, 1H, J = 9.0 Hz), 7.53 (m, 1H), 7.36 (m, 1H), 7.21 (t, 1H, J = 9.0 Hz); HRMS calcd for C16H10N5OSBrF [M+H]+: 417.9769, found 417.9769.
9-(3-Chloro-4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carboxamide (10h). Orange solid (yield: 98%); mp > 260 °C. IR (KBr) νmax/cm−1 3453, 1684, 1624, 1601, 1576, 1534, 1506, 1487, 1376, 1348, 1282, 1260, 1209, 1124, 1085, 1057, 993, 969, 825, 810; 19F-NMR (DMSO-d6) δ −129.1; 1H-NMR (DMSO-d6) δ 8.26–8.21 (m, 2H), 8.12 (d, 1H, J = 9.0 Hz), 8.02–7.99 (m, 1H), 7.80 (s, 1H), 7.53 (d, 1H, J = 9.0 Hz), 7.47 (m, 1H), 7.18 (t, 1H, J = 9.0 Hz); HRMS calcd for C16H10N5OSClF [M+H]+: 374.0279, found 374.0280.
3.2.9. Synthesis of Methylimidates 7i–10i
General procedure: a stirred mixture of carbonitriles 7–10 (0.13 mmol) and NaOCH3 (0.5 M sol. in MeOH, 130 μL) in methanol (4 mL) was heated under microwaves at 65 °C (600 W) for 30 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM–EtOAc) to afford imidates 7i–10i.
Methyl 9-(4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7i). Purified by flash chromatography (DCM–EtOAc, 5:5) as orange solid (yield: 82%); mp = 240–242 °C. IR (cm−1); νmax 2833, 2354, 1644, 1614, 1567, 1504, 1439, 1397, 1373, 1349, 1325, 1286, 1240, 1215, 1181, 1155, 1104, 1069, 1033, 965, 935, 859, 832, 814; 1H-NMR (MeOD-d4) δ 8.09 (d, 1H, J = 9.0 Hz), 7.93 (s, 1H), 7.50 (d, 1H, J = 9.0 Hz), 7.14 (d, 2H, J = 9.0 Hz), 6.82 (d, 2H, J = 9.0 Hz), 3.98 (s, 3H), 3.74 (s, 3H); HRMS calcd for C18H16N5O2S [M+H]+: 366.1025, found 366.1034.
Methyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8i). Purified by flash chromatography (DCM–EtOAc, 5:5) as yellow solid (yield: 92%); mp = 230–232 °C. IR (cm−1) νmax/3287, 2902, 1648, 1617, 1575, 1528, 1499, 1483, 1452, 1432, 1385, 1322, 1272, 1196, 1125, 1043, 936, 885, 834, 817; 1H-NMR (DMSO-d6) δ 8.36 (d, 1H, J = 9 Hz), 7.96 (s, 1H), 7.71 (d, 1H, J = 9 Hz), 6.87 (d, 1H, J = 8 Hz), 6.74 (m, 1H), 6.63 (d, 1H, J = 8 Hz), 5.96 (s, 2H), 4.05 (s, 3H); HRMS calcd for C18H14N5O3S [M+H]+: 380.0817, found 380.0805.
Methyl 9-(4-bromo-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9i). Purified by flash chromatography eluent (DCM–EtOAc, 2:8) as pale yellow solid (yield: 94%); mp > 260 °C. IR (cm−1) νmax 2950, 1638, 1617, 1595, 1555, 1507, 1479, 1434, 1398, 1352, 1325, 1288, 1224, 1197, 1159, 1115, 1070, 988, 965, 942, 882, 818; 19F-NMR (DMSO-d6) δ −119.8; 1H-NMR (DMSO-d6) δ 8.46 (d, 1H, J = 9.0 Hz), 8.02 (s, 1H), 7.69 (d, 1H, J = 9.0 Hz), 7.53 (m, 1H), 7.35 (m, 1H), 7.20 (t, 1H, J = 9.0 Hz), 3.94 (s, 3H); HRMS calcd for C17H12N5OSBrF [M+H]+: 431.9930, found 431.9937.
Methyl 9-(3-chloro-4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (10i). Purified by flash chromatography (DCM–EtOAc, 5:5) as orange solid (yield: 98%); mp = 210–212 °C. IR (KBr) νmax/cm−1 1642, 1559, 1479, 1352, 1260, 1201, 1156, 1073, 942, 817; 19F-NMR (DMSO-d6) δ −126.9; 1H-NMR (DMSO-d6) δ 8.29 (d, 1H, J = 9.0 Hz), 8.18 (s, 1H), 7.73 (m, 1H), 7.61 (d, 1H, J = 9.0 Hz), 7.30 (m, 1H), 7.27 (t, 1H, J = 9.0 Hz), 3.95 (s, 3H); HRMS calcd for C17H12N5OSClF [M+H]+: 388.0435, found 388.0447.