Oxidized Fatty Acids as Inter-Kingdom Signaling Molecules
Abstract
:1. Introduction
2. Inter-Kingdom Signaling between Animals and Plants
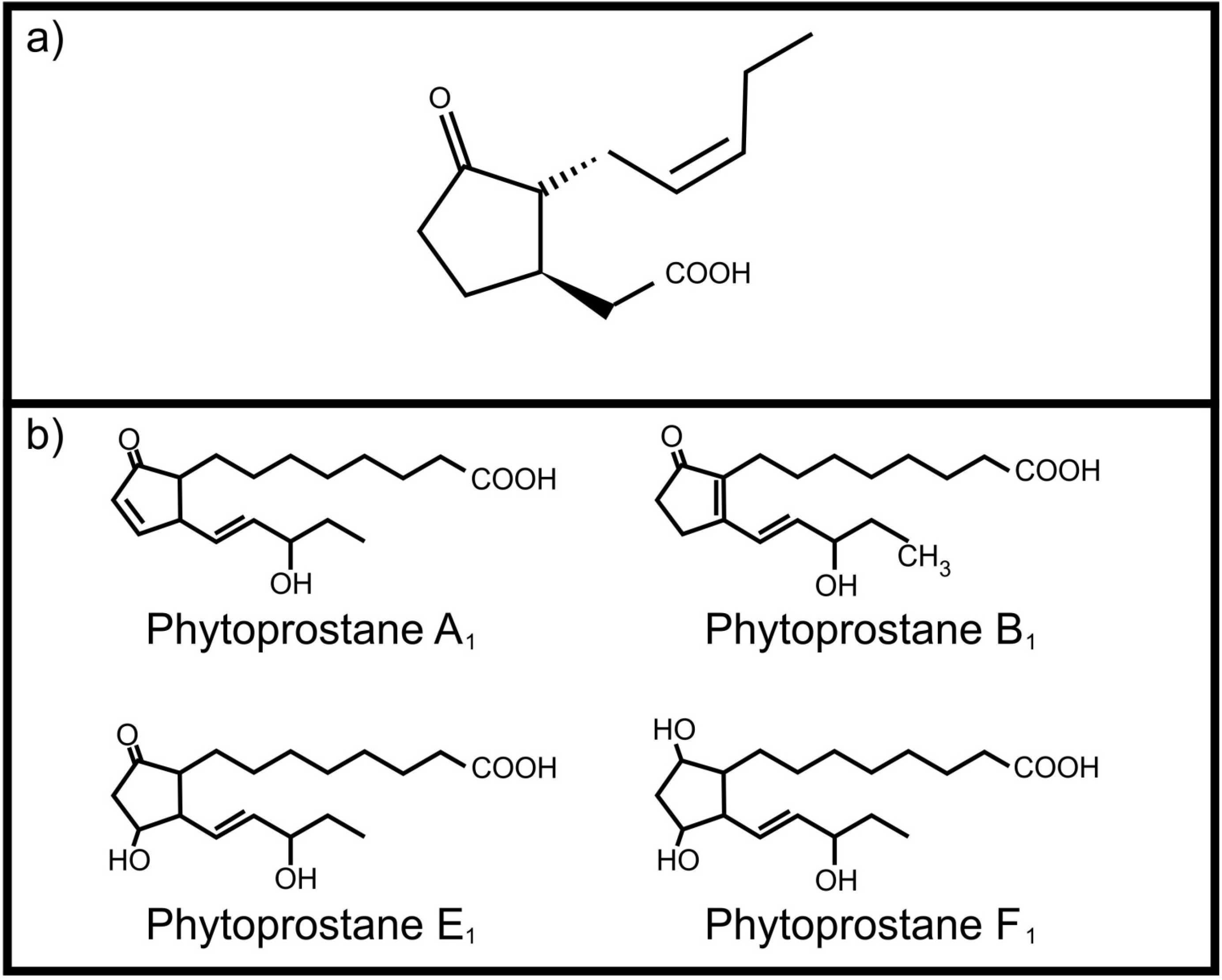
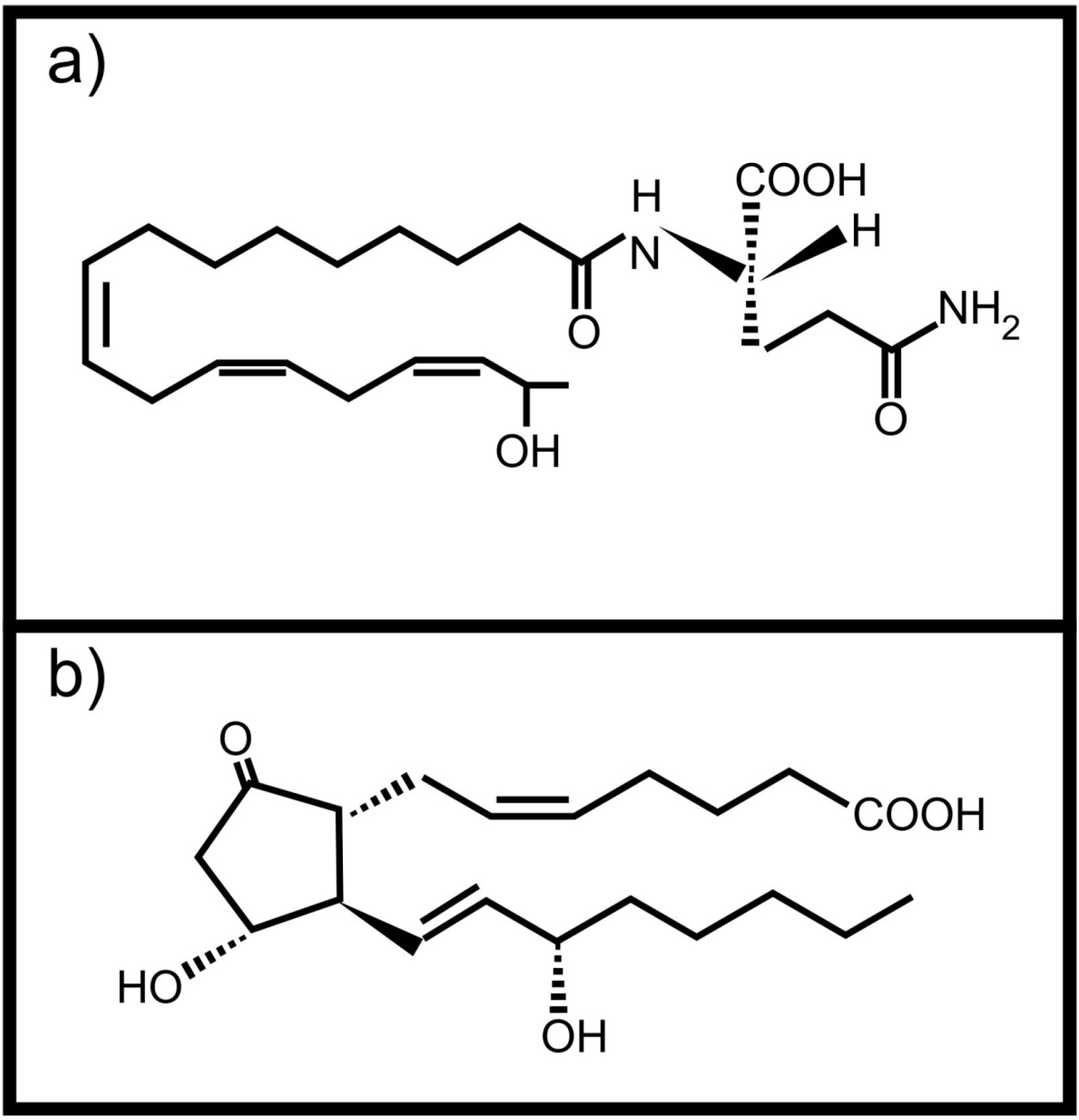
3. Inter-Kingdom Signaling between Animals and Fungi

4. Inter-Kingdom Signaling between Animals and Bacteria

5. Inter-Kingdom Signaling between Plants and Fungi
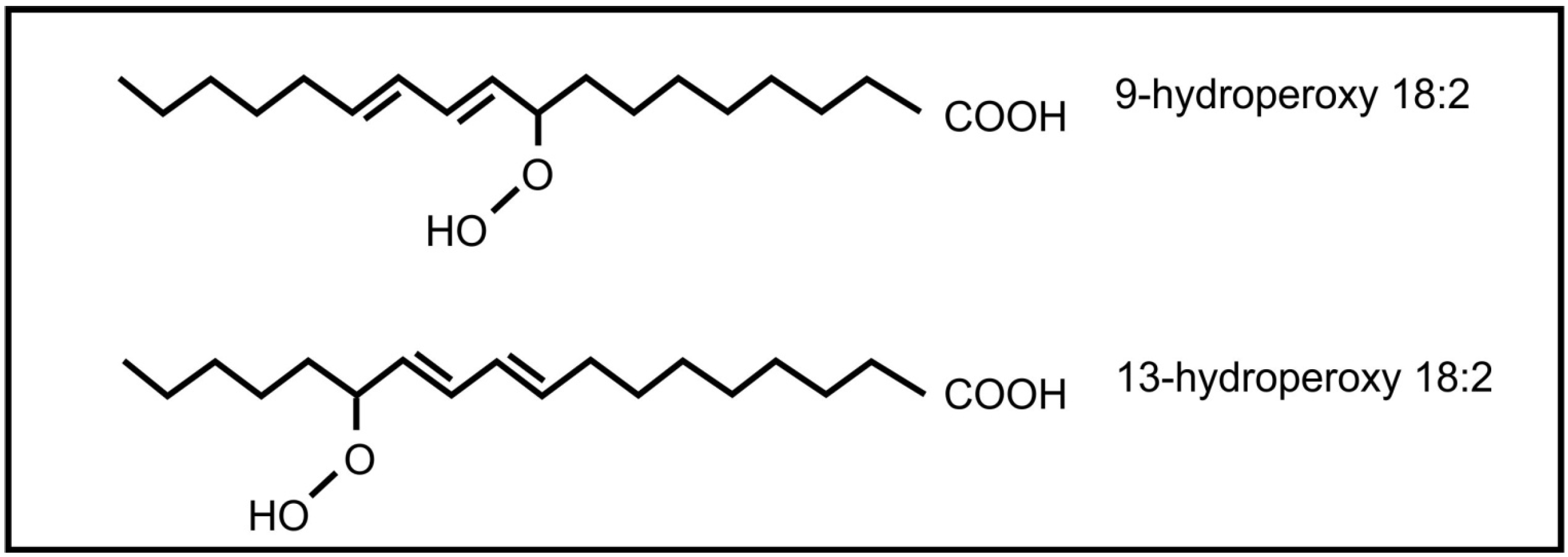

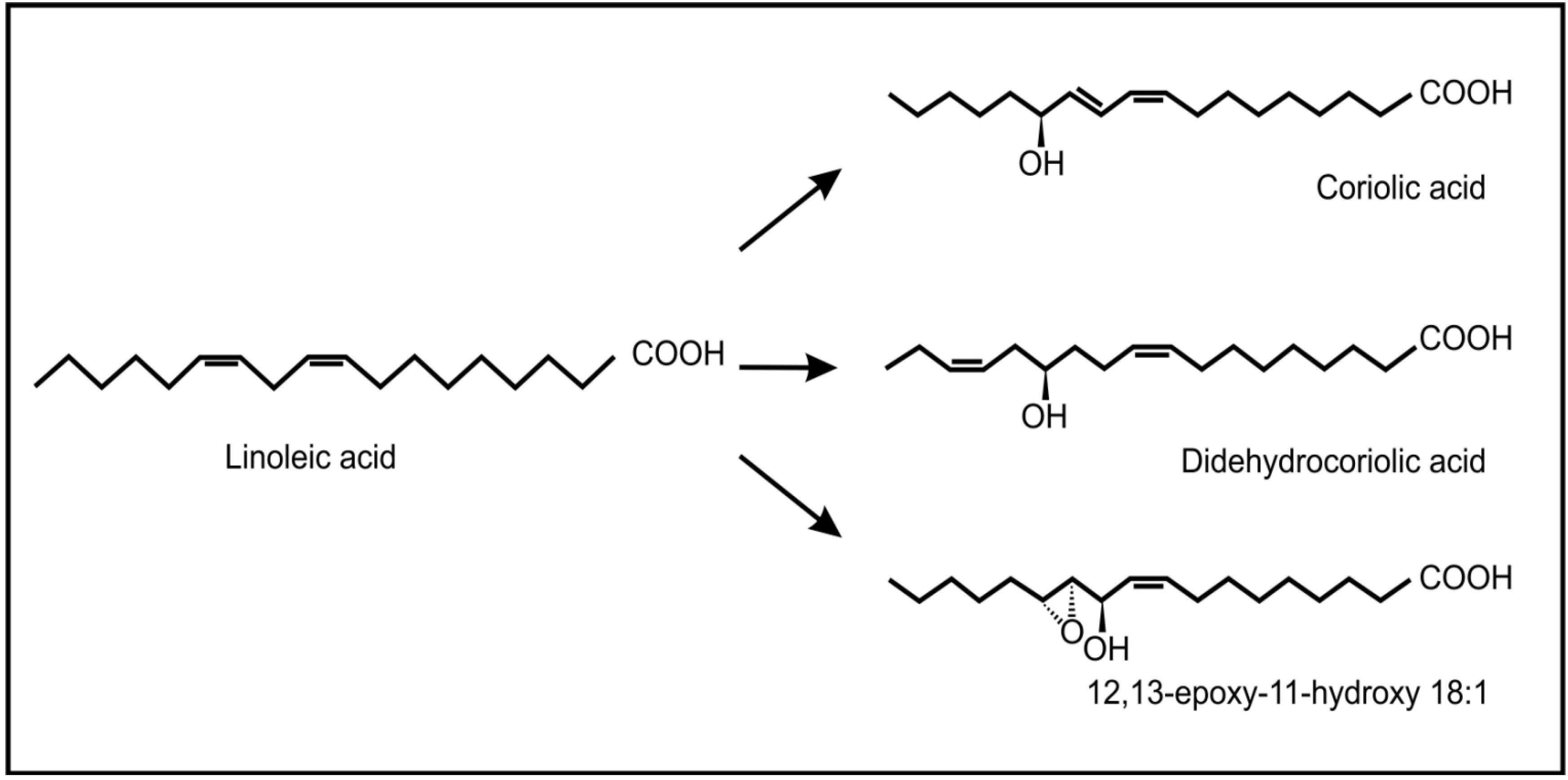
6. Conclusions
Conflicts of Interest
References
- Schultz, J.C. Shared signals and the potential for phylogenetic espionage between plants and animals. Integr. Comp. Biol. 2002, 42, 454–462. [Google Scholar] [CrossRef]
- Schultz, J.C.; Appel, H.M. Cross-kingdom cross-talk: Hormones shared by plants and insects and their herbivores. Ecology 2004, 85, 70–77. [Google Scholar] [CrossRef]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionary conserved signaling molecule modulates plant stress signaling networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef]
- Hernandez-Oñate, M.A.; Esquivel-Naranjo, E.U.; Mendoza-Mendoza, A.; Stewart, A.; Herrera-Estrella, A.H. An injury-response mechanism conserved across kingdoms determines entry of the fungus Trichoderma atroviride into development. Proc. Natl. Acad. Sci. USA 2012, 109, 14918–14923. [Google Scholar] [CrossRef]
- Haas-Stapleton, E.J.; Lu, Y.; Hong, S.; Arita, M.; Favoreto, S.; Nigam, S.; Serhan, C.N.; Agabian, N. Candida albicans modulates host defence by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One 2007, 12, e1316. [Google Scholar]
- Weber, H. Fatty acid-derived signals in plants. Trends Plant. Sci. 2002, 7, 217–224. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Jasmonate and salicylate induce expression of herbivore cytochrome 450 genes. Nature 2002, 419, 712–715. [Google Scholar] [CrossRef]
- Parchmann, S.; Meuller, M.J. Evidence for the formation of dinor isoprostanes E1 from linolenic acid in plants. J. Biol. Chem. 1998, 273, 32650–32655. [Google Scholar] [CrossRef]
- Imbusch, R.; Meuller, M.J. Formation of isoprostane F2-like compounds (phytoprostanes F1) from α-linolenic acid in plants. Free Radic. Biol. Med. 2000, 28, 720–726. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Mariani, V.; Hochrein, H.; Karg, K.; Wagner, H.; Ring, J.; Meuller, M.J.; Jakob, T.; Behrendt, H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 2005, 20, 627–636. [Google Scholar]
- Gilles, S.; Mariani, V.; Bryce, M.; Meuller, M.J.; Ring, J.; Behrendt, H.; Jakob, T.; Traidl-Hoffmann, C. Pollen allergens do not come alone: Pollen associated lipid mediators (PALMS) shift the human immune systems towards a Th2-dominated response. Allergy Asthma Clin. Immunol. 2009, 5, 3. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Kasche, A.; Jakob, T.; Huger, M.; Plötz, S.; Feussner, I.; Ring, J.; Behrendt, H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J. Allergy Clin. Immunol. 2002, 109, 831–838. [Google Scholar] [CrossRef]
- Gilles, S.; Mariani, V.; Bryce, M.; Meuller, M.J.; Ring, J.; Jakob, T.; Pastore, S.; Behrendt, H.; Traidl-Hoffmann, C. Pollen derived E1 phytoprostanes signal via PPAR-γ and NF-κB dependent mechanisms. J. Immunol. 2009, 182, 6653–6658. [Google Scholar] [CrossRef]
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Alborn, H.T.; Loughrin, J.H.; Tumlinson, J.H. Volicitin, an elicitor of maize volatiles in oral secretions of Spodoptera exigua: Isolation and bioactivity. J. Chem. Ecol. 2000, 26, 189–202. [Google Scholar] [CrossRef]
- Mori, N.; Yoshinaga, N. Function and evolutionary diversity of fatty acid amino acid conjugates in insects. J. Plant Interact. 2011, 6, 103–107. [Google Scholar] [CrossRef]
- Häring, D.A.; Huber, M.J.; Suter, D.; Edwards, P.J.; Lüscher, A. Plant enemy-derived elicitors increase the foliar tannin concentration of Onobrychis viciifolia without a trade-off to growth. Ann. Bot. 2008, 102, 979–987. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Romano, G.; Lamari, N.; Massa Gallucci, A.; Cimino, A.; Miralto, G.; Ianora, A. A metabolic mechanism for the detrimental effect of marine diatoms on zooplankton grazers. ChemBioChem 2007, 8, 1810–1818. [Google Scholar] [CrossRef]
- Ianora, A.; Romano, G.; Carotenuto, Y.; Esposito, F.; Roncalli, V.; Buttino, I.; Miralto, A. Impact of the diatom oxylipin 15S-HEPE on the reproductive success of the copepod Temora stylifera. Hydrobiologia 2011, 666, 265–275. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar]
- Smith, W.L. The eicosanoids and their biochemical mechanism of action. Biochem. J. 1989, 259, 315–324. [Google Scholar]
- Zeldin, D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001, 276, 36059–36062. [Google Scholar]
- Murakami, M.; Kudo, I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog. Lipid Res. 2004, 43, 3–35. [Google Scholar]
- Henderson, W.R. The role of leukotrienes in inflammation. Ann. Intern. Med. 1994, 121, 684–697. [Google Scholar]
- Carroll, M.A.; McGiff, J.C. A new class of lipid mediators: Cytochrome P450 arachidonate metabolites. Thorax 2000, 55, S13–S16. [Google Scholar]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.D. Thematic review: An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar]
- Serhan, C.N.; Arita, M.; Hong, S.; Gotlinger, K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 2004, 39, 1125–1132. [Google Scholar]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar]
- Hatae, N.; Sugimoto, Y.; Ichikawa, A. Prostaglandin receptors: Advances in the study of EP3 receptor signalling. J. Biochem. 2002, 131, 781–783. [Google Scholar]
- Tsitsigiannis, D.I.; Keller, N.P. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007, 15, 109–118. [Google Scholar]
- Ells, R.; Kock, J.L.F.; Albertyn, J.; Pohl, C.H. Arachidonic acid metabolites of pathogenic yeasts. Lipids Health Dis. 2012, 11. [Google Scholar] [CrossRef]
- Noverr, M.C.; Phare, S.M.; Toews, G.B.; Coffey, M.J.; Huffnagle, G.B. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 2001, 69, 2957–2963. [Google Scholar] [CrossRef]
- Shiraki, Y.; Ishbashi, Y.; Hiruma, M.; Nashikawa, A.; Ikeda, S. Candida albicans abrogates the expression of interferon-γ-inducible protein-10 in human keratinocytes. FEMS Immunol. Med. Microbiol. 2008, 54, 122–128. [Google Scholar] [CrossRef]
- Ells, R.; Kock, J.L.F.; Albertyn, J.; Kemp, G.; Pohl, C.H. Effect of inhibitors of arachidonic acid metabolism on prostaglandin E2 production by Candida albicans and Candida dubliniensis. Med. Microbiol. Immunol. 2011, 200, 23–28. [Google Scholar]
- Noverr, M.C.; Toews, G.B.; Huffnagle, G.B. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 2002, 70, 400–402. [Google Scholar] [CrossRef]
- Bordon, A.P.; Dias-Melicio, L.A.; Acorci, M.J.; Calvi, S.A.; Peraçoli, M.T.S.; Soares, A.M.V.C. Prostalandin E2 inhibits Paracoccidioides brasiliensis killing by human monocytes. Microbes Infect. 2007, 9, 744–747. [Google Scholar] [CrossRef]
- Biondo, G.A.; Dias-Melicio, L.A.; Bordon-Graciani, A.P.; Acorci-Valério, M.J.; Soares, A.M.V.C. Paracoccidioides brasiliensis uses endogenous and exogenous arachidonic acid for PGEx production. Mycopathologia 2010, 170, 123–130. [Google Scholar] [CrossRef]
- Tsitsigiannis, D.I.; Bok, J.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Keller, N.P. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect. Immun. 2005, 73, 4548–4559. [Google Scholar] [CrossRef]
- Kupfahl, C.; Tsikas, D.; Niemann, J.; Geginat, G.; Hof, H. Production of prostaglandins, isoprostanes and thromboxane by Aspergillus fumigatus: Identification by gas chromatography-tandem mass spectrometry and quantification by enzyme immunoassay. Mol. Immunol. 2012, 49, 621–627. [Google Scholar] [CrossRef]
- Betz, M.; Fox, B.S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 1991, 146, 108–113. [Google Scholar]
- Soares, A.M.V.C.; Calvi, S.A.; Peraçoli, M.T.S.; Fernandez, A.C.; Dias, L.A.; Dos Anjos, A.R. Modulatory effect of prostaglandins on human monocyte activiation for killing of high- and low-virulence strains of Paracoccidioides brasiliensis. Immunology 2001, 102, 480–485. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef]
- Shibata, Y.; Hendersen, R.A.; Honda, I.; Nakamura, R.M.; Myrvik, Q.N. Splenic PGE2-releasing macrophages regulate Th1 and Th2 immune responses in mice treated with heat-killed BCG. J. Leukoc. Biol. 2005, 78, 1281–1290. [Google Scholar] [CrossRef]
- Kalo-Klein, A.; Witkin, S.S. Prostaglandin E2 enhances and gamma-interferon inhibits germ tube formation in Candida albicans. Infect. Immun. 1990, 58, 260–262. [Google Scholar]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef]
- Yang, J.; Eiserich, J.P.; Cross, C.E.; Morrissey, B.M.; Hammock, B.D. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic. Biol. Med. 2012, 53, 160–171. [Google Scholar] [CrossRef]
- Eiserich, J.P.; Yang, J.; Morrissey, B.M.; Hammock, B.D.; Cross, C.E. Omics approaches in cystic fibrosis research: A focus on oxylipin profiling in airway secretions. Ann. N. Y. Acad. Sci. 2012, 1259, 1–9. [Google Scholar]
- Ciofu, O.; Hansen, C.R.; Høiby, N. Respiratory bacterial infections in cystic fibrosis. Curr. Opin. Pulm. Med. 2013, 19, 251–258. [Google Scholar] [CrossRef]
- Saliba, A.M.; Nascimento, D.O.; Silva, M.C.A.; Assis, M.C.; Gayer, C.R.M.; Raymond, B.; Coelho, M.G.P.; Marques, E.A.; Touqui, L.; Albano, R.M.; et al. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell. Microbiol. 2005, 7, 1811–1822. [Google Scholar] [CrossRef]
- Vance, R.E.; Hong, S.; Gronert, K.; Serhan, C.N.; Mekalanos, J.J. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 2135–2139. [Google Scholar] [CrossRef]
- Kühn, H.; O’Donnell, V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006, 45, 334–356. [Google Scholar] [CrossRef]
- Kühn, H.; Walther, M.; Kuban, R.J. Mammalian arachidonate 15-lipoxygenases, structure, function and biological implications. Prostaglandins Other Lipid Mediat. 2002, 68, 263–290. [Google Scholar]
- Weil, K.; Gruber, P.; Heckel, F.; Harmsen, D.; Schreier, P. Selective (R)-3-hydroxylation of FA by Stenotrophomonas maltophilia. Lipids 2002, 37, 317–323. [Google Scholar] [CrossRef]
- Ciccoli, R.; Sahi, S.; Singh, S.; Prakash, H.; Zafiriou, M.-P.; Ishdorj, G.; Kock, J.L.F.; Nigam, S. Oxygenation by COX-2 (cyclo-oxygenase-2) of 3-HETE (3-hydroxyeicosatetraenoic acid), a fungal mimetic of arachidonic acid, produces a cascade of novel bioactive 3-hydroxyeicosanoids. Biochem. J. 2005, 390, 737–747. [Google Scholar] [CrossRef]
- Tirelli, A.S.; Colombo, C.; Torresani, E.; Fortunato, F.; Biffi, A.; Cariani, L.; Daccò, V.; Carbone, A.; Edefonti, A.; Paglialonga, F.; et al. Effects of treatment in the levels of circulating cytokines and growth factors in cystic fibrosis and dialyzed patients by multi-analytical determination with a biochip array platform. Cytokine 2013, 62, 413–420. [Google Scholar] [CrossRef]
- Beiersdorf, N.; Schien, M.; Hentschel, J.; Pfister, W.; Markert, U.R.; Mainz, J.G. Soluble inflammation markers in nasal lavage from CF patients and healthy controls. J. Cystic Fibrosis 2013, 12, 249–257. [Google Scholar] [CrossRef]
- King, S.J.; Nyulasi, I.B.; Bailey, M.; Kotsimbos, T.; Wilson, J.W. Loss of fat-free mass over four years in adult cystic fibrosis is associated with high serum interleukin-6 levels but not tumour necrosis factor-alpha. Clin. Nutrit. 2013. [Google Scholar] [CrossRef]
- Christensen, S.A.; Kolomiets, M.V. The lipid language of plant-fungal interactions. Fungal Genet. Biol. 2011, 48, 4–14. [Google Scholar] [CrossRef]
- Calvo, A.; Hinze, L.; Gardner, H.; Keller, N.P. Sporogenic effect of polyunsaturated fatty acids on Aspergillus ssp. development. Appl. Environ. Microbiol. 1999, 65, 3668–3673. [Google Scholar]
- Burow, G.B.; Nesbitt, T.C.; Dunlap, J.; Keller, N.P. Seed lipoxygenase products modulate Aspergillus mycotoxin biosynthesis. Mol. Plant.Microbe Interact. 1997, 10, 380–387. [Google Scholar] [CrossRef]
- Gao, X.Q.; Kolomiets, M.V. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 2009, 28, 79–88. [Google Scholar] [CrossRef]
- Goodrich-Tanrikulu, M.; Mahoney, N.E.; Rodriques, S.B. The plant-growth regulator methyl jasmonate inhibits aflatoxin production by Aspergillus flavus. Microbiology 1995, 141, 2831–2837. [Google Scholar] [CrossRef]
- Vergopoulou, S.; Galanopoulou, D.; Markaki, P. Methyl jasmonate stimulates aflatoxin B-1 biosynthesis by Aspergillus parasiticus. J. Agric. Food Chem. 2001, 49, 3494–3498. [Google Scholar] [CrossRef]
- Brodhagen, M.; Tsitsigiannis, D.I.; Hornung, E.; Goebel, C.; Feussner, I.; Keller, N.P. Reciprocal oxylipin-mediated cross-talk in the Aspergillus-seed pathosystem. Mol. Microbiol. 2008, 67, 378–391. [Google Scholar]
- Tsukada, K.; Takahashi, K.; Nabeta, K. Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodia theobromae. Phytochemistry 2010, 71, 2019–2023. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Ocampo, J.A.; García Garrido, J.M. Plant 9-lox oxylipin metabolism in response to arbuscular mycorrhiza. Plant. Signal. Behav. 2012, 7, 1–5. [Google Scholar] [CrossRef]
- Ding, L.; Peschel, G.; Hertwick, C. Biosynthesis of archetypal plant self-defensive oxylipins by an endophytic fungus in mangrove embryos. ChemBioChem 2012, 13, 2661–2664. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pohl, C.H.; Kock, J.L.F. Oxidized Fatty Acids as Inter-Kingdom Signaling Molecules. Molecules 2014, 19, 1273-1285. https://doi.org/10.3390/molecules19011273
Pohl CH, Kock JLF. Oxidized Fatty Acids as Inter-Kingdom Signaling Molecules. Molecules. 2014; 19(1):1273-1285. https://doi.org/10.3390/molecules19011273
Chicago/Turabian StylePohl, Carolina H., and Johan L.F. Kock. 2014. "Oxidized Fatty Acids as Inter-Kingdom Signaling Molecules" Molecules 19, no. 1: 1273-1285. https://doi.org/10.3390/molecules19011273



