Oxetane Synthesis through the Paternò-Büchi Reaction
Abstract
:1. Introduction
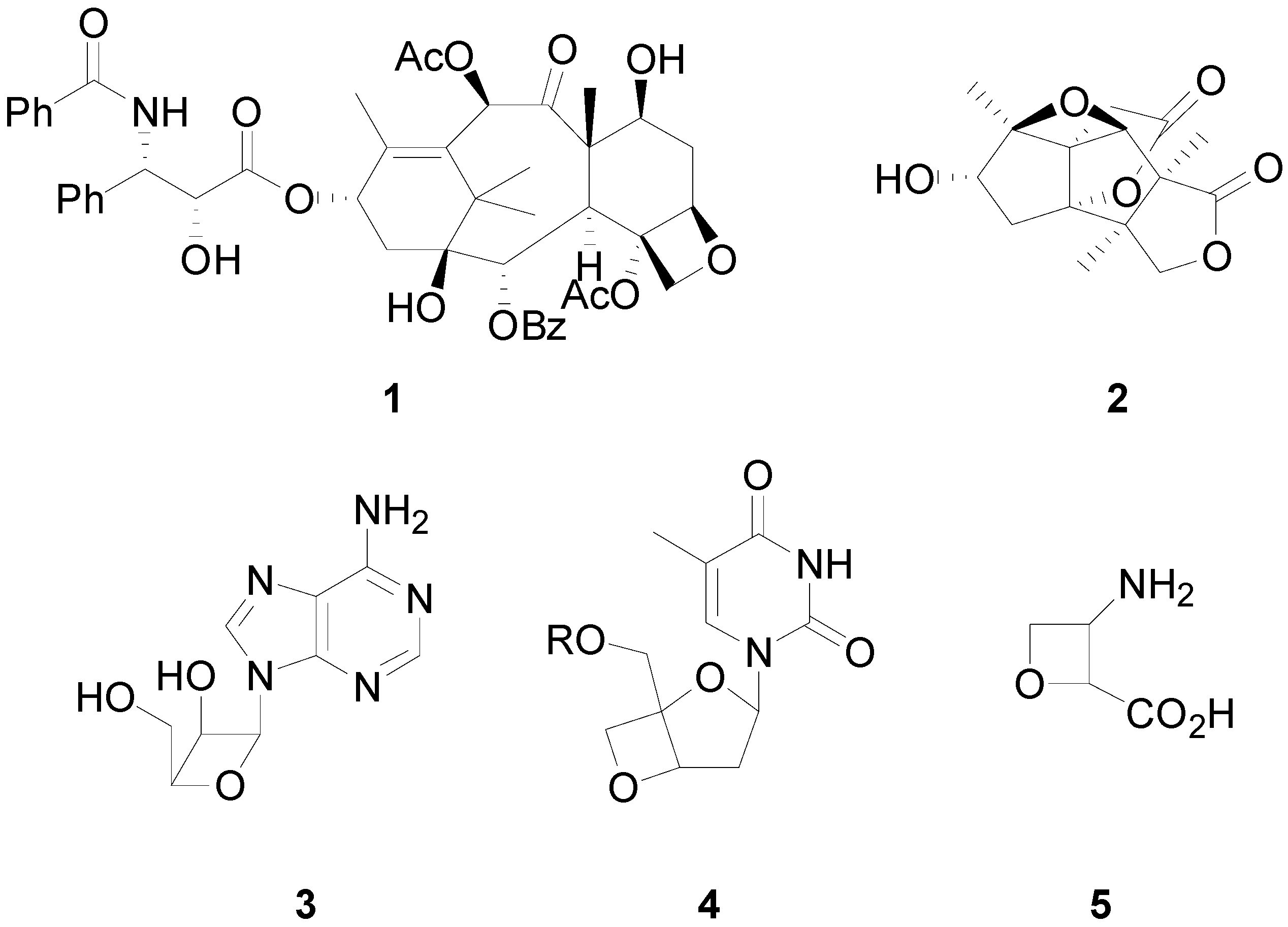
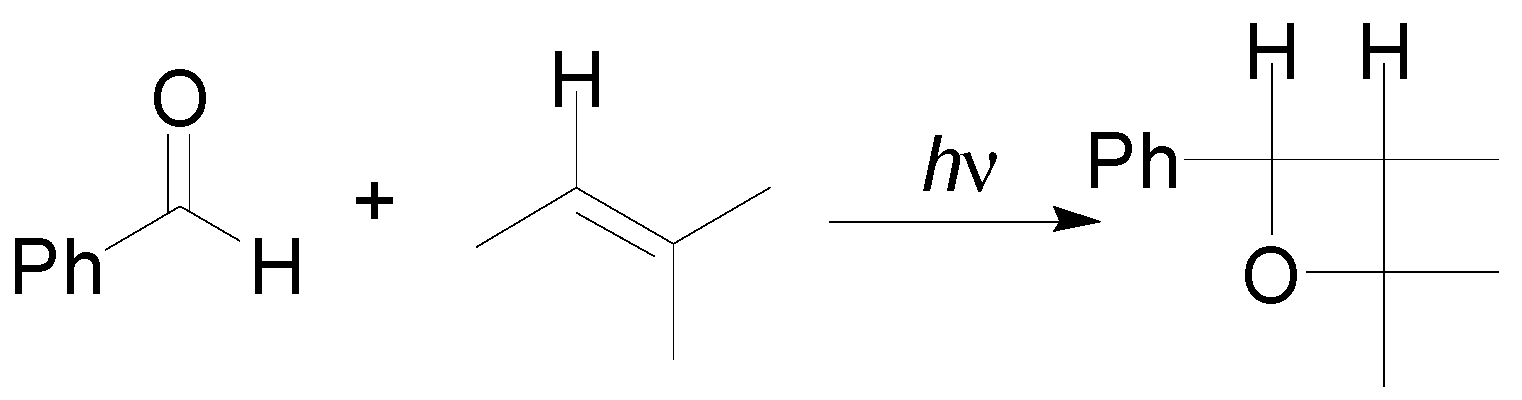
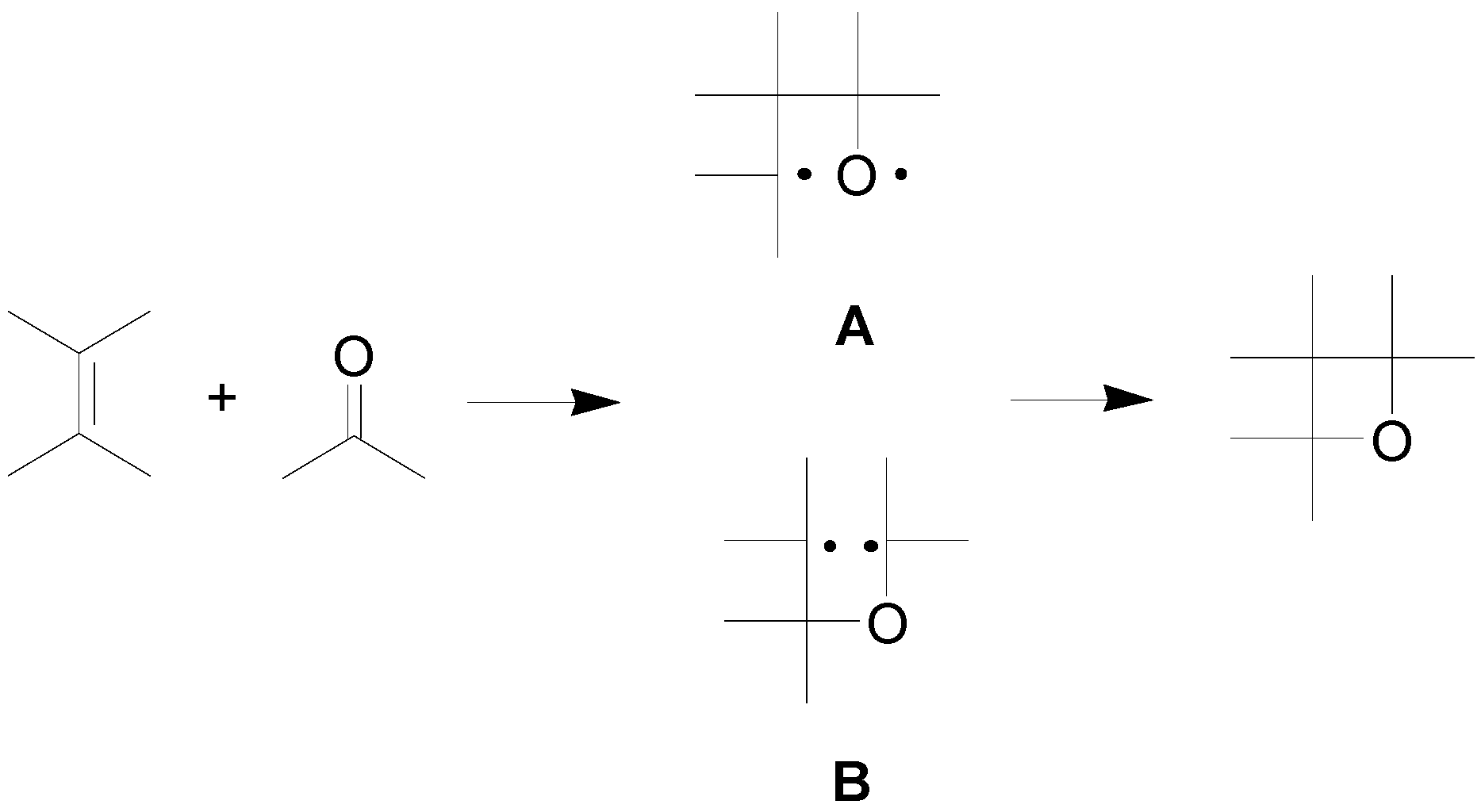
2. How to Perform the Reaction
3. Reactions with Electron Rich Unsaturated Compounds
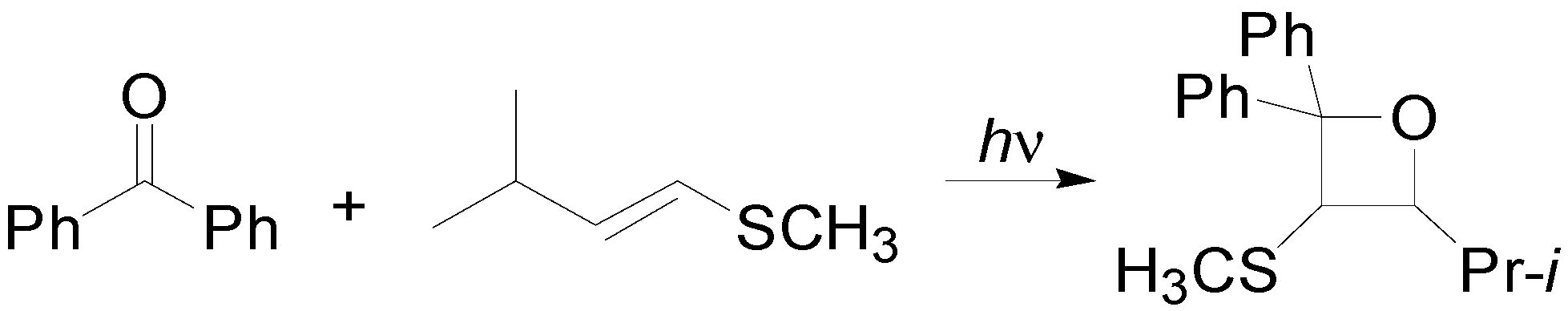
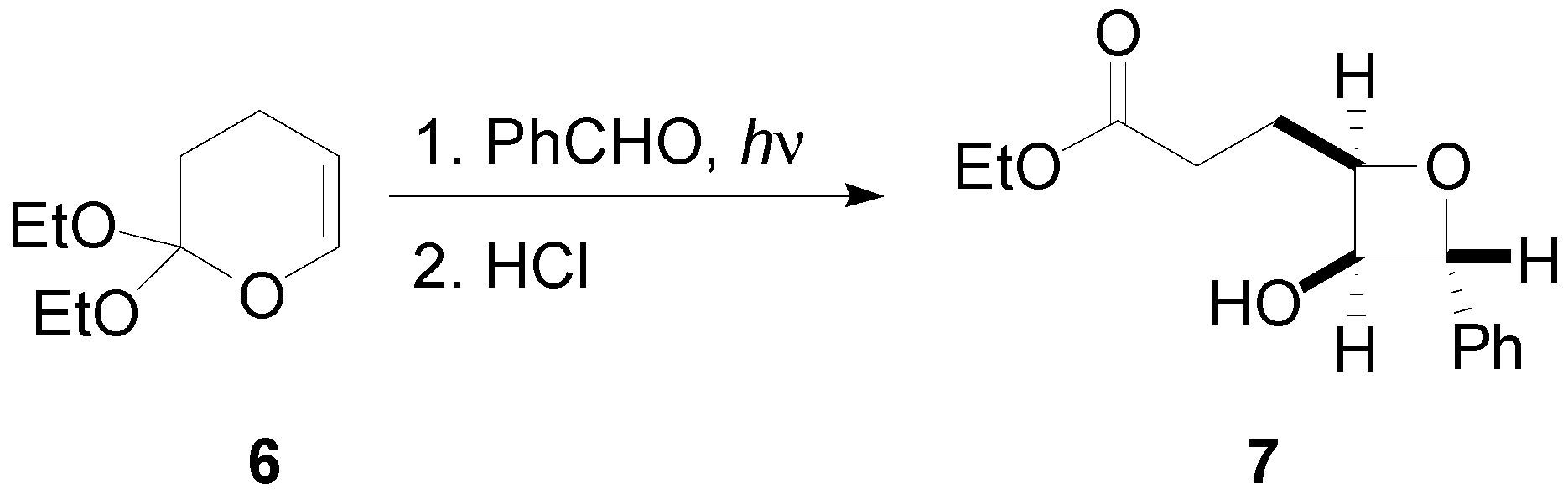
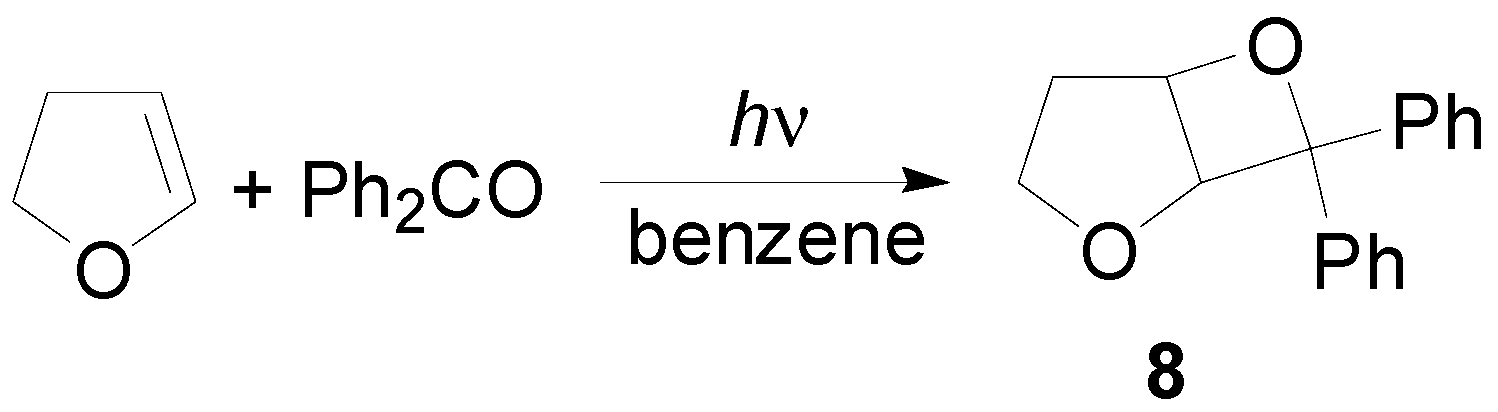
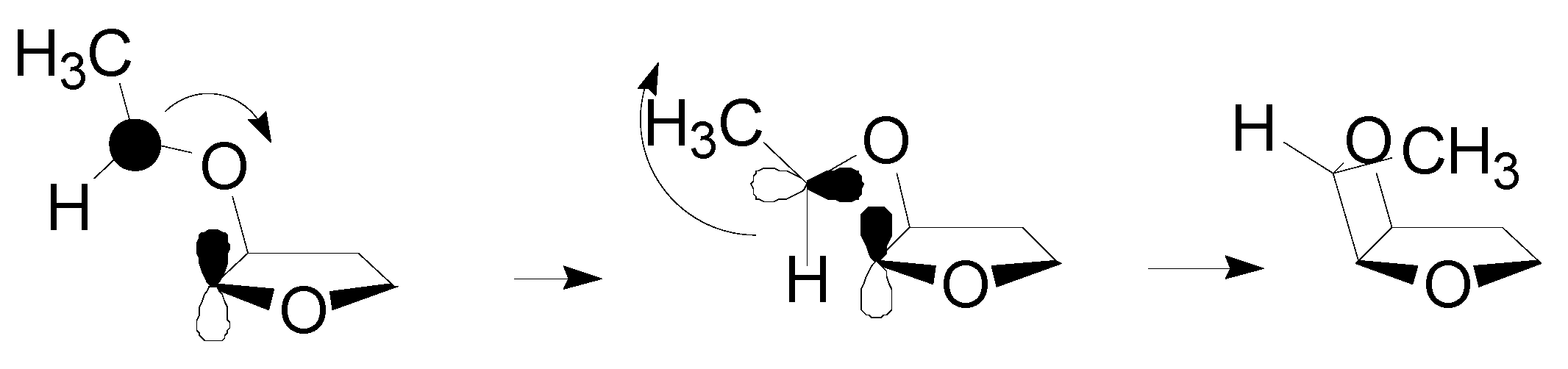
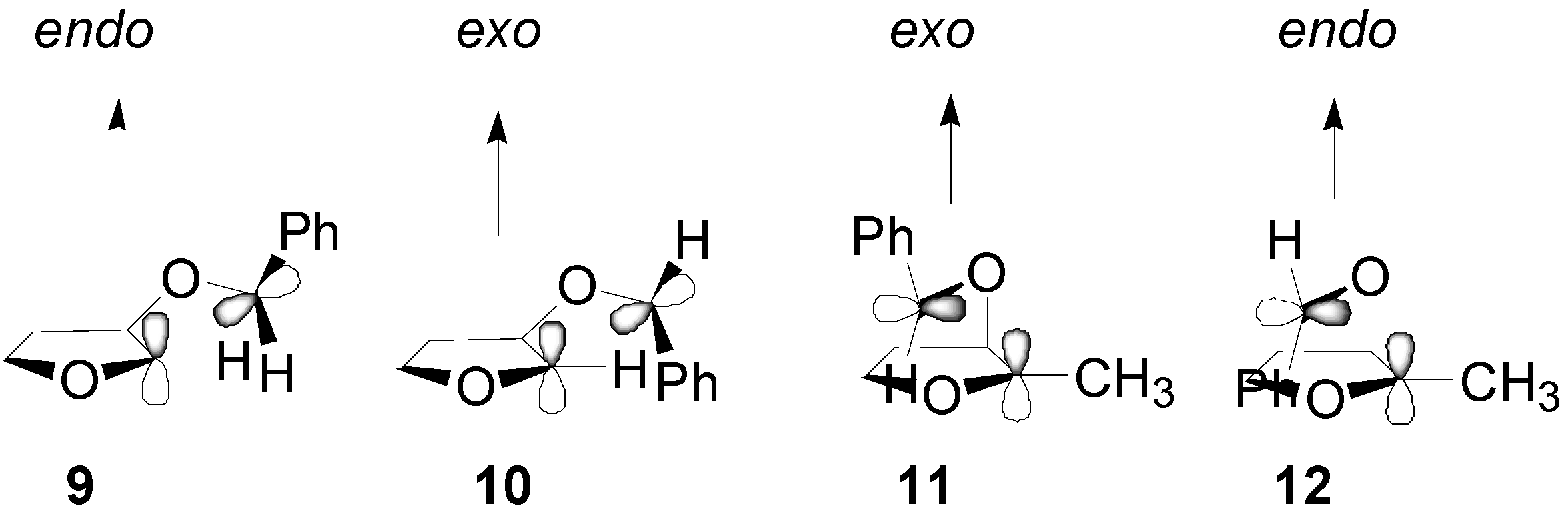
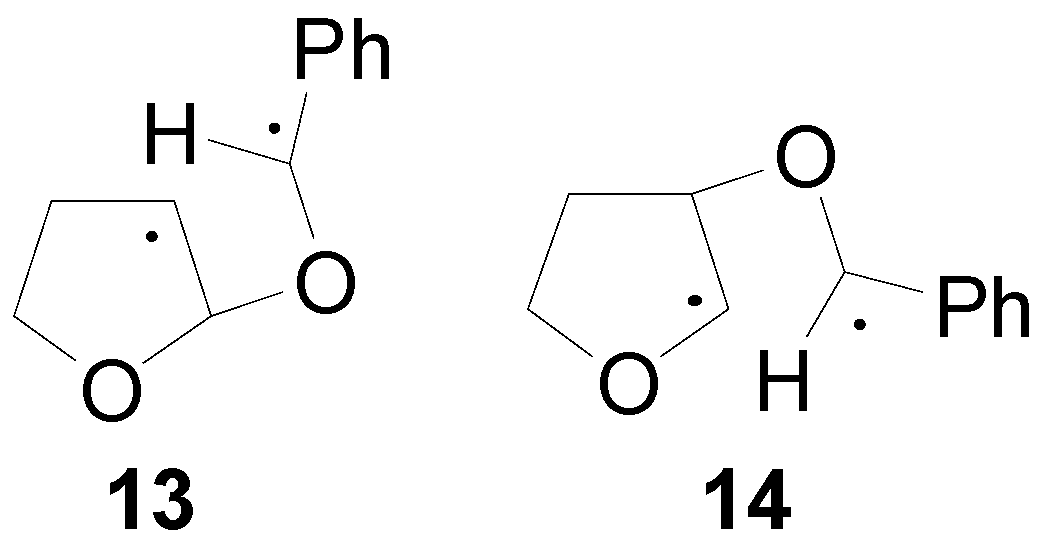
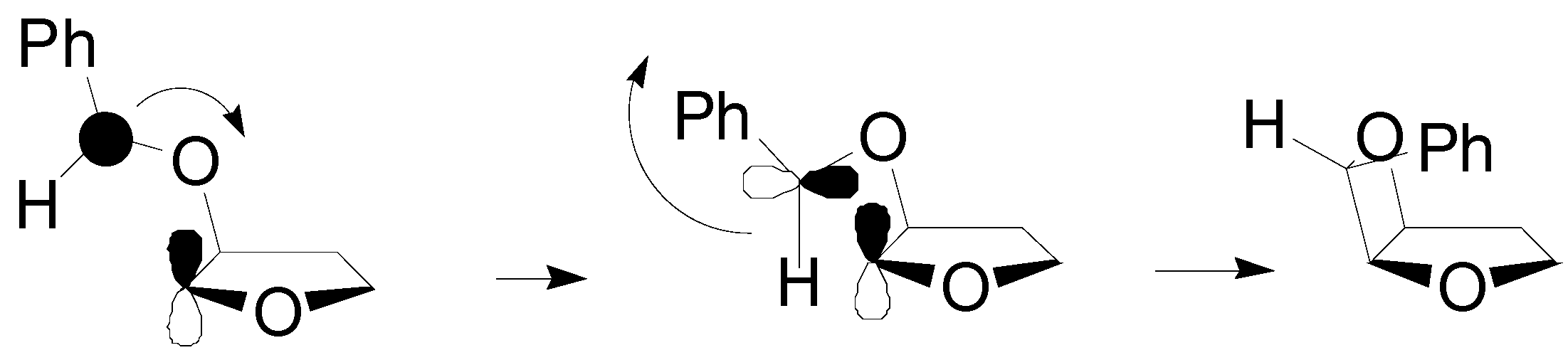
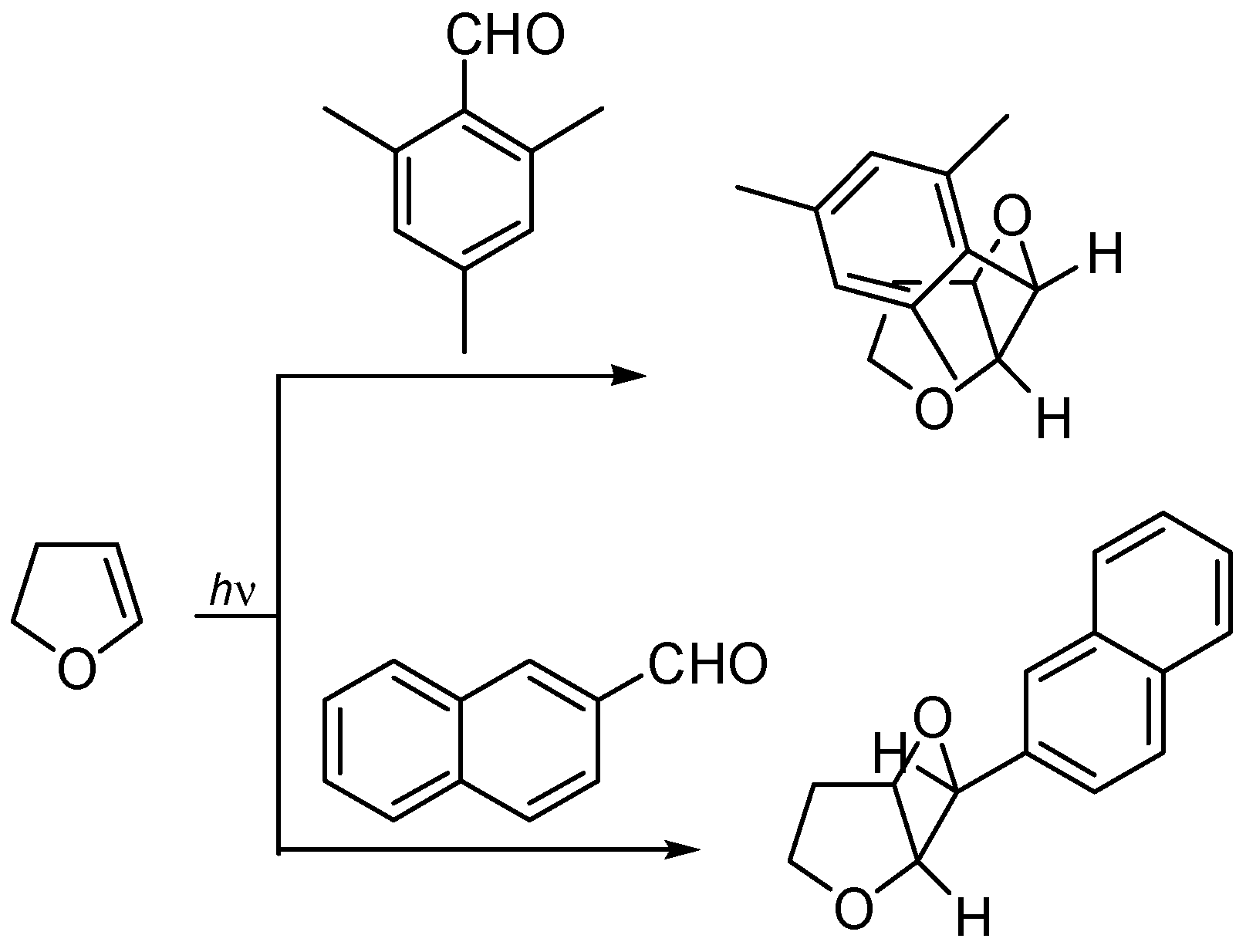
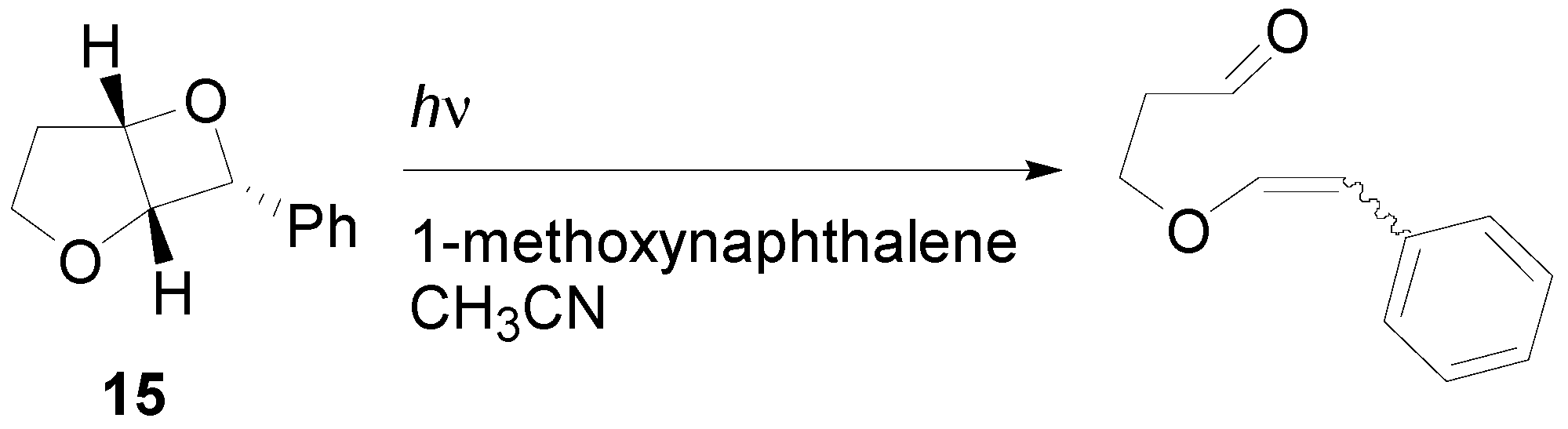
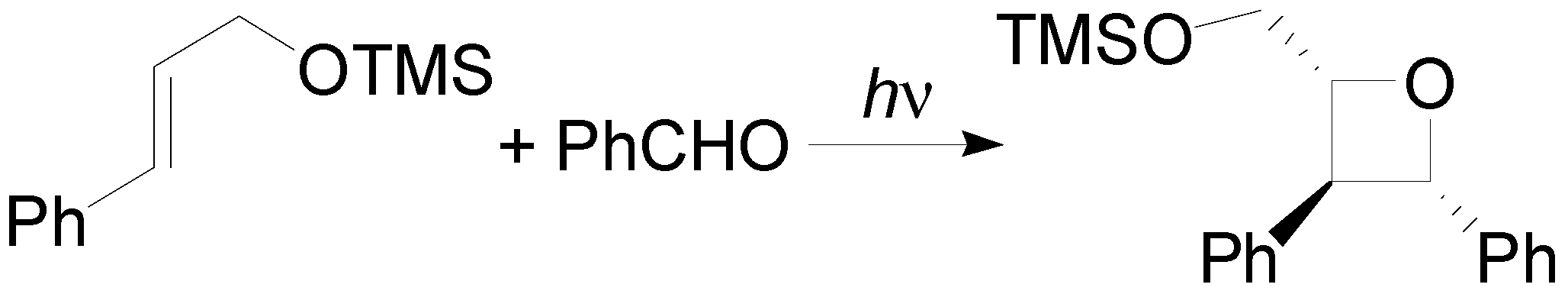


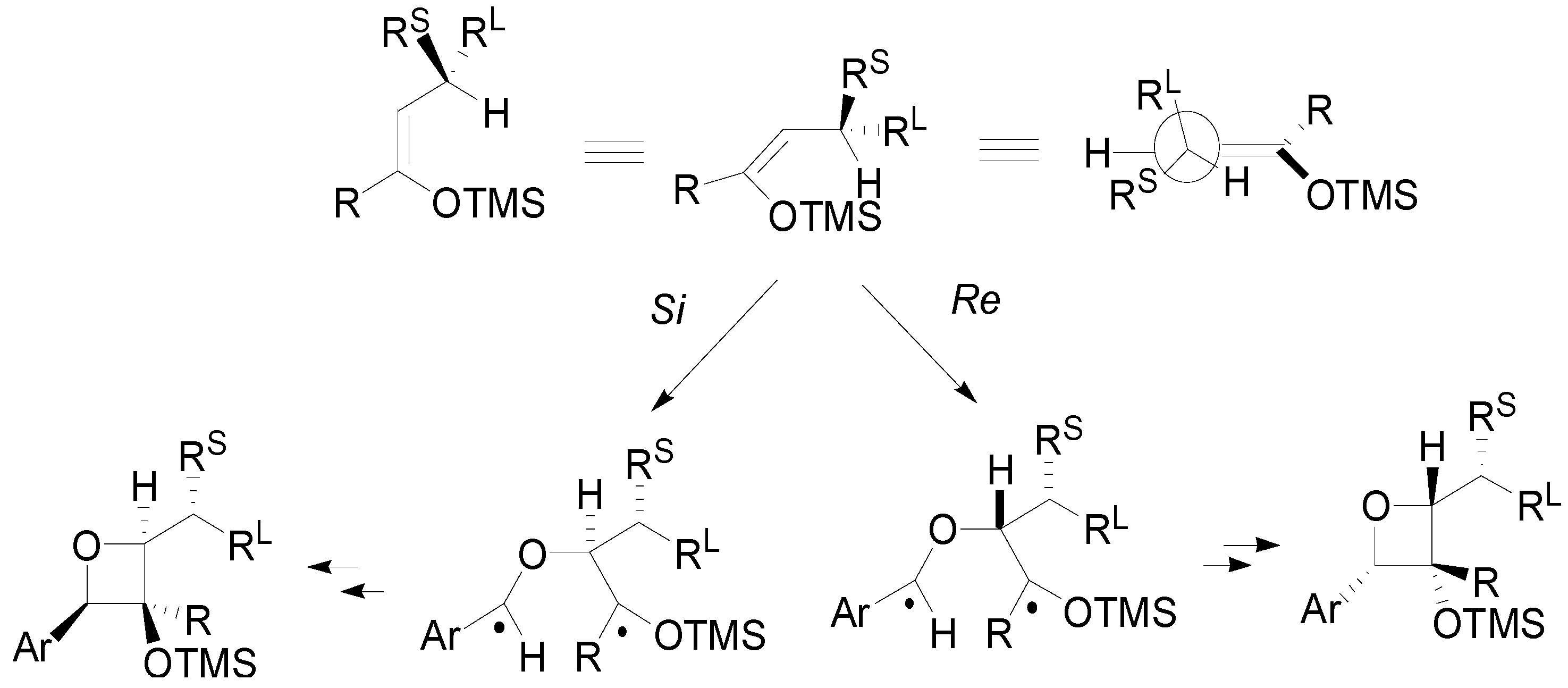
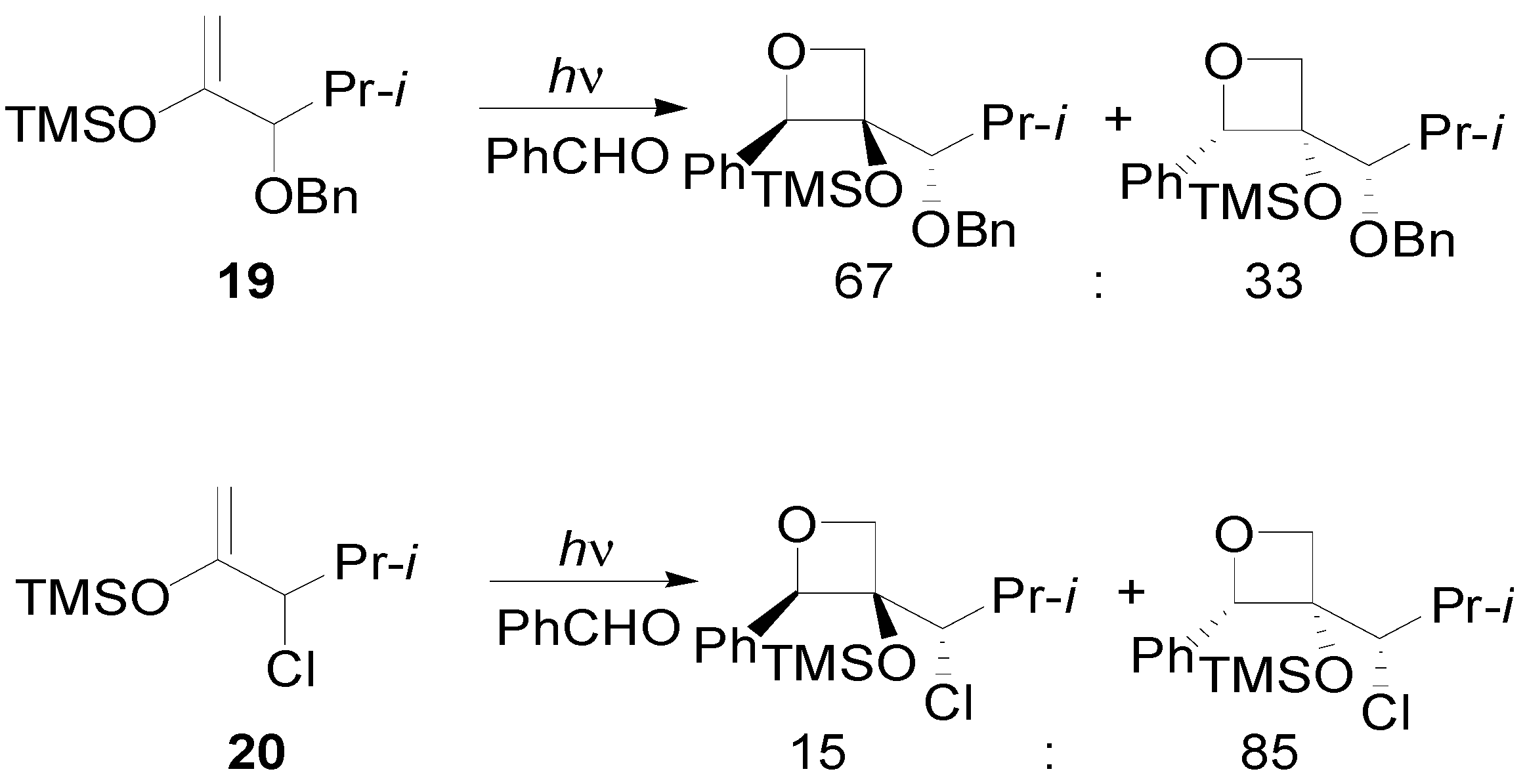
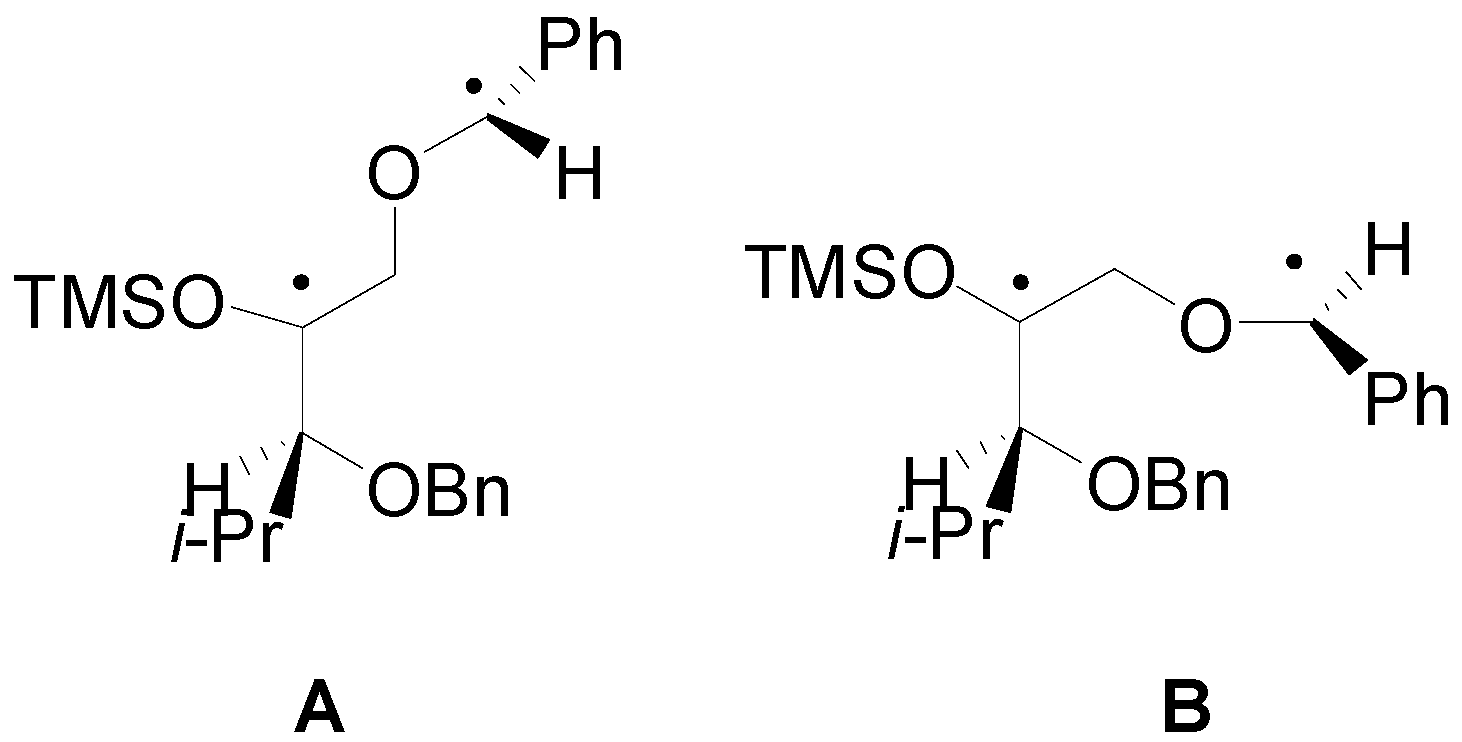

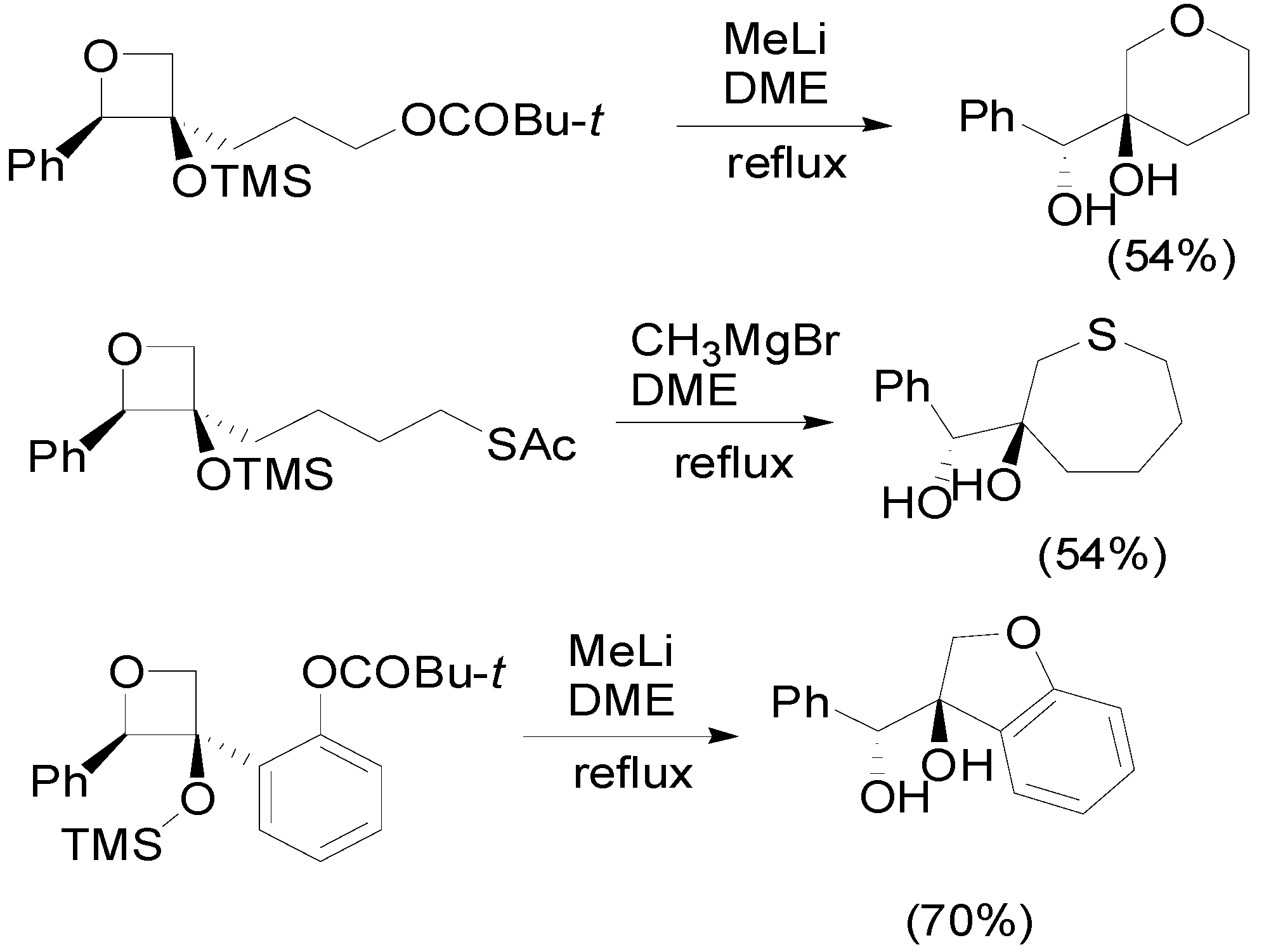
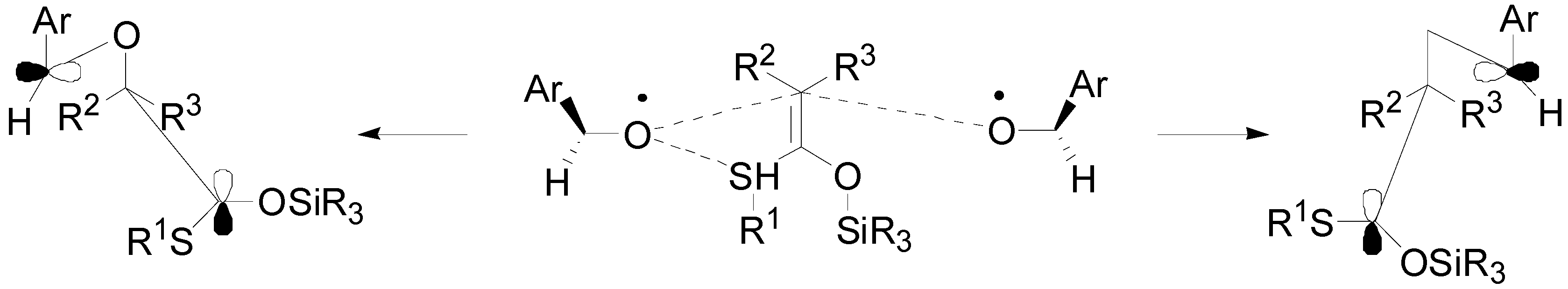
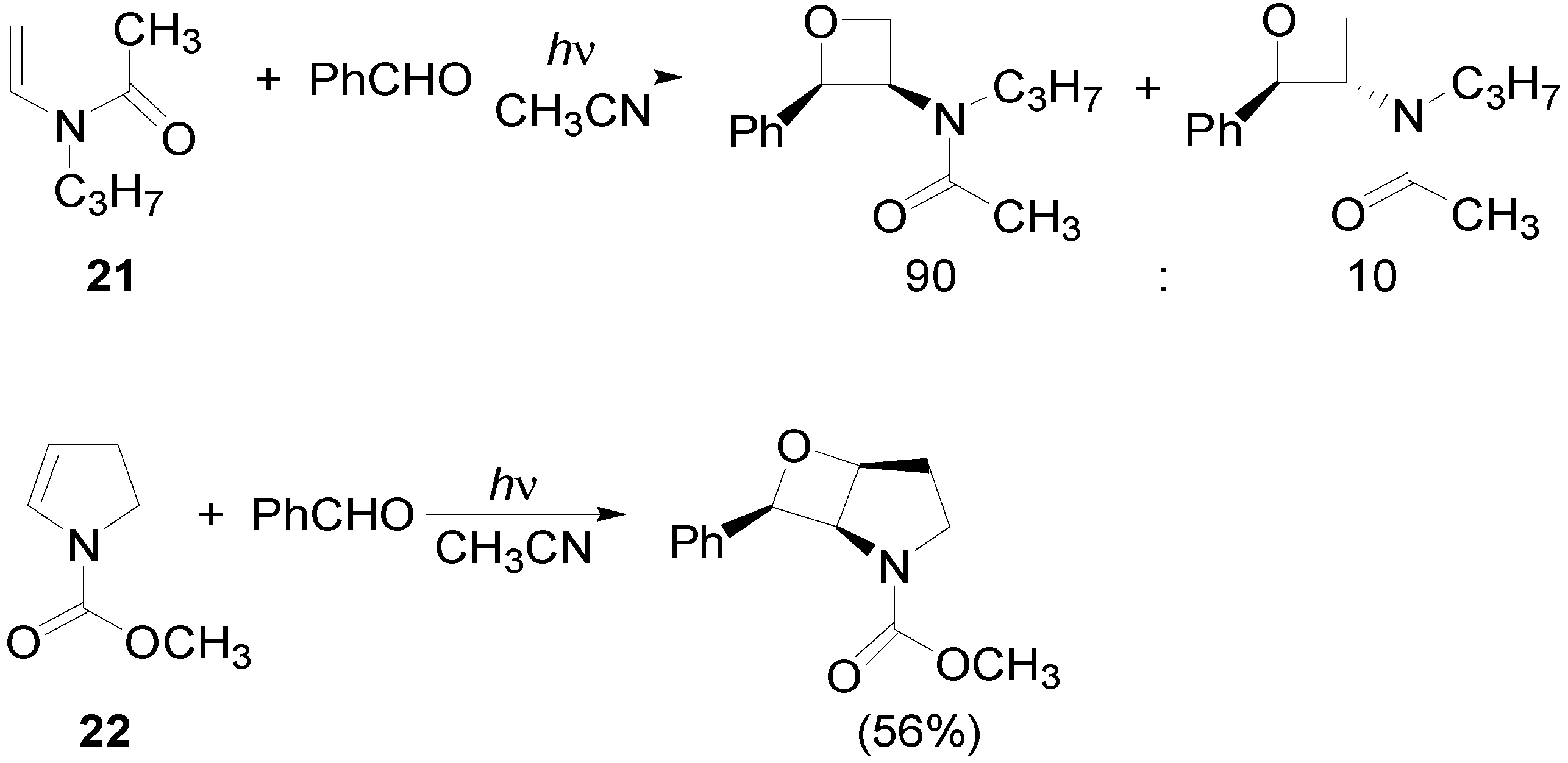
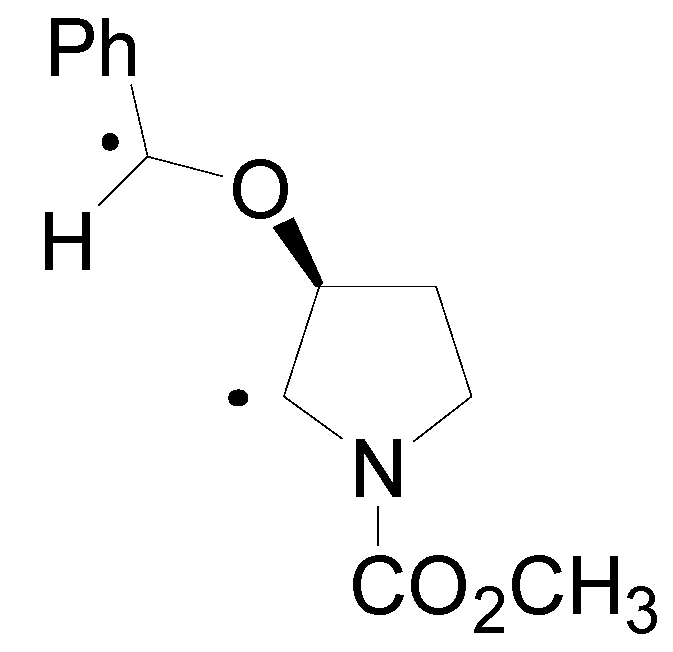

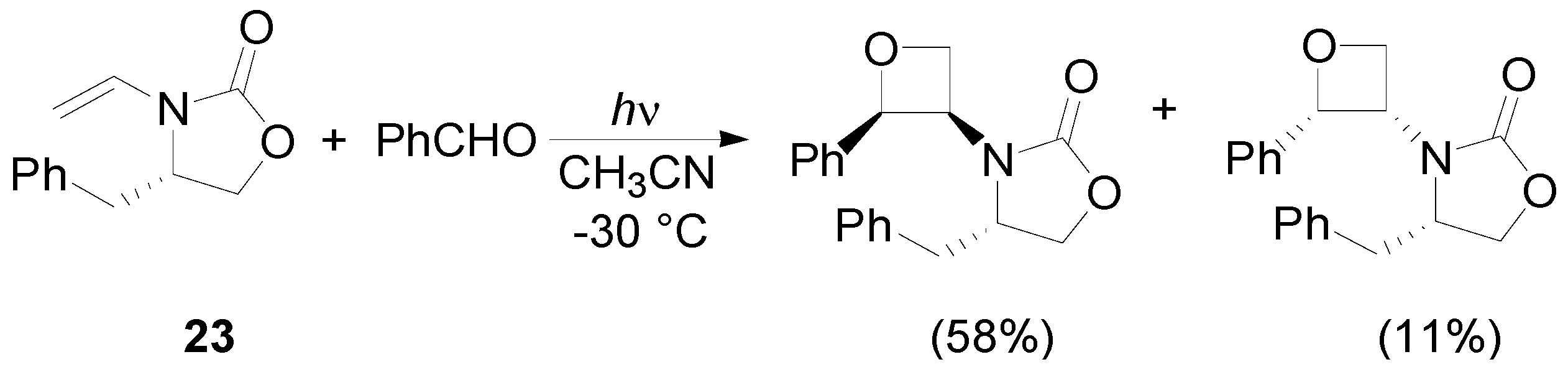
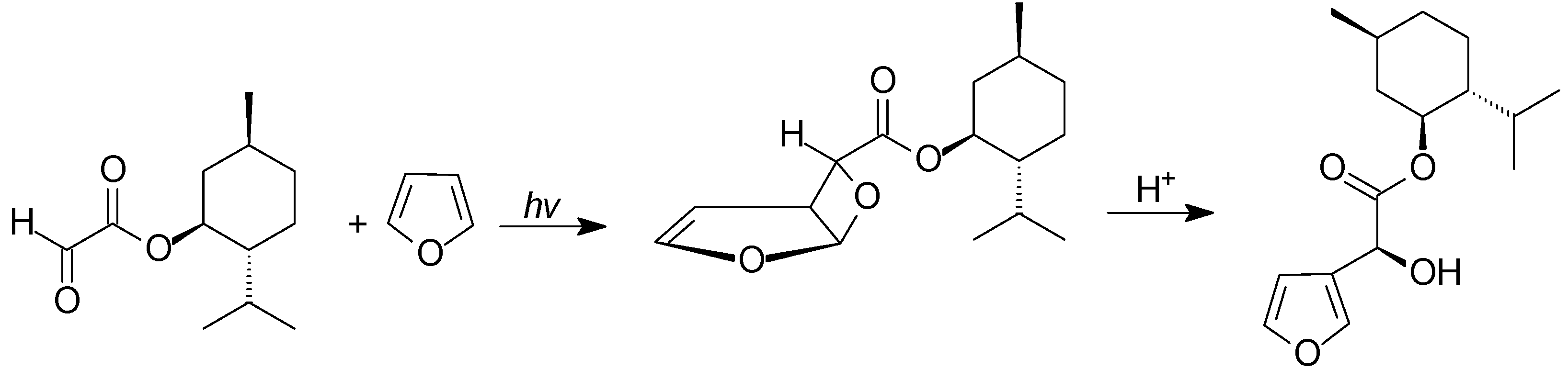
4. Reactions with Heterocyclic Compounds
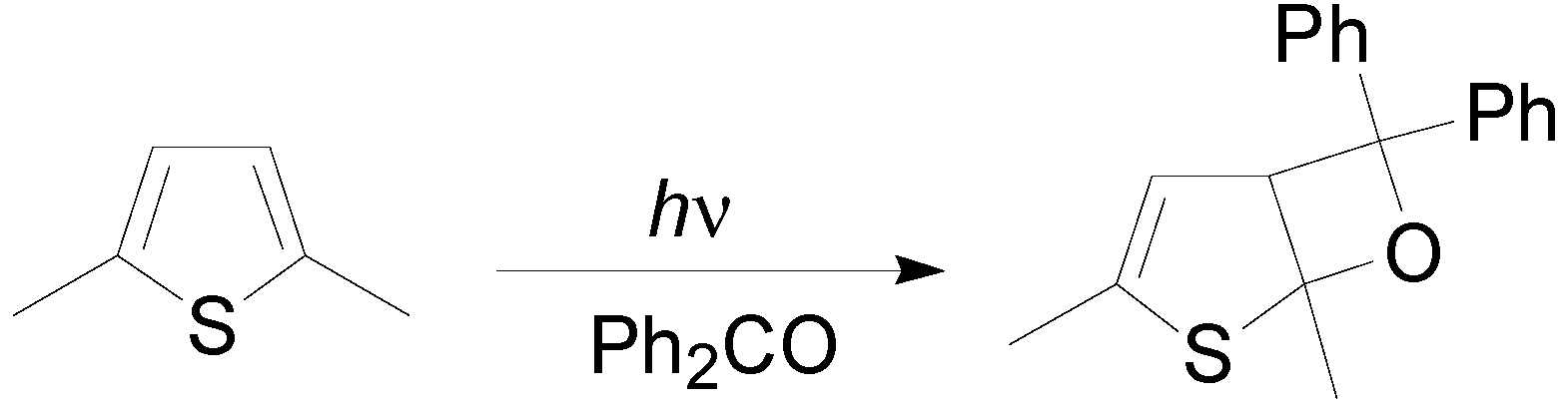
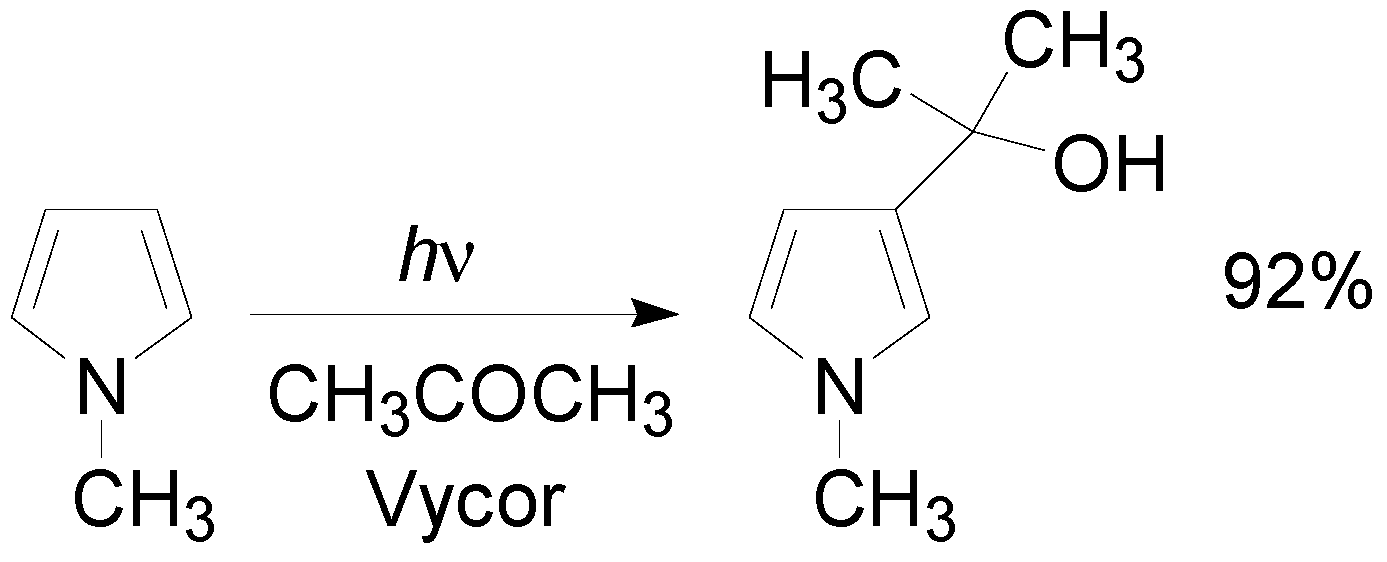

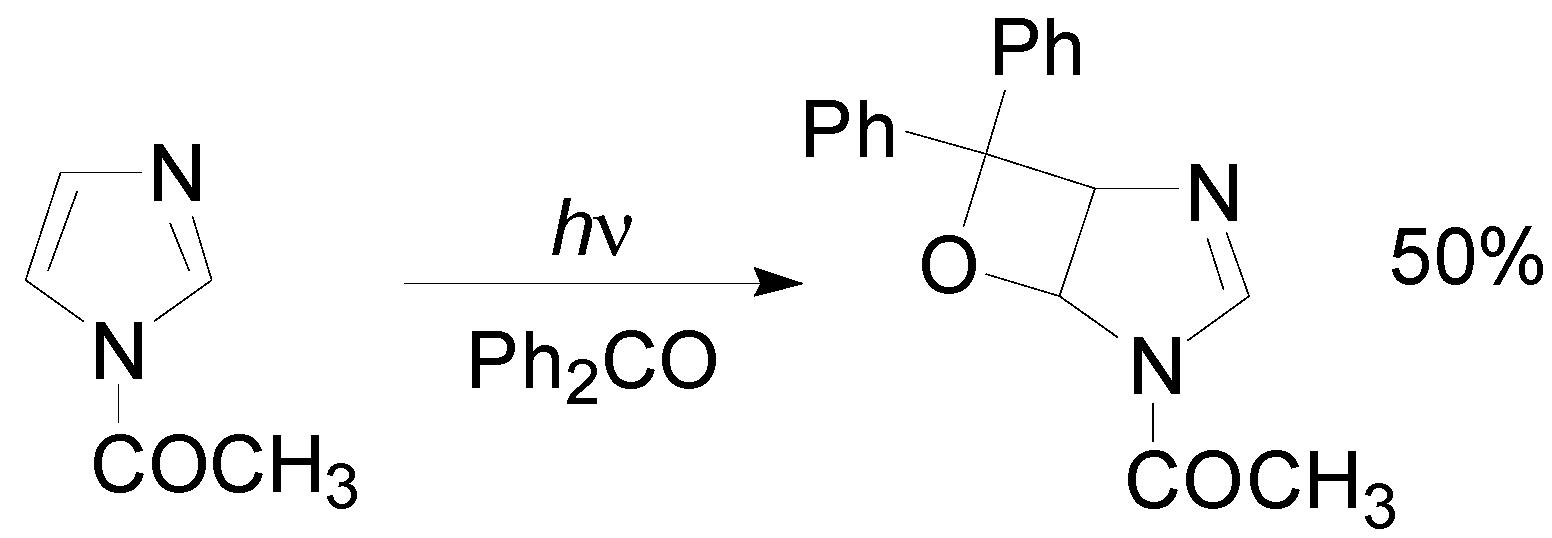
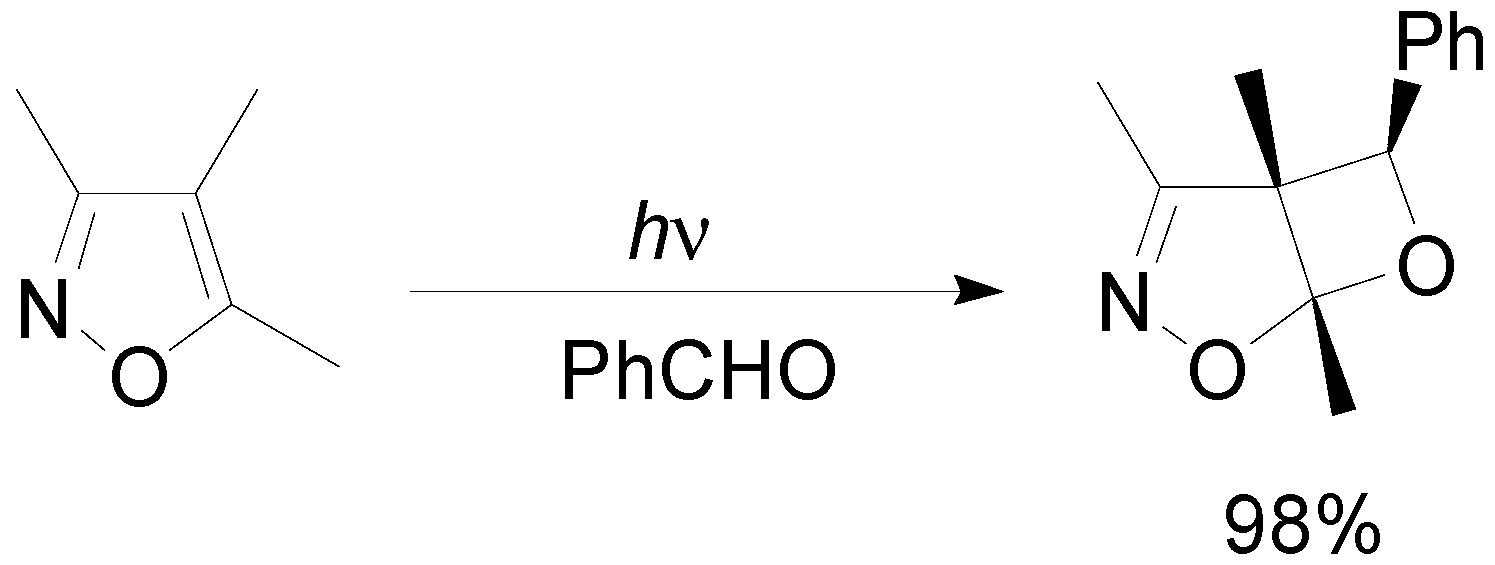
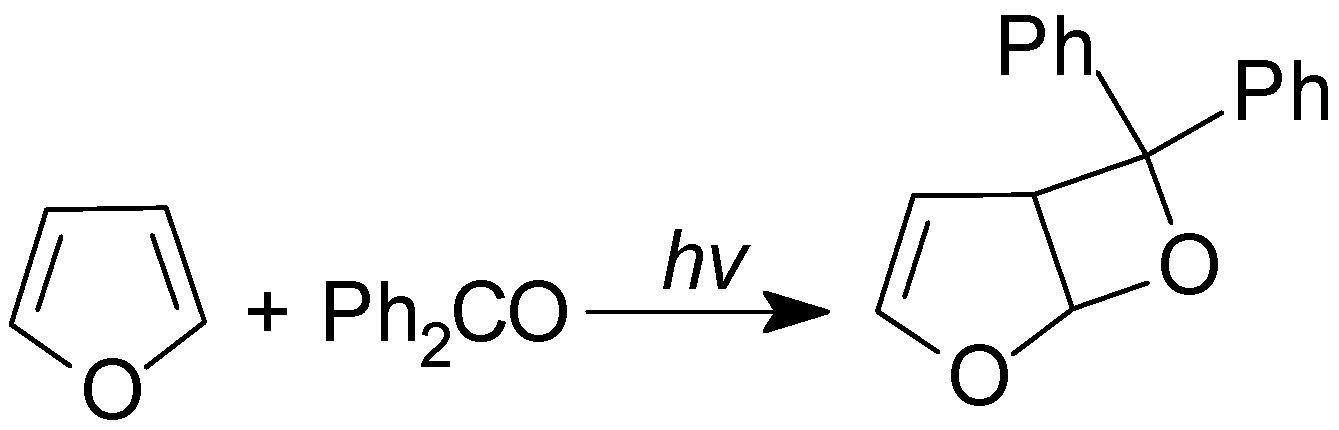
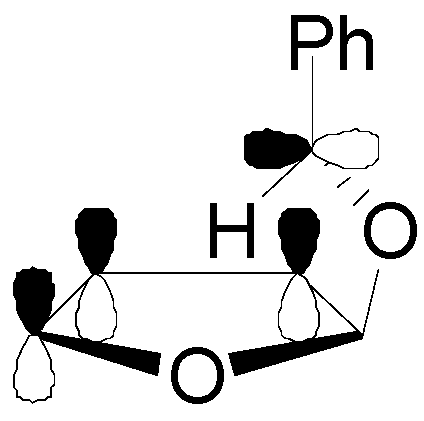
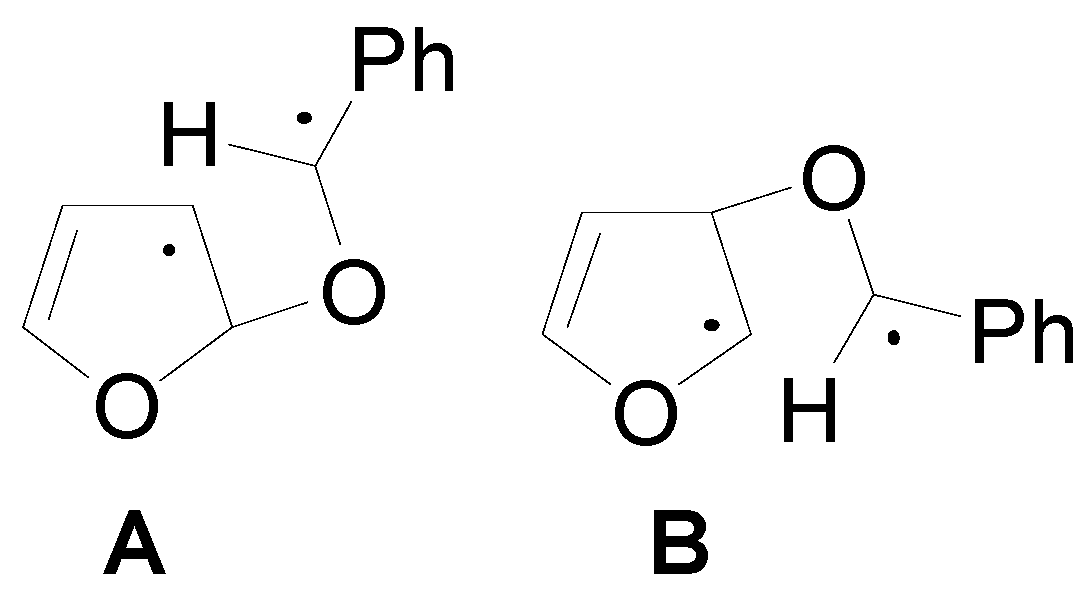
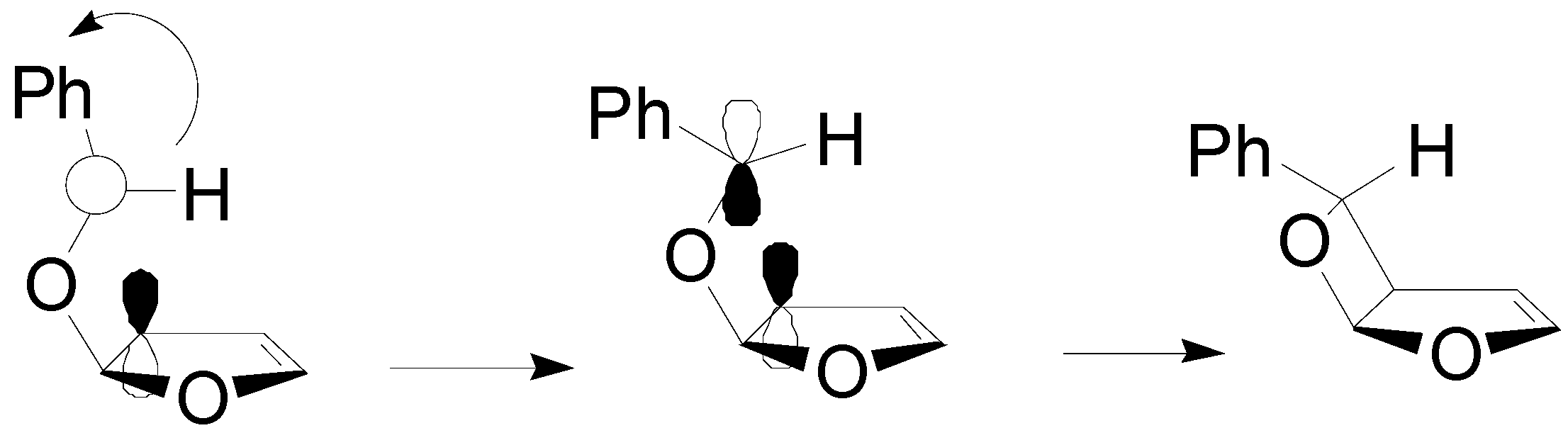
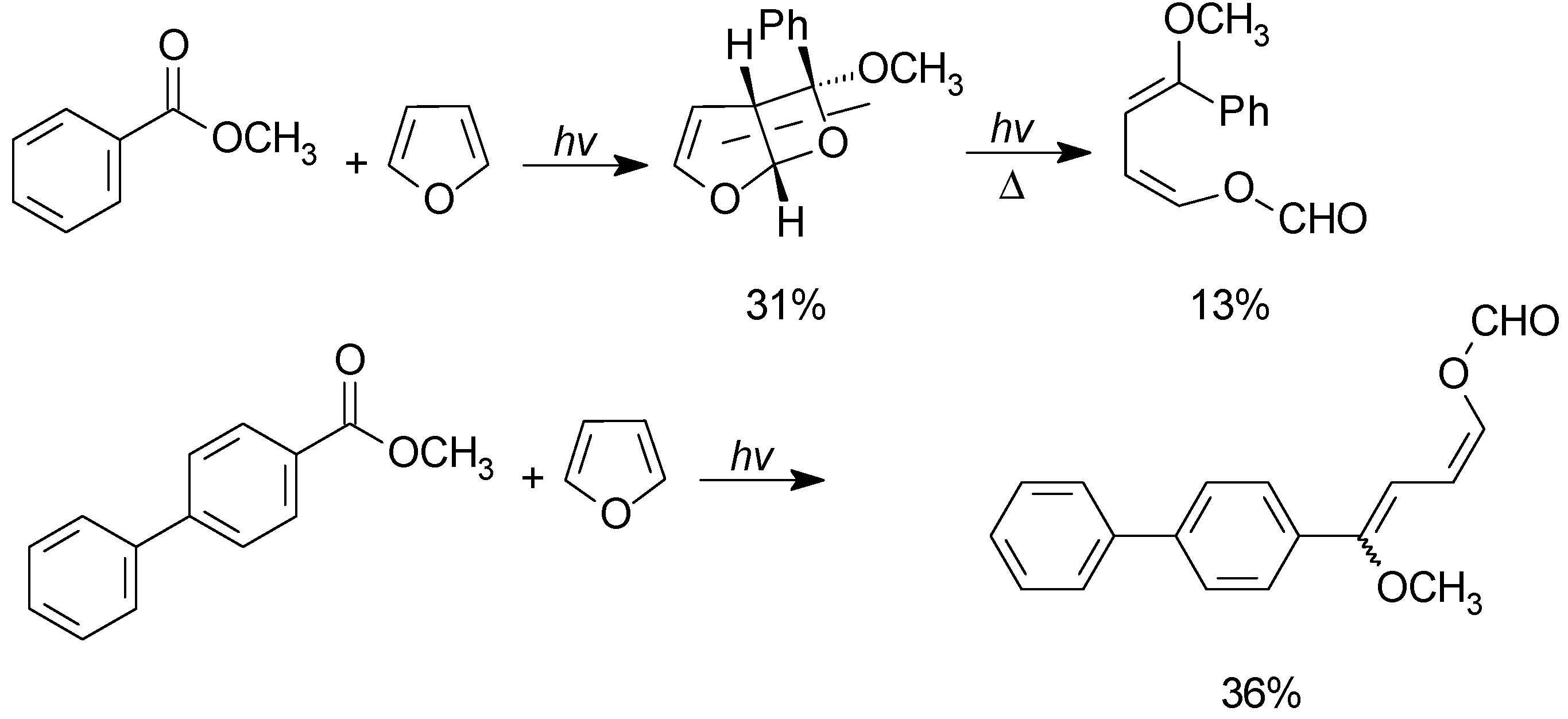
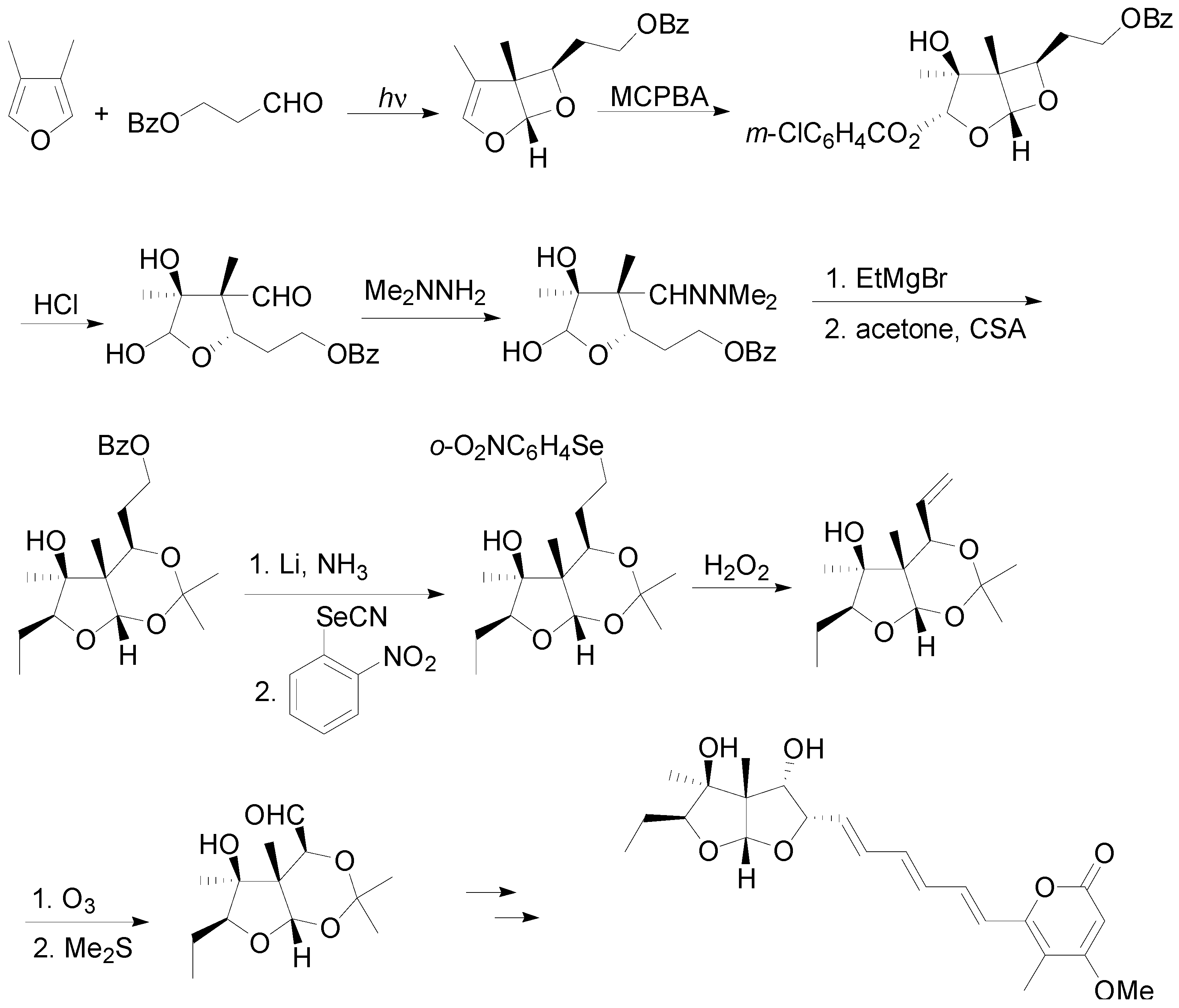
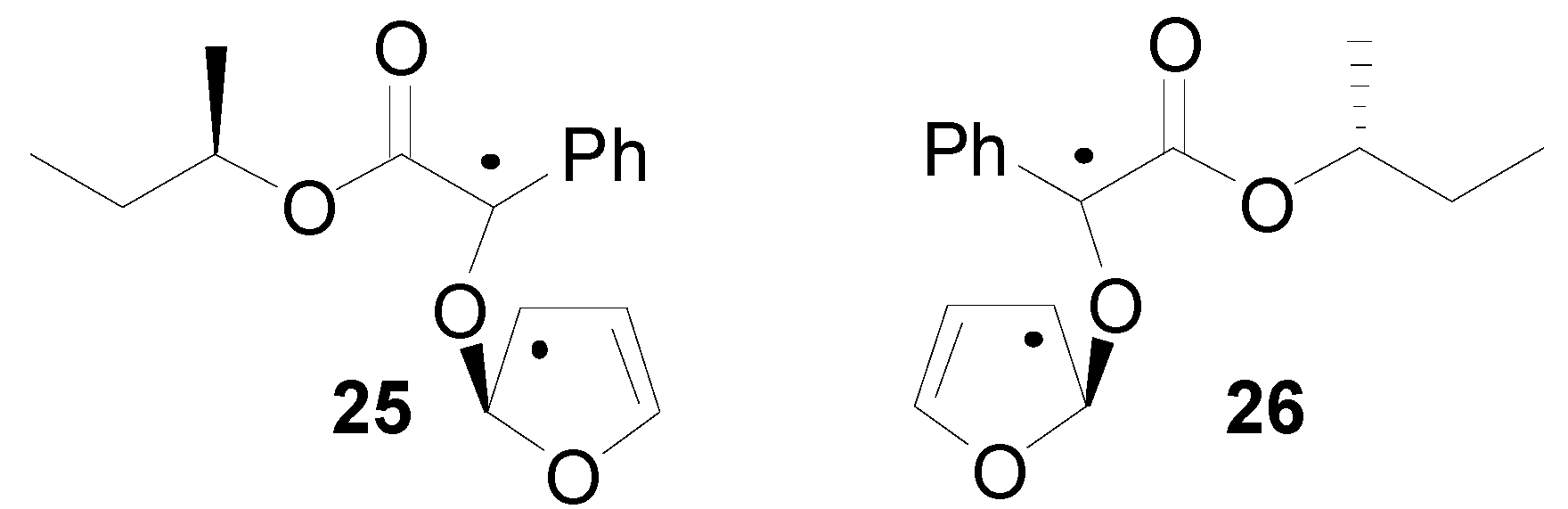
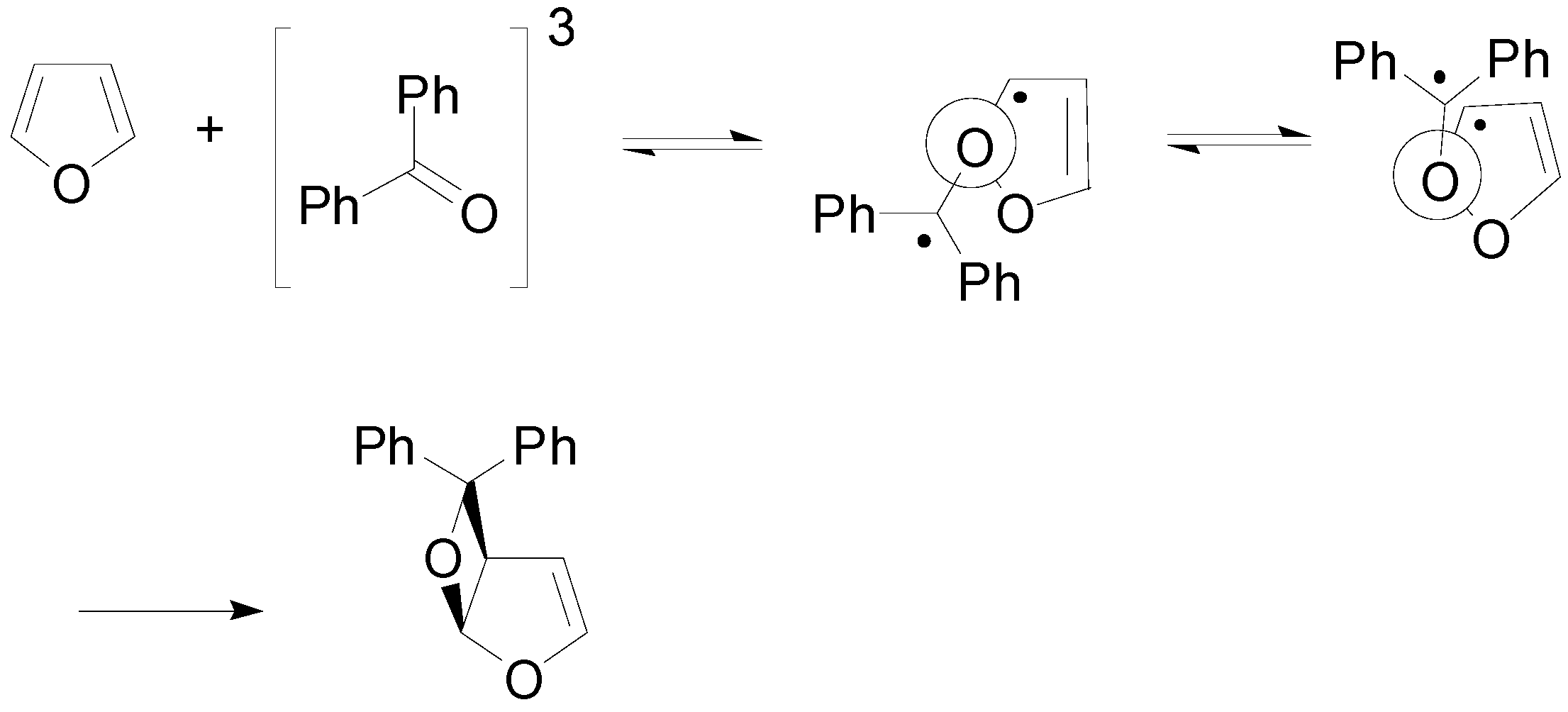
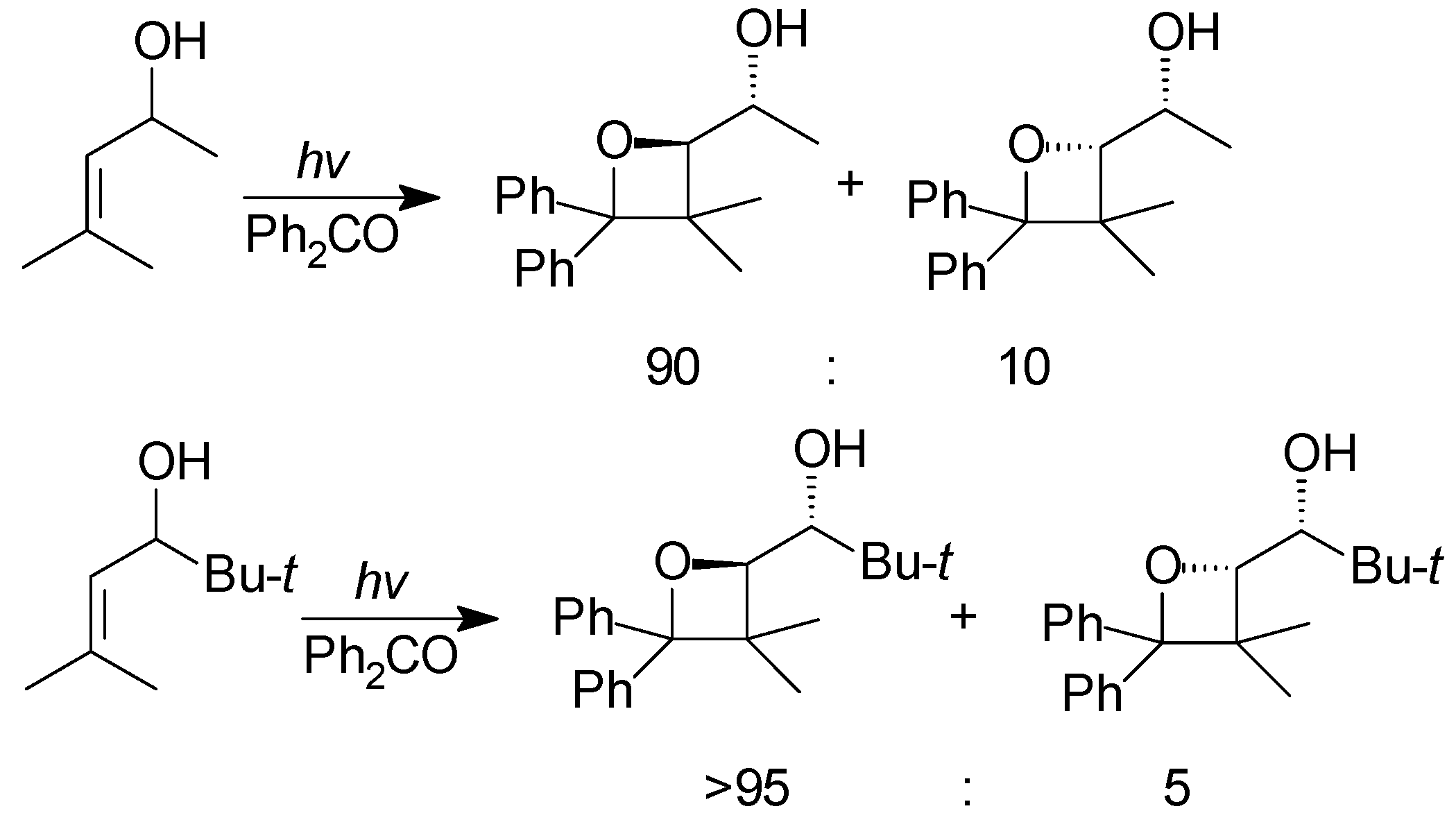

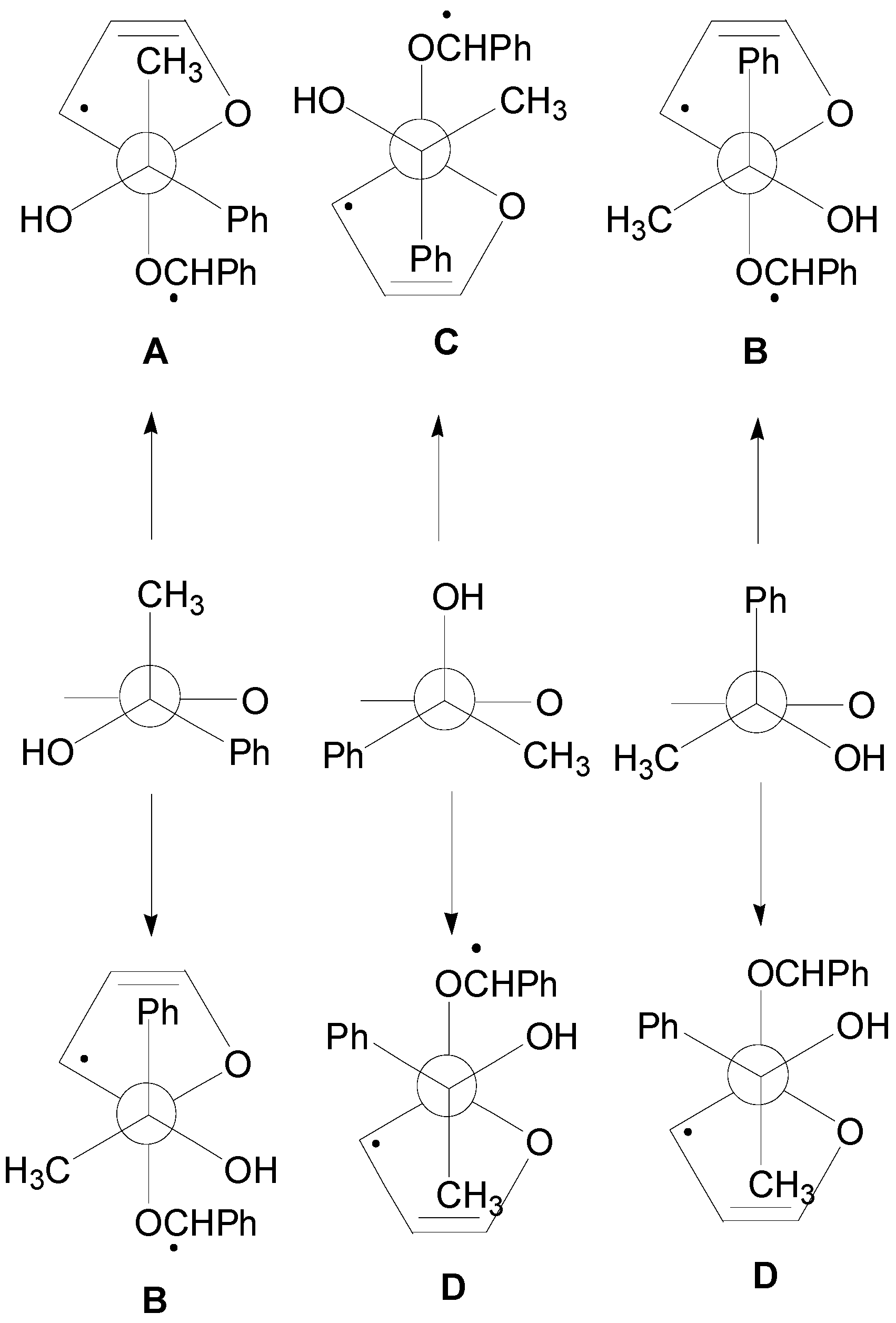

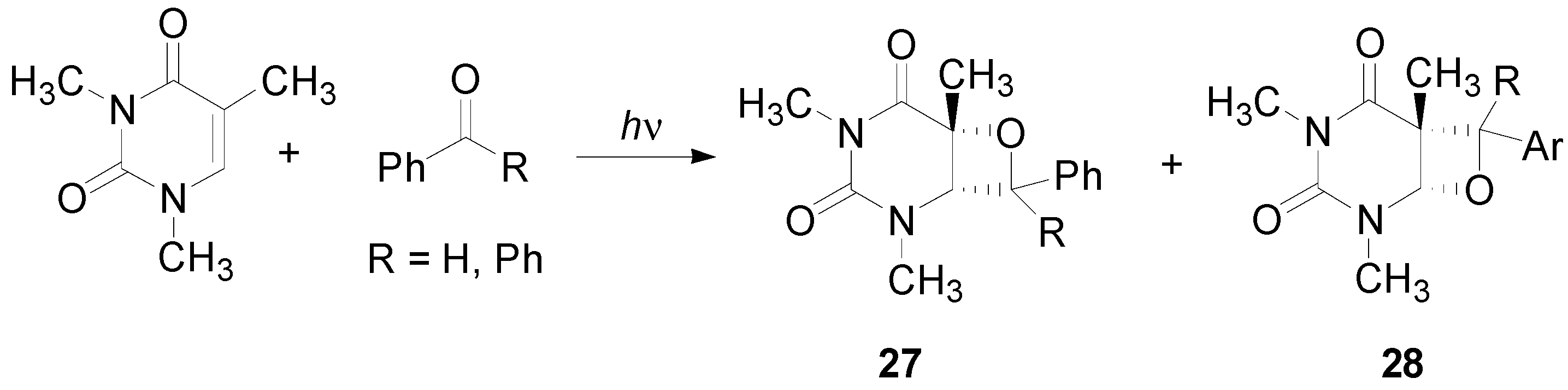
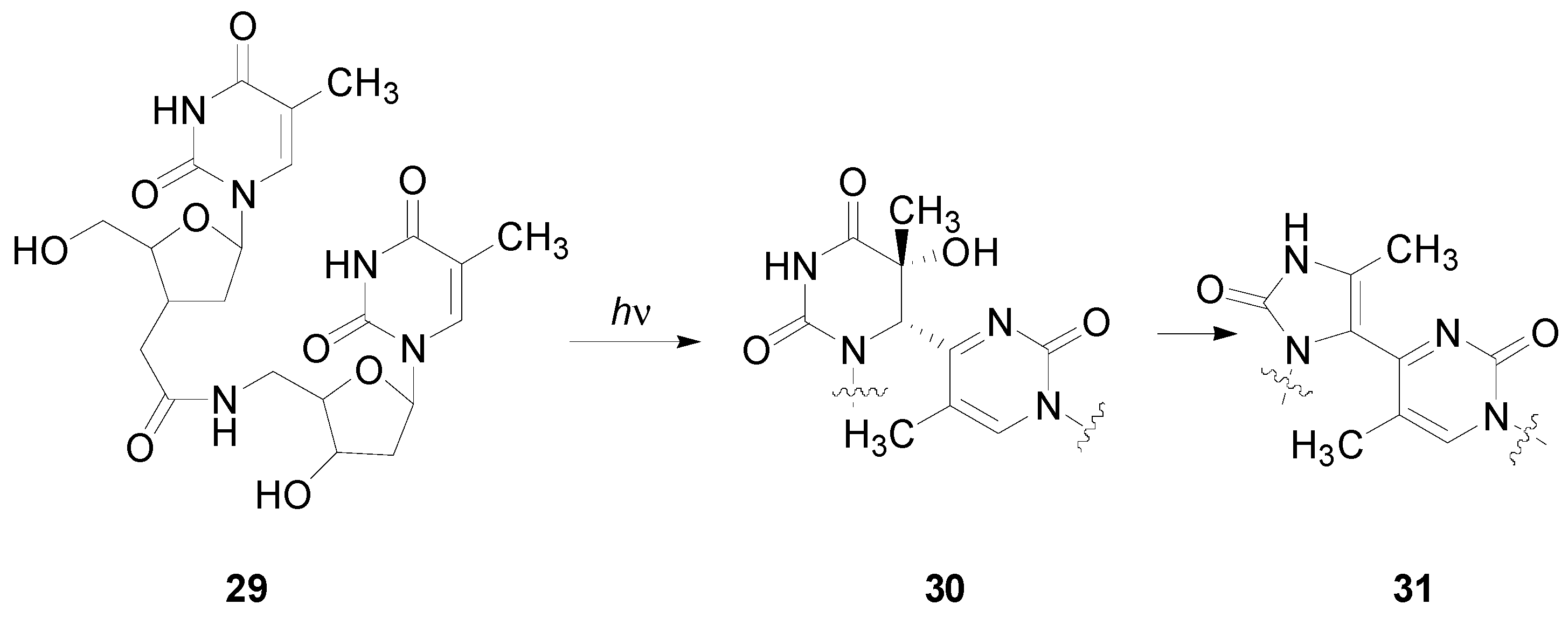
5. Other intermolecular reactions
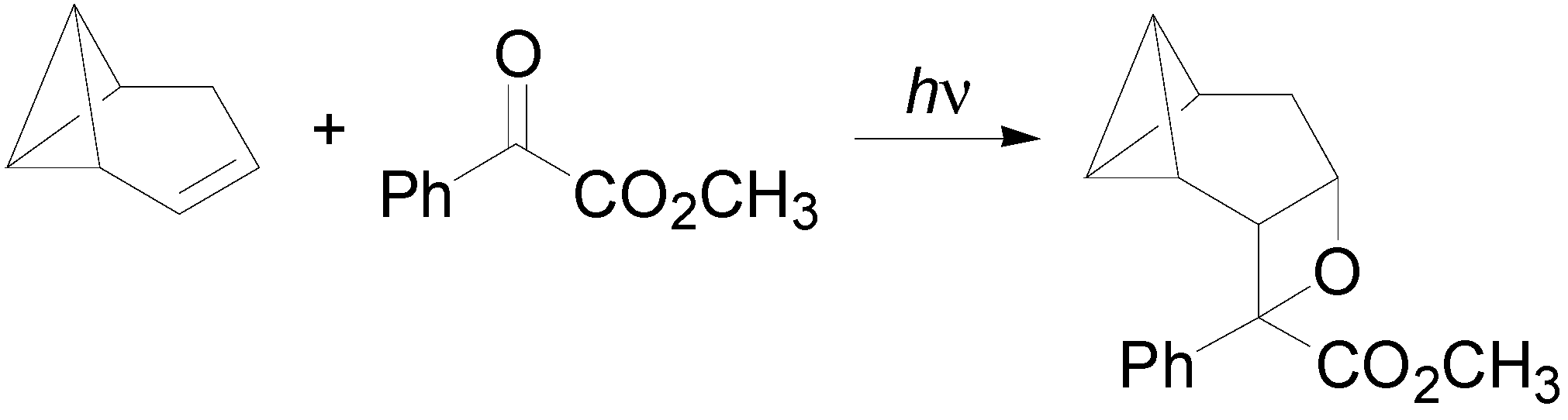
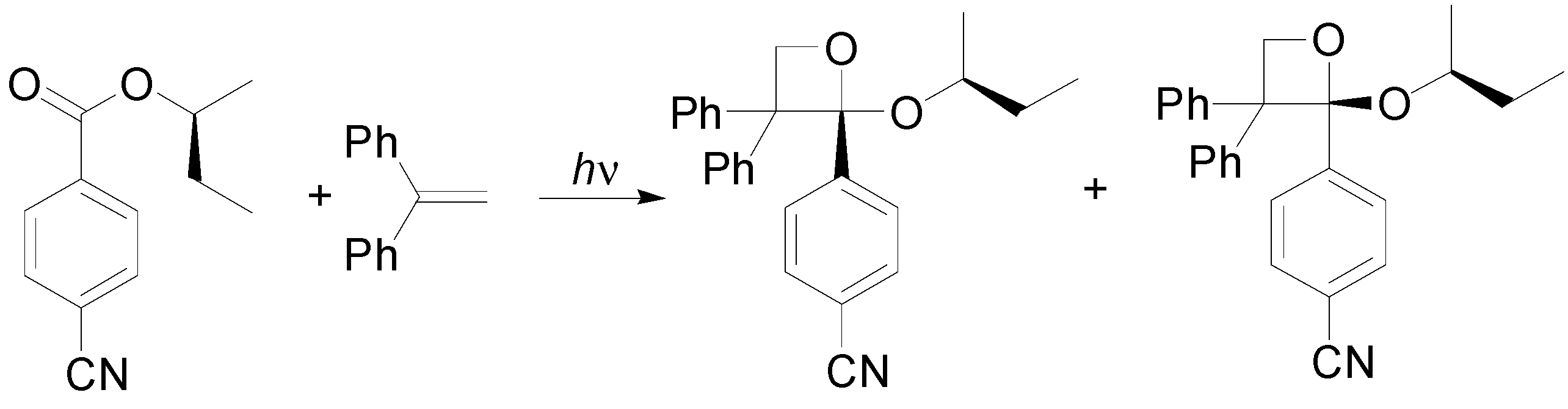
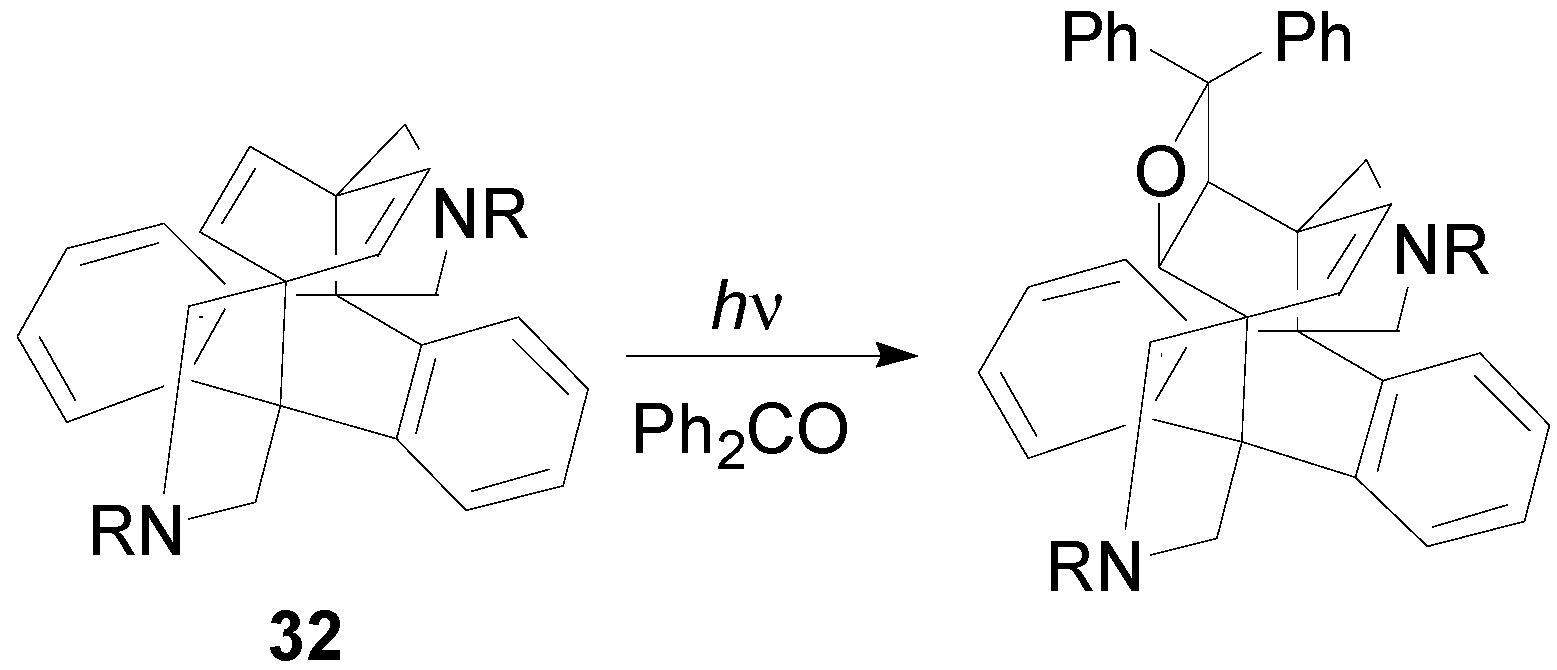
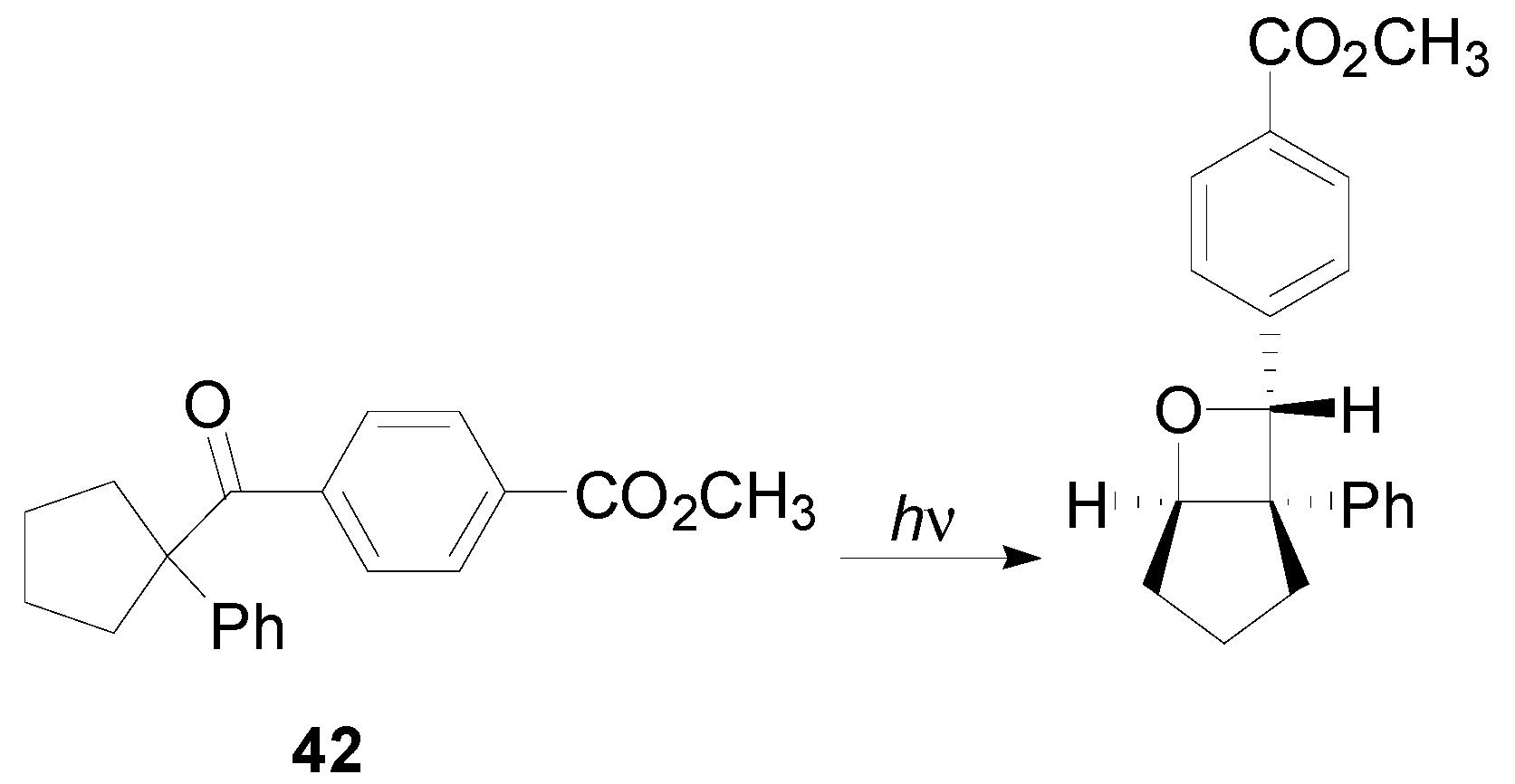
6. Intramolecular Reactions

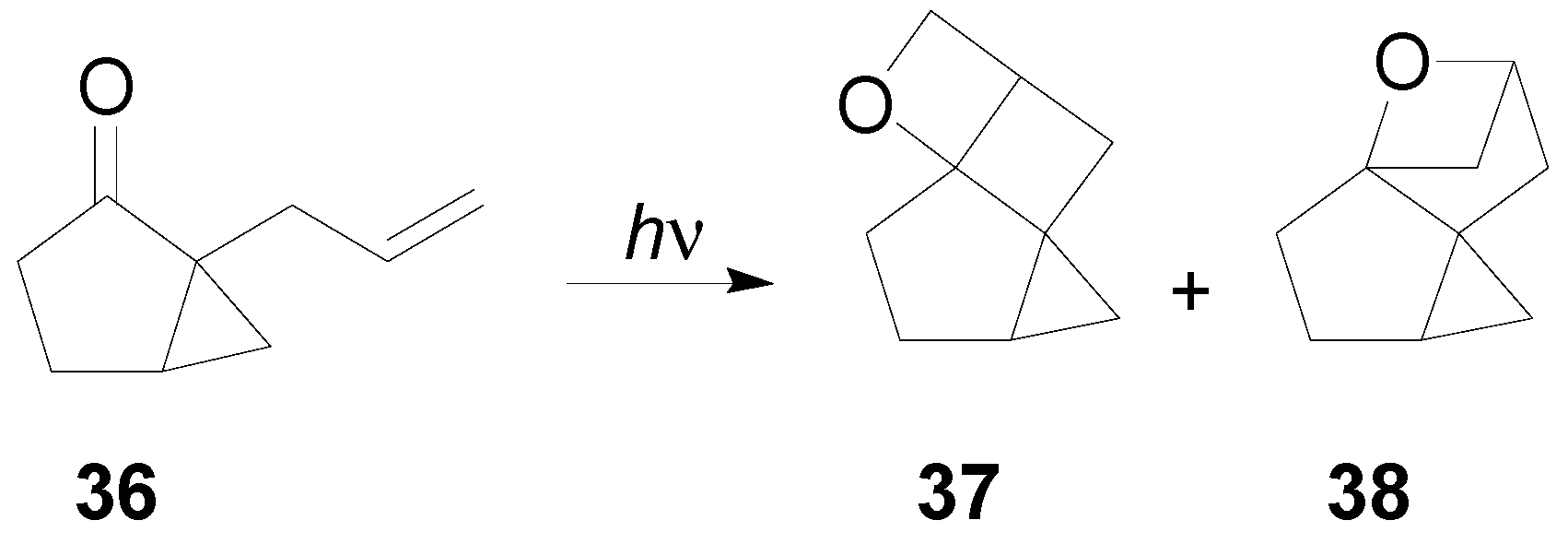
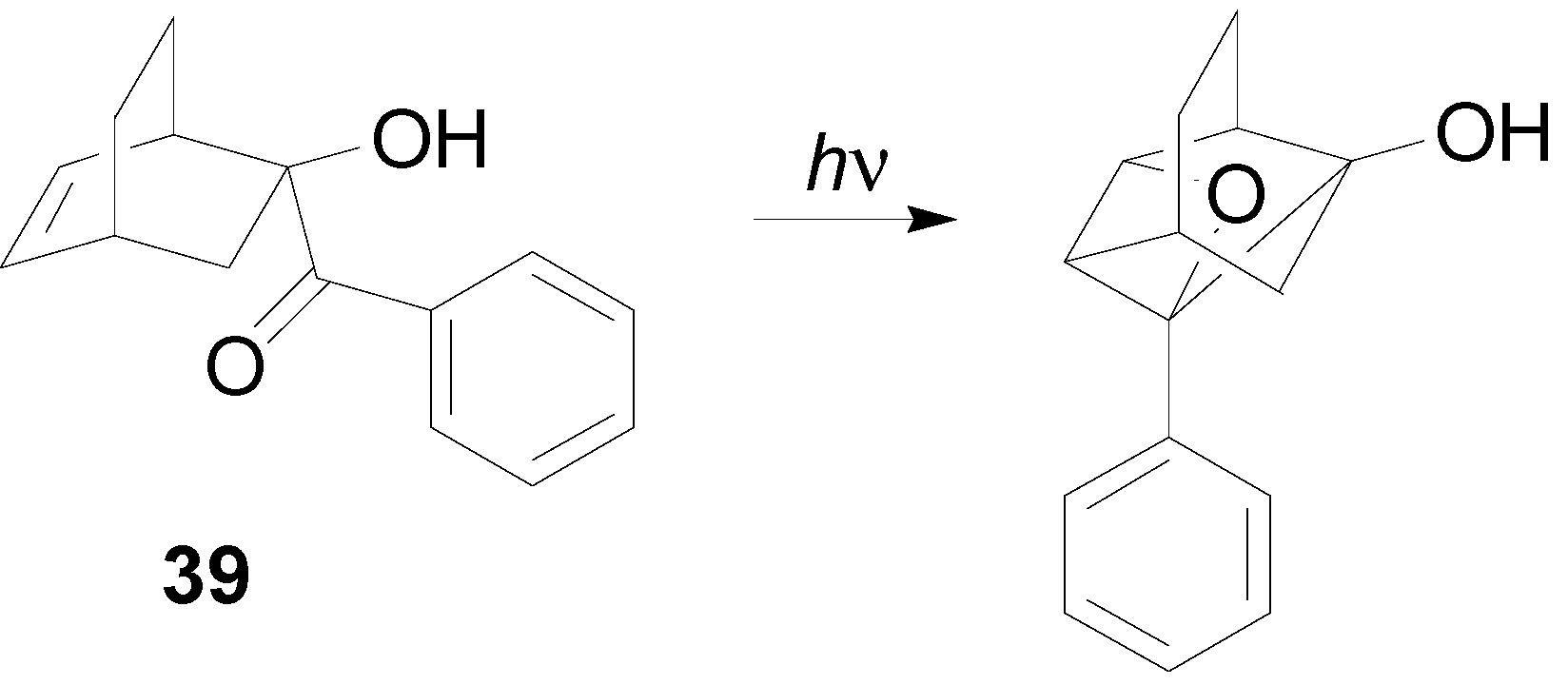
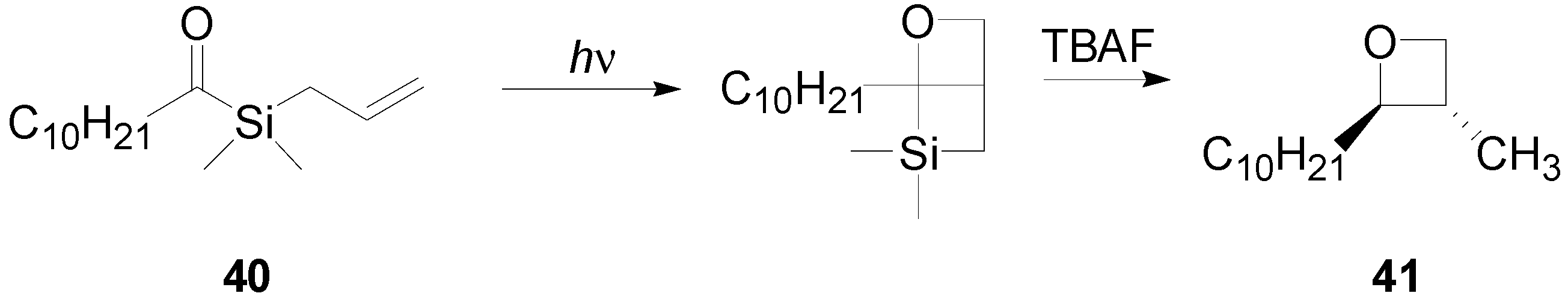
7. The Future of the Paternò-Büchi Reaction
8. Conclusions
Conflicts of Interest
References
- Kingston, D.G.I. The chemistry of taxol. Pharmacol. Ther. 1991, 52, 1–34. [Google Scholar] [CrossRef]
- Huang, J.; Yokoyama, R.; Yang, C.; Fukuyama, Y. Merrilactone A, a novel neurotrophic sesquiterpene dilactone from Illicium merrillianum. Tetrahedron Lett. 2000, 41, 6111–6114. [Google Scholar] [CrossRef]
- Wang, Y.; Fleet, G.W.J.; Storer, R.; Myers, P.L.; Wallis, C.J.; Doherty, O.; Watkin, D.J.; Vogt, K.; Witty, D.R.; Wilson, F.X.; Peach, J.M. Synthesis of the potent antiviral oxetane nucleoside epinoroxetanocin from D-lyxonolactone. Tetrahedron Asymmetry 1990, 1, 527–530. [Google Scholar] [CrossRef]
- Yang, C.O.; Kurz, W.; Eugui, E.M.; Mc Roberts, M.J.; Verheyden, J.P.H.; Kurz, L.J.; Walker, K.A.M. 4′-Substituted nucleosides as inhibitors of HIV: An unusual oxetane derivative. Tetrahedron Lett. 1992, 33, 41–44. [Google Scholar]
- Kawahata, Y.; Takatsuto, S.; Ikekawa, N.; Murata, M.; Omura, S. Synthesis of a new amino acid-antibiotic, oxetin and its three stereoisomers. Chem. Pharm. Bull. 1986, 34, 3102–3110. [Google Scholar] [CrossRef]
- Paternò, E. Sintesi in chimica organica per mezzo della luce. Nota II. Composti degli idrocarburi non saturi con aldeidi e chetoni. In Synthesis in Organic Chemistry by Means of Light; D’Auria, M., Ed.; Società Chimica Italiana: Rome, Italy, 2009; pp. 85–105. [Google Scholar]
- Büchi, G.; Inman, C.G.; Lipinsky, E.S. Light-catalyzed organic reactions. I. The reaction of carbonyl compounds with 2-methyl-2-butene in the presence of ultraviolet light. J. Am. Chem. Soc. 1954, 76, 4327–4331. [Google Scholar] [CrossRef]
- Arnold, D.R. The photocycloaddition of carbonyl compounds to unsaturated systems: The syntheses of oxetanes. Adv. Photochem. 1968, 6, 301–423. [Google Scholar] [CrossRef]
- Jones, G. Synthetic applications of the Paternò–Büchi reaction. Org. Photochem. 1981, 5, 1–122. [Google Scholar]
- Carless, H.A.J. Photochermical synthesis of oxetanes. In Synthetic Organic Photochemistry; Horspool, W.M., Ed.; Plenum: New York, NY, USA, 1984; pp. 425–487. [Google Scholar]
- Porco, J.A.; Schreiber, S.L. The Paternò–Büchi reaction. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Paquette, L.A., Eds.; Plenum: New York, NY, USA, 1991; Volume 5, pp. 151–192. [Google Scholar]
- Griesbeck, A.G. Oxetane formation stereocontrol. In Handbook of Photochemistry and Photobiology; Horspool, W.A., Song, P.-S., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 522–535, 550–559. [Google Scholar]
- Bach, T. Stereoselective intermolecular [2+2]-photocycloaddition reactions and their application in synthesis. Synthesis 1998, 1998, 683–703. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R.; Romaniello, G. The Paternò–Büchi reaction on furan derivatives. Curr. Org. Chem. 2003, 7, 1443–1459. [Google Scholar] [CrossRef]
- D’Auria, M. Paternò-Büchi reaction on furan: Regio- and stereochemistry. In Targets in Heterocyclic Systems, Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society of Chemistry: Rome, Italy, 2003; Volume 7, pp. 157–173. [Google Scholar]
- Griesbeck, A.G.; Bondock, S. Oxetane formation: Stereocontrol. In Handbook of Photochemistry and Photobiology; Horspool, W.A., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 59:1–59:9. [Google Scholar]
- Griesbeck, A.G.; Bondock, S. Oxetane formation: Intermolecular additions. In Handbook of Photochemistry and Photobiology; Horspool, W.A., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; p. 60. [Google Scholar]
- Abe, M. Photochemical oxetane formation: Addition to heterocycles. In Handbook of Photochemistry and Photobiology; Horspool, W.A., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; p. 62. [Google Scholar]
- D’Auria, M.; Emanuele, L.; Racioppi, R. 1,2-Cycloaddition reaction of carbonyl compounds and pentaatomic heterocyclic compounds. In Advances in Photochemistry; Neckers, D.C., Jenks, W.S., Wolff, T., Eds.; John Wiley & Sons: Hoboken, New Jersey, NJ, USA, 2005; Volume 28, pp. 81–127. [Google Scholar]
- D’Auria, M.; Emanuele, L.; Racioppi, R. 2+2 Cycloaddition on furan derivatives. In Photochemistry Research Progress; Sanchez, A., Gutierrez, S.J., Eds.; Nova Science Publishers Inc.: Hauppage, NY, USA, 2008; pp. 373–438. [Google Scholar]
- D’Auria, M.; Racioppi, R. Concepts of stereoselective photochemistry and a case study: The Paternò-Büchi reaction. Curr. Org. Chem. 2009, 13, 939–954. [Google Scholar] [CrossRef]
- Abe, M. Formation of a 4-membered ring III (oxetanes). In Handbook of Synthetic Photochemistry; Albini, A., Fagnoni, M., Eds.; Wiley: New York, NY, USA, 2010; pp. 217–239. [Google Scholar]
- Griesbeck, A.G. Photocycloadditions of alkenes to excited carbonyls. In Synthetic Organic Photochemistry; Griesbeck, A.G., Mattay, J., Eds.; Marcel Dekker: New York, NY, USA, 2005; pp. 89–139. [Google Scholar]
- D’Auria, M. Paternò–Büchi Reaction. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd Edition; Griesbeck, A., Oelgemöller, M., Ghetti, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 653–681. [Google Scholar]
- Kopecký, J. Organic Photochemistry; VCH: New York, NY, USA, 1992; p. 126. [Google Scholar]
- Turro, N.J.; Dalton, J.C.; Dawes, K.; Farrington, G.; Hautala, R.; Morton, D.; Niemczyk, M.; Schore, N. Molecular photochemistry. L. Molecular photochemistry of alkanones in solution. α-Cleavage, hydrogen abstraction, cycloaddition, and sensitization reactions. Acc. Chem. Res. 1972, 5, 92–101. [Google Scholar] [CrossRef]
- Freilich, S.C.; Peters, K.S. Observation of the 1,4 biradical in the Paterno-Buchi reaction. J. Am. Chem. Soc. 1981, 103, 6255–6257. [Google Scholar] [CrossRef]
- Caldwell, R.A.; Majima, T.; Pac, C. Some structural effects on triplet biradical lifetimes. Norrish II and Paterno-Buchi biradicals. J. Am. Chem. Soc. 1982, 104, 629–630. [Google Scholar] [CrossRef]
- Freilich, S.C.; Peters, K.S. Picosecond dynamics of the Paterno-Buechi reaction. J. Am. Chem. Soc. 1985, 107, 3819–3822. [Google Scholar] [CrossRef]
- Abe, M.; Kawakami, T.; Ohata, S.; Nozaki, K.; Nojima, M. Mechanism of stereo- and regioselectivity in the Paternò−Büchi reaction of furan derivatives with aromatic carbonyl compounds: Importance of the conformational distribution in the intermediary triplet 1,4-diradicals. J. Am. Chem. Soc. 2004, 126, 2838–2846. [Google Scholar]
- Wilson, R.M.; Wunderly, S.W.; Walsh, T.F.; Musser, A.K.; Outcalt, R.; Geiser, F.; Gee, S.K.; Brabender, W.; Yerino, L., Jr.; Conrad, T.T.; et al. Laser photochemistry: Trapping of quinone-olefin preoxetane intermediates with molecular oxygen and chemistry of the resulting 1,2,4-trioxanes. J. Am. Chem. Soc. 1982, 104, 4429–4446. [Google Scholar] [CrossRef]
- Adam, W.; Kliem, U.; Lucchini, V. Preparative UV-VIS laser photochemistry; molecular oxygen trapping of the Paterno-Büchi triplet diradicals derived from 1,4-dioxene. Tetrahedron Lett. 1986, 27, 2953–2956. [Google Scholar] [CrossRef]
- Adam, W.; Kliem, U.; Mosandl, T.; Peters, E.-M.; Peters, K.; von Schnering, H.G. Preparative visible-laser photochemistry: Qinghaosu-type 1,2,4-trioxanes by molecular oxygen trapping of Paterno-Buechi triplet 1,4-diradicals derived from 3,4-dihydro-4,4-dimethyl-2H-pyran-2-one and quinones. J. Org. Chem. 1988, 53, 4986–4992. [Google Scholar]
- Adam, W.; Kliem, U.; Lucchini, V. Preparative UV-VIS laser photochemistry: Photocycloadditions of methylenelactones with benzophenone and p-benzoquinone. Oxygen trapping of paterno-Büchi triplet 1,4-diradicals as model reactions for quinghaosu-type 1,2,4-trioxanes. Liebigs Ann. 1988, 869–875. [Google Scholar]
- Pinter, B.; de Proft, F.; Veszprémi, T.; Geerlings, P. Regioselectivity in the [2 + 2] cyclo-addition reaction of triplet carbonyl compounds to substituted alkenes (Paterno-Büchi reaction): A spin-polarized conceptual DFT approach. J. Chem. Sci. 2005, 117, 561–571. [Google Scholar] [CrossRef]
- Noorizadeh, S. Minimum electrophilicity principle in photocycloaddition formation of oxetanes. J. Phys. Org. Chem. 2007, 20, 514–524. [Google Scholar] [CrossRef]
- Palmer, I.J.; Ragazos, I.N.; Bernardi, F.; Olivucci, M.; Robb, M.A. An MC-SCF Study of the (Photochemical) Paterno-Buchi Reaction. J. Am. Chem. Soc. 1994, 116, 2121–2132. [Google Scholar] [CrossRef]
- Kutateladze, A.G. Conformational analysis of singlet-triplet state mixing in Paternò-Büchi diradicals. J. Am. Chem. Soc. 2001, 123, 9279–9282. [Google Scholar] [CrossRef]
- Kutateladze, A.G.; McHale, W.A., Jr. Toward parameterization of spin-orbit coupling in triplet organic diradicals separated by a partially conjugated spacer. Arkivoc 2005, 88–101. [Google Scholar] [CrossRef]
- Minaev, B.F. Spin–orbit coupling in oxygen containing diradicals. J. Mol. Struct. 1998, 434, 193–206. [Google Scholar] [CrossRef]
- Minaev, B.F.; Ågren, H. The role of one-center spin-orbit coupling in organic chemical reactions. EPA Newslett. 1999, 65, 7–38. [Google Scholar]
- Mattay, J. Charge transfer and radical ions in photochemistry. Angew. Chem. Int. Ed. Engl. 1987, 26, 825–845. [Google Scholar] [CrossRef]
- Paternò, E.; Chieffi, G. Sintesi in chimica organica per mezzo della luce. Nota V. Comportamento degli acidi e degli eteri col benzofenone. Gazz. Chim. Ital. 1910, 40, 321–331. [Google Scholar]
- Gáplovský, A.; Donovalová, J.; Toma, S.; Kubinec, R. Ultrasound effects on photochemical reactions, Part 1: Photochemical reactions of ketones with alkenes. Ultrasonics Sonochem. 1997, 4, 109–115. [Google Scholar] [CrossRef]
- Schroeter, S.H.; Orlando, C.M. Photocycloaddition of various ketones and aldehydes to vinyl ethers and ketene diethyl acetal. J. Org. Chem. 1969, 34, 1181–1187. [Google Scholar] [CrossRef]
- Khan, N.; Morris, T.H.; Smith, E.H.; Walsh, R. Alkenyl sulphides and ketene S,S-dithioacetals as olefin components in the Paterno-Büchi reaction: A regioselective synthesis of oxetanes. J. Chem. Soc. Perkin Trans. 1 1991, 1991, 865–870. [Google Scholar]
- Mattay, J.; Buchkremer, K. Thermal and photochemical oxetane formation. A contribution to the synthesis of Branched-Chain aldonolactone. Helv. Chim. Acta 1988, 71, 981–987. [Google Scholar] [CrossRef]
- Mattay, J.; Buchkremer, K. Thermal and photochemical oxetane formation with α-Ketoesters. Heterocycles 1988, 27, 2153–2166. [Google Scholar] [CrossRef]
- Buhr, S.; Griesbeck, A.G.; Lex, J.; Mattay, J.; Schröer, J. Stereoselectivity in the Paternò-Büchi reaction of 2,2-diisopropyl-1,3-dioxol with methyl trimethylpyruvate. Tetrahedron Lett. 1996, 37, 1195–1196. [Google Scholar] [CrossRef]
- Abe, M.; Taniguchi, K.; Hayashi, T. Exo-selective formation of bicyclic oxetanes in the photocycloaddition reaction of carbonyl compounds with vinylene carbonate: The important role of intermediary triplet diradicals in the stereoselectivity. Arkivoc 2007, 2007, 58–65. [Google Scholar]
- Gan, C.Y.; Lambert, J.N. The tandem intermolecular Paternò–Büchi reaction: Formation of tetrahydrooxepins. J. Chem. Soc. Perkin Trans. 1 1998, 1998, 2363–2372. [Google Scholar]
- Park, S.-K.; Lee, S.-J.; Baek, K.; Yu, C.-M. Diastereoselective routes in the Paterno-Büchi reactions of cyclic enol ortho ester with aldehydes. Bull. Korean Chem. Soc. 1998, 19, 35–36. [Google Scholar]
- Netto-Ferreira, J.C.; Silva, M.T.; Puget, F.P. Photochemistry of cyclic vicinal tricarbonyl compounds: [2+2] Photocycloaddition of 1,2,3-indanetrione to electron rich olefins. J. Photochem. Photobiol. A 1998, 119, 165–170. [Google Scholar] [CrossRef]
- Silva, M.T.; Braz-Filho, R.; Netto-Ferreira, J.C. Photochemistry of cyclic vicinal tricarbonyl compounds. Photochemical reaction of 1,2,3- indanetrione with 2,3-Dimethyl-2-butene: Hydrogen abstraction and photocycloaddition. J. Braz. Chem. Soc. 2000, 11, 479–485. [Google Scholar] [CrossRef]
- Ogata, M.; Watanabe, H.; Kanō, H. Photochemical cycloaddition of benzophenone to furans. Tetrahedron Lett. 1967, 8, 533–537. [Google Scholar] [CrossRef]
- Carless, H.A.; Haywood, D.J. Photochemical syntheses of 2,6-dioxabicyclo[3.2.0]heptanes. J. Chem. Soc. Chem. Commun. 1980, 1067–1068. [Google Scholar] [CrossRef]
- Bondock, S.; Griesbeck, A.G. Spin-dependent diastereoselectivity in the photocycloaddition of aldehydes to 2,2-dimethyl-2,3-dihydrofuran. Int. J. Photoenergy 2005, 7, 23–25. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Fiege, M.; Bondock, S.; Gudipati, M.S. Spin-directed stereoselectivity of carbonyl-alkene photocycloadditions. Org. Lett. 2000, 2, 3623–3625. [Google Scholar] [CrossRef]
- Griesbeck, A.G. Stereoselective and spin-selective photochemical reactions. J. Photoscience 2003, 10, 49–60. [Google Scholar]
- Griesbeck, A.G. Spin-selectivity in photochemistry: A tool for organic synthesis. Synlett 2003, 2003, 451–472. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Abe, M.; Bondock, S. Selectivity control in electron spin inversion processes: Regio- and stereochemistry of Paternò−Büchi photocyclo- additions as a powerful tool for mapping intersystem crossing processes. Acc. Chem. Res. 2004, 37, 919–928. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Bondock, S.; Gudipati, M.S. Temperature and viscosity dependence of the spin-directed stereoselectivity of the carbonyl-alkene photocycloaddition. Angew. Chem. Int. Ed. 2001, 40, 4684–4687. [Google Scholar] [CrossRef]
- D’Auria, Maurizio. University of Basilicata: Potenza, Italy, Unpublished work. 2013.
- Griesbeck, A.G.; Stadtmüller, S. Photocycloaddition of benzaldehyde to cyclic olefins: Electronic control of endo stereoselectivity. J. Am. Chem. Soc. 1990, 112, 1281–1283. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Stadtmüller, S. Electronic control of stereoselectivity in photocycloaddition reactions. 4. Effects of methyl substituents at the donor olefin. J. Am. Chem. Soc. 1991, 113, 6923–6928. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Stadtmüller, S. Regio- und stereoselektive photocycloadditionen aromatischer aldehyde an furan und 2,3-dihydrofuran. Chem. Ber. 1990, 123, 357–362. [Google Scholar] [CrossRef]
- Lhiaubet-Vallet, V.; Encinas, S.; Miranda, M.A. Excited state enantiodifferentiating interactions between a chiral benzophenone derivative and nucleosides. J. Am. Chem. Soc. 2005, 127, 12774–12775. [Google Scholar] [CrossRef]
- Abe, M. Recent progress regarding regio-, site-, and stereoselective formation of oxetanes in Paternò-Büchi reactions. J. Chin. Chem. Soc. 2008, 55, 479–486. [Google Scholar]
- D’Auria, M.; Emanuele, L.; Racioppi, R. Regio- and stereoselectivity in the Paterno-Buchi reaction between 2,3-dihydrofuran and furan with benzaldehyde. Lett. Org. Chem. 2006, 3, 244–246. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Mauder, H.; Peters, K.; Peters, E.-M.; von Schnering, H.G. Photocycloadditionen mit α- und β-naphthaldehyd: Vollständige umkehr der diastereoselektivität als konsequenz unterschiedlich konfigurierter elektronischer Zustände. Chem. Ber. 1991, 124, 407–410. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R. A DFT study of 1,4-biradical intermediates involved in stereoselective Paternò–Büchi reactions. Eur. J. Org. Chem. 2010, 3831–3836. [Google Scholar]
- Smith, A.B., III; Sulikowski, G.A.; Sulikowski, M.M.; Fujimoto, K. Applications of an asymmetric [2 + 2]-photocycloaddition. Total synthesis of (−)-echinosporin. Construction of an advanced 11-deoxyprostaglandin intermediate. J. Am. Chem. Soc. 1992, 114, 2567–2576. [Google Scholar] [CrossRef]
- Thopate, S.R.; Kulkarni, M.G.; Puranik, V.G. The Paternò–Büchi reaction of l-ascorbic acid. Angew. Chem. Int. Ed. Engl. 1998, 37, 1110–1112. [Google Scholar] [CrossRef]
- Perez-Ruiz, R.; Miranda, M.A.; Alle, R.; Meerholz, K.; Griesbeck, A.G. An efficient carbonyl-alkene metathesis of bicyclic oxetanes: Photoinduced electron transfer reduction of the Paternò–Büchi adducts from 2,3-dihydrofuran and aromatic aldehydes. Photochem. Photobiol. Sci. 2006, 5, 51–55. [Google Scholar] [CrossRef]
- Rodina, L.L.; Galkina, O.S.; Supurgibekov, M.B.; Grigov'ev, Y.M.; Utsal, V.A. Photolysis of regioisomeric α,α-diphenyl-substituted diazotetrahydrofuranones: Primary and secondary photochemical processes. Russian J. Org. Chem. 2010, 46, 1542–1545. [Google Scholar] [CrossRef]
- Vasudeva, S.; Brock, C.P.; Watt, D.S.; Morita, H. Diastereoselectivity in the Paterno-Buechi reaction of enol acetates and benzaldehydes. J. Org. Chem. 1994, 59, 4677–4679. [Google Scholar] [CrossRef]
- Kollenz, G.; Terpetschnig, E.; Sterk, H.; Peters, K.; Peters, E.-M. Regio- and stereoselective photocycloadditions of heterocyclic 2,3-diones—Evidence for an unexpected 1,2-aroyl migration. Tetrahedron 1999, 55, 2973–2984. [Google Scholar] [CrossRef]
- Fleming, S.A.; Gao, J.J. Stereocontrol of Paterno-Büchi photocycloadditions. Tetrahedron Lett. 1997, 38, 5407–5410. [Google Scholar] [CrossRef]
- Bach, T.; Kather, K. The β-silicon effect as a control element for the regioselective ring opening of oxetanes. Tetrahedron 1994, 50, 12319–12328. [Google Scholar] [CrossRef]
- Bach, T. 3-Trimethylsilyloxy-oxetanes via a highly selective Paterno-Büchi reaction. Tetrahedron Lett. 1991, 32, 7037–7038. [Google Scholar] [CrossRef]
- Vogt, F.; Jödicke, K.; Schröder, J.; Bach, T. Paternò-Büchi reactions of silyl enol ethers and enamides. Synthesis 2009, 4268–4273. [Google Scholar]
- Bach, T. Silyl enol ethers in Paternò-Büchi reactions. Functional group tolerance and the effect of β-alkyl substitution. Liebigs Ann. 1995, 855–865. [Google Scholar] [CrossRef]
- Bach, T. Regioselective ring opening of oxetanes by hydrogenolysis—A convenient method for the carbohydroxylation of enol ethers. Liebigs Ann. 1995, 1045–1053. [Google Scholar] [CrossRef]
- Bach, T.; Jödicke, K.; Kather, K.; Fröhlich, R. 1,3-Allylic strain as a control element in the Paternò-Büchi reaction of chiral silyl enol ethers: Synthesis of diastereomerically pure oxetanes containing four contiguous stereogenic centers. J. Am. Chem. Soc. 1997, 119, 2437–2445. [Google Scholar] [CrossRef]
- Bach, T.; Jödicke, K.; Kather, K.; Hecht, J. Facial diastereoselectivity in the Paternò–Büchi reaction of chiral silyl enol ethers. Angew. Chem. Int. Ed. Engl. 1995, 34, 2271–2273. [Google Scholar] [CrossRef]
- Bach, T.; Jödicke, K.; Wibbeling, B. Reversal of the facial diastereoselectivity in the Paternò-Büchi reaction of silyl enol ethers carrying a chiral substituent in α-position. Tetrahedron 1996, 52, 10861–10878. [Google Scholar] [CrossRef]
- Bach, T.; Kather, K.; Krämer, O. Synthesis of five-, six-, and seven-membered heterocycles by intramolecular ring opening reactions of 3-Oxetanol derivatives. J. Org. Chem. 1998, 63, 1910–1918. [Google Scholar] [CrossRef]
- Cho, D.W.; Lee, H.-Y.; Oh, S.W.; Choi, J.H.; Park, H.J.; Mariano, P.S.; Yoon, U.C. Photoaddition reactions of 1,2-diketones with silyl ketene acetals. Formation of β-hydroxy-γ-ketoesters. J. Org. Chem. 2008, 73, 4539–4547. [Google Scholar] [CrossRef]
- Yoon, U.C.; Kim, M.J.; Moon, J.J.; Oh, S.W.; Kim, H.J.; Mariano, P.S. Photoaddition reactions of silyl ketene acetals with aromatic carbonyl compounds: A new procedure for β-hydroxyester synthesis. Bull. Korean Chem. Soc. 2002, 23, 1218–1228. [Google Scholar] [CrossRef]
- Abe, M.; Ikeda, M.; Shirodai, Y.; Nojima, M. Regio- and stereo-selective formation of 2-siloxy-2-alkoxyoxetanes in the photoreaction of cyclic ketene silyl acetals with 2-naphthaldehyde and their transformation to aldol-type adducts. Tetrahedron Lett. 1996, 37, 5901–5904. [Google Scholar] [CrossRef]
- Abe, M.; Ikeda, M.; Nojima, M. A stereoselective, tandem [2+2] photocycloaddition-hydrolysis route to aldol-type adducts. J. Chem. Soc. Perkin Trans. 1 1998, 1998, 3261–3266. [Google Scholar]
- Abe, M.; Shirodai, Y.; Nojima, M. Regioselective formation of 2-alkoxyoxetanes in the photoreaction of aromatic carbonyl compounds with β,β-dimethyl ketene silyl acetals: Notable solvent and silyl group effects. J. Chem. Soc., Perkin Trans. 1 1998, 1998, 3253–3260. [Google Scholar]
- Abe, M.; Fujimoto, K.; Nojima, M. Notable sulfur atom effects on the regio- and stereoselective formation of oxetanes in Paternò−Büchi photocycloaddition of aromatic aldehydes with silyl O,S-ketene Acetals. J. Am. Chem. Soc. 2000, 122, 4005–4010. [Google Scholar] [CrossRef]
- Abe, M.; Tachibana, K.; Fujimoto, K.; Nojima, M. Regioselective formation of 3-selanyl-3-siloxyoxetanes in the Paternò-Büchi reaction of silyl O,Se-Ketene acetals (O,Se-SKA). Synthesis 2001, 2001, 1243–1247. [Google Scholar] [CrossRef]
- Bach, T. N-Acyl enamines in the Paternò-Büchi reaction: Stereoselective preparation of 1,2-amino alcohols by CC bond formation. Angew. Chem. Int. Ed. Engl. 1996, 35, 884–886. [Google Scholar] [CrossRef]
- Bach, T.; Schröder, J. Photocycloaddition of N-Acyl enamines to aldehydes and its application to the synthesis of diastereomerically pure 1,2-amino alcohols. J. Org. Chem. 1999, 64, 1265–1273. [Google Scholar] [CrossRef]
- Bach, T.; Bergmann, H.; Brummerhop, H.; Lewis, W.; Harms, K. The [2+2]-photocycloaddition of aromatic aldehydes and ketones to 3,4-dihydro-2-pyridones: Regioselectivity, diastereoselectivity, and reductive ring opening of the product oxetanes. Chem. Eur. J. 2001, 7, 4512–4521. [Google Scholar] [CrossRef]
- Bach, T. The Paternò-Büchi reaction of 3-heteroatom-substituted alkenes as a stereoselective entry to polyfunctional cyclic and acyclic molecules. Liebigs Ann. Recueil 1997, 1627–1634. [Google Scholar] [CrossRef]
- Bach, T.; Schröder, J. The Paternò-Büchi reaction of α-alkyl-substituted enecarbamates and benzaldehyde. Synthesis 2001, 2001, 1117–1124. [Google Scholar] [CrossRef]
- Bach, T.; Schröder, J.; Brandl, T.; Hecht, J.; Harms, K. Facial diastereoselectivity in the photocycloaddition of chiral N-acyl enamines to benzaldehyde. Tetrahedron 1998, 54, 4507–4520. [Google Scholar] [CrossRef]
- Bach, T. The Paternò-Büchi reaction of N-acyl enamines and aldehydes—The development of a new synthetic method and its application to total synthesis and molecular recognition studies. Synlett 2000, 2000, 1699–1707. [Google Scholar]
- Bach, T.; Schröder, J. Synthesis of syn- and anti-1,2-amino alcohols by regioselective ring opening reactions of cis-3-aminooxetanes. Tetrahedron Lett. 1997, 38, 3707–3710. [Google Scholar] [CrossRef]
- Bach, T.; Schröder, J. A Short Synthesis of (±)-Oxetin. Liebigs Ann. Recueil 1997, 2265–2267. [Google Scholar] [CrossRef]
- Bach, T.; Brummerhop, H. Unprecedented facial diastereoselectivity in the Paternò–Büchi reaction of a chiral dihydropyrrole—A short total synthesis of (+)-preussin. Angew. Chem. Int. Ed. 1998, 37, 3400–3402. [Google Scholar] [CrossRef]
- Bach, T.; Brummerhop, H.; Harms, K. The Synthesis of (+)-preussin and related pyrrolidinols by diastereoselective Paternò–Büchi reactions of chiral 2-substituted 2,3-dihydropyrroles. Chem. Eur. J. 2000, 6, 3838–3848. [Google Scholar] [CrossRef]
- Kugelberg, A.; Döpp, D.; Görner, H. Photoadditions of 2-morpholinopropenenitrile to naphthalenecarboxylic acids. J. Inf. Recording 2000, 25, 187–194. [Google Scholar]
- Van Wolven, C.; Döpp, D.; Fischer, M.A. α-Cyanoenamines in the Paterno-Buchi reaction. J. Inf. Recording 2000, 25, 209–214. [Google Scholar]
- Döpp, D.; Fischer, M.-A. [2+2] Photoaddition of 2-aminopropenenitriles to diarylethanediones. A product study. Recl. Trav. Chim. Pays-Bas 1995, 114, 498–503. [Google Scholar]
- Rivas, C.; Vargas, F. Oxetane formation: Addition to heterocycles. In CRC Handbook of Organic Photochemistry and Photobiology; Horspool, W.M., Song, P.-S, Eds.; CRC Press: Boca Raton, FL, USA, 1995; p. 536. [Google Scholar]
- Vargas, F.; Rivas, C.; Navarro, M.; Alvarado, Y. Synthesis of an elusive oxetane by photoaddition of benzophenone to thiophene in the presence of a Lewis acid. J. Photochem. Photobiol. A Chem. 1996, 93, 169–171. [Google Scholar] [CrossRef]
- Rivas, C.; Bolivar, R.A.; Cucarella, M. Photoaddition of carbonyl compounds to five-membered ring heterocycles. J. Heterocyclic Chem. 1982, 19, 529–535. [Google Scholar] [CrossRef]
- Rivas, C.; Velez, M.; Crescente, O. Synthesis of an oxetan by photoaddition of benzophenone to a thiophen derivative. J. Chem. Soc. Chem. Commun. 1970, 1474. [Google Scholar]
- Rivas, C.; Bolivar, R.A. Synthesis of oxetanes by photoaddition of carbonyl compounds to 2,5-dirnethylthiophene. J. Heterocyclic Chem. 1973, 10, 967–971. [Google Scholar] [CrossRef]
- Rivas, C.; Pacheco, D.; Vargas, F.; Ascanio, J. Synthesis of oxetanes by photoaddition of carbonyl compounds to thiophene derivatives. J. Heterocyclic Chem. 1981, 18, 1065–1067. [Google Scholar] [CrossRef]
- Jones, G.; Gilow, H.M.; Low, J. Regioselective photoaddition of pyrroles and aliphatic carbonyl compounds. A new synthesis of 3(4)-substituted pyrroles. J. Org. Chem. 1979, 44, 2949–2951. [Google Scholar] [CrossRef]
- Rivas, C.; Velez, M.; Cucarella, M.; Bolivar, R.A.; Flores, S.E. Synthesis of an oxetane by photoaddition of benzophenone to a pyrrole derivative. Acta Cient. Venezolana 1971, 22, 145–146. [Google Scholar]
- Rivas, C.; Bolivar, R.A. Synthesis of oxetanes by photoaddition of carbonyl compounds to pyrrole derivatives. J. Heterocyclic Chem. 1976, 13, 1037–1040. [Google Scholar] [CrossRef]
- Rivas, C.; Pacheco, D.; Vargas, F. Synthesis of an oxetane by photoaddition of benzophenone to a selenophene derivative. Acta Sud Am. Quim. 1982, 2, 1–3. [Google Scholar]
- Matsuura, T.; Banba, A.; Ogura, K. Photoinduced reactions—XLV: Photoaddition of ketones to methylimidazoles. Tetrahedron 1971, 27, 1211–1219. [Google Scholar] [CrossRef]
- Nakano, T.; Rivas, C.; Perez, C.; Larrauri, J.M. Photoaddition of ketones to imidazoles: Synthesis of oxetanes. J. Heterocyclic Chem. 1976, 13, 173–174. [Google Scholar] [CrossRef]
- Nakano, T.; Rodriguez, W.; de Roche, S.Z.; Larrauri, J.M.; Rivas, C.; Perez, C. Photoaddition of ketones to imidazoles, thiazoles, isothiazoles and isoxazoles. Synthesis of their oxetanes. J. Heterocyclic Chem. 1980, 17, 1777–1780. [Google Scholar] [CrossRef]
- Ito, Y.; Ji-Ben, M.; Suzuki, S.; Kusunaga, Y.; Matsuura, T.; Fukuyama, K. Efficient photochemical oxetane formation from 1-methyl-2,4,5-triphenylimidazole and benzophenones. Tetrahedron Lett. 1985, 26, 2093–2096. [Google Scholar] [CrossRef]
- Julian, D.R.; Tringham, G.D. Photoaddition of ketones to indoles: Synthesis of oxeto[2,3-b]indoles. J. Chem. Soc. Chem. Commun. 1973, 13. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Franke, M.; Neudörfl, J.; Kotaka, H. Photocycloaddition of aromatic and aliphatic aldehydes to isoxazoles: Cycloaddition reactivity and stability studies. Beilstein J. Org. Chem. 2011, 7, 127–134. [Google Scholar] [CrossRef]
- D'Auria, M. Unified Approach to the photochemical isomerization of five-membered heteroaromatic compounds. In Targets in Heterocyclic Systems, Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society of Chemistry: Rome, Italy, 1999; Volume 2, pp. 233–279. [Google Scholar]
- D'Auria, M. Towards a unitary description of the photochemical isomerization reactions in pentaatomic aromatic heterocycles: The case of furan, thiophene, pyrrole, isoxazole, imidazole, and pyrazole derivatives. Heterocycles 1999, 50, 1115–1136. [Google Scholar] [CrossRef]
- D'Auria, M. Photochemical isomerization of pentaatomic heterocycles. Adv. Heterocycl. Chem. 2001, 79, 41–48. [Google Scholar] [CrossRef]
- D'Auria, M. Photochemical and photophysical behavior of thiophene. Adv. Heterocycl. Chem. 2011, 104, 127–390. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Fiege, M.; Lex, J. Oxazole–carbonyl photocycloadditions: Selectivity pattern and synthetic route to erythro α-amino, β-hydroxy ketones. Chem. Commun. 2000, 589–590. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Bondock, S.; Lex, J. Synthesis of erythro-α-amino β-hydroxy carboxylic acid esters by diastereoselective photocycloaddition of 5-methoxyoxazoles with aldehydes. J. Org. Chem. 2003, 68, 9899–9906. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Bondock, S.; Lex, J. Stereoselective generation of vicinal stereogenic quaternary centers by photocycloaddition of 5-methoxy oxazoles to α-keto esters: Synthesis of erythro β-hydroxy dimethyl aspartates. Org. Biomol. Chem. 2004, 2, 1113–1115. [Google Scholar] [CrossRef]
- Bondock, S.; Griesbeck, A.G. Diastereoselective photochemical synthesis of α-amino-β-hydroxyketones by photocycloaddition of carbonyl compounds to 2,5-dimethyl-4-isobutyloxazole. Monatsh. Chem. 2006, 137, 765–777. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Bondock, S. Photocycloaddition of 5-methoxyoxazoles to aldehydes and α-keto esters: A comprehensive view on stereoselectivity, triplet biradical conformations, and synthetic applications of Paternò–Büchi adducts. Aust. J. Chem. 2008, 61, 573–580. [Google Scholar] [CrossRef]
- Nakano, T.; Santana, M. Photoaddition of benzophenone to azaindole, synthesis of the oxetane of 7-azaindole. J. Heterocyclic Chem. 1976, 13, 585–587. [Google Scholar] [CrossRef]
- Schenck, G.O.; Hartman, W.; Steinmetz, R. Vierringsynthesen durch photosensibilisierte Cycloaddition von Dimethylmaleinsäureanhydrid an Olefine. Chem. Ber. 1963, 96, 498–508. [Google Scholar] [CrossRef]
- Gagnaire, D.; Payo-Subiza, E. Nuclear magnetic resonance coupling of methyl protons over five bonds: Heterocyclic vinyl ethers. Bull. Soc. Chim. Fr. 1963, 2623–2631. [Google Scholar]
- Toki, S.; Shima, K.; Sakurai, H. Organic photochemical reactions. I. The synthesis of substituted oxetanes by the photoaddition of aldehydes to furans. Bull Chem. Soc. Jpn 1965, 38, 760–762. [Google Scholar] [CrossRef]
- Nakano, T.; Rivas, C.; Perez, C. Configuration and stereochemistry of photoproducts by application of the nuclear Overhauser effect. Adducts of benzophenone with methyl-substituted furans and 2,5-dimethylthiophen, and of methyl-substituted maleic anhydrides with thiophen and its methyl derivatives, and benzo-[b]thiophen. J. Chem. Soc. Perkin Trans. 1 1973, 2322–2327. [Google Scholar] [CrossRef]
- Rivas, C.; Payo, E. Synthesis of oxetanes by photoaddition of benzophenone to furans. J. Org. Chem. 1967, 32, 2918–2920. [Google Scholar] [CrossRef]
- Shima, K.; Sakurai, H. Organic photochemical reactions. IV. Photoaddition reactions of various carbonyl compounds to furan. Bull. Chem. Soc. Jpn 1966, 39, 1806–1808. [Google Scholar] [CrossRef]
- Toki, S.; Sakurai, H. Kinetic studies of the photoreaction of benzophenone with furan. Bull. Soc. Chim. Jpn 1967, 40, 2885–2889. [Google Scholar] [CrossRef]
- Zamojski, A.; Koźluk, T. Synthesis of 3-substituted furans. J. Org. Chem. 1977, 42, 1089–1090. [Google Scholar] [CrossRef]
- Whipple, E.B.; Evanega, G.R. The assignment of configuration to the photoaddition products of unsymmetrical carbonyls to furan using pseudocontact shifts. Tetrahedron 1968, 24, 1299–1310. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Desmaele, D.; Porco, J.A. On the use of unsymmetrically substituted furans in the furan-carbonyl photocycloaddition reaction: Synthesis of a kadsurenone-ginkgolide hybrid. Tetrahedron Lett. 1988, 29, 6689–6693. [Google Scholar]
- Schreiber, S.L.; Desmaele, D.; Porco, J.A. On the use of unsymmetrically substituted furans in the furan-carbonyl photocycloaddition reaction: Synthesis of a kadsurenone-ginkgolide hybrid. Tetrahedron Lett. 1988, 29, 6689–6693. [Google Scholar] [CrossRef]
- Carless, H.A. J.; Halfhide, A.F. Highly regioselective [2 + 2] photocycloaddition of aromatic aldehydes to acetylfurans. J. Chem. Soc., Perkin Trans. 1 1992, 1081–1082. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Mauder, H.; Stadtmüller, S. Intersystem crossing in triplet 1,4-biradicals: Conformational memory effects on the stereoselectivity of photocycloaddition reactions. Acc. Chem. Res. 1994, 27, 70–76. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Buhr, S.; Fiege, M.; Schmickler, H.; Lex, J. Stereoselectivity of triplet photocycloadditions: Diene−carbonyl reactions and solvent effects. J. Org. Chem. 1998, 63, 3847–3854. [Google Scholar]
- Leitich, J. Comments on the paper “Photochemical cycloaddition of benzophenone to furans”. Tetrahedron Lett. 1967, 1937–1939. [Google Scholar] [CrossRef]
- Evanega, G.R.; Whipple, E.B. The photochemical addition of benzophenone to furan. Tetrahedron Lett. 1967, 2163–2168. [Google Scholar] [CrossRef]
- Toki, S.; Sakurai, H. On the structure of the 2:1-adduct of benzophenone and furan. Tetrahedron Lett. 1967, 4119–4122. [Google Scholar] [CrossRef]
- Sekretar, S.; Rudā, J.; Štibranyi, L. Photochemical reactions of 2-substituted furans with some carbonyl compounds. Coll. Czech. Chem. Commun. 1984, 49, 71–77. [Google Scholar] [CrossRef]
- Cantrell, T.S.; Allen, A.; Ziffer, H. Photochemical 2 + 2 cycloaddition of arenecarboxylic acid esters to furans and 1,3-dienes. 2 + 2 Cycloreversion of oxetanes to dienol esters and ketones. J. Org. Chem. 1989, 54, 140–145. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R.; Viggiani, L. Paternò–Büchi reaction between furan and heterocyclic aldehydes: Oxetane formation vs. metathesis. Photochem. Photobiol. Sci. 2010, 9, 1134–1138. [Google Scholar] [CrossRef]
- Kubo, Y.; Suto, M.; Tojo, S.; Araki, T. Photochemical reaction of N-methylnaphthalene-1,8-dicarboximide with alkenes, dienes, and furans. Cyclobutane and oxetane formation. J. Chem. Soc. Perkin Trans. 1 1986, 771–779. [Google Scholar] [CrossRef]
- Krauch, C.H.; Metzner, W.; Schenck, G.O. Photochemische C4- und C3O-Cycloadditionen an Cumaron. Chem. Ber. 1966, 99, 1723–1731. [Google Scholar] [CrossRef]
- Farid, S.; Hartman, S.E.; DeBoer, C.D. Reversible energy transfer and oxetane formation in the photoreactions of carbonyl compounds with benzofurans. J. Am. Chem. Soc. 1975, 97, 808–812. [Google Scholar]
- Capozzo, M.; D’Auria, M.; Emanuele, L.; Racioppi, R. Synthesis and photochemical reactivity towards the Paternó–Büchi reaction of benzo[b]furan derivatives: Their use in the preparation of 3-benzofurylmethanol derivatives. J. Photochem. Photobiol. A: Chem. 2007, 185, 38–43. [Google Scholar] [CrossRef]
- Jarosz, S.; Zamojski, A. Rearrangement of 6-substituted 2,7-dioxabicyclo[3.2.0]hept-3-enes to furans. J. Org. Chem. 1979, 44, 3720–3723. [Google Scholar]
- Kitamura, T.; Kawakami, Y.; Imagawa, T.; Kawanisi, M. One-pot synthesis of 3-substituted furan: A synthesis of perillaketone. Synth. Commun. 1977, 7, 521–528. [Google Scholar] [CrossRef]
- Kozluk, T.; Zamojski, A. The synthesis of 3-deoxy-dl-streptose. Tetrahedron 1983, 39, 805–810. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Hoveyda, A.H.; Wu, H.-J. A photochemical route to the formation of threo aldols. J. Am. Chem. Soc. 1983, 105, 660–661. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Satake, K. Application of the furan carbonyl photocycloaddition reaction to the synthesis of the bis(tetrahydrofuran) moiety of asteltoxin. J. Am. Chem. Soc. 1983, 105, 6723–6724. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Satake, K. Total synthesis of (±)-asteltoxin. J. Am. Chem. Soc. 1984, 106, 4186–4188. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Hoveyda, A.H. Synthetic studies of the furan-carbonyl photocycloaddition reaction. A total synthesis of (±)-avenaciolide. J. Am. Chem. Soc. 1984, 106, 7200–7202. [Google Scholar] [CrossRef]
- Schreiber, S.L.; Porco, J.A. Studies of the furan-carbonyl photocycloaddition reaction: Vinylic substitution reactions. J. Org. Chem. 1989, 54, 4721–4723. [Google Scholar] [CrossRef]
- Hambalek, R.; Just, G. Trisubstituted oxetanes from 2,7-dioxa-bicyclo-[3,2,0]-hept-3-enes. Tetrahedron Lett. 1990, 31, 4693–4696. [Google Scholar] [CrossRef]
- Hambalek, R.; Just, G. A short synthesis of (±)-oxetanocin. Tetrahedron Lett. 1990, 31, 5445–5448. [Google Scholar] [CrossRef]
- Jarosz, S.; Zamojski, A. Asymmetric photocycloaddition between furan and chiral alkyl glyoxylates. Tetrahedron 1982, 38, 1447–1451. [Google Scholar] [CrossRef]
- Pelzer, R.; Jütten, P.; Scharf, H.-D. Chirale Induktion bei photochemischen Reaktionen, IX. Isoselektivität bei der asymmetrischen Paterno-Büchi-Reaktion unter Verwendung von Kohlenhydraten als chirale Auxiliare. Chem. Ber. 1989, 122, 487–491. [Google Scholar] [CrossRef]
- Pelzer, R.; Scharf, H. –D.; Buschmann, H.; Runsink, J. Chirale Induktion bei Photochemischen Reaktionen, XI. Derivate der (+)-Menthyl-glyoxylate in der Paternò-Büchi-Reaktion. Einfluß von Substituenten im Glyoxylsäurerest auf die Diastereoselektivität. Chem. Ber. 1989, 122, 1187–1192. [Google Scholar] [CrossRef]
- Hu, S.; Neckers, D.C. Photocycloaddition and ortho-hydrogen abstraction reactions of methyl arylglyoxylates: Structure dependent reactivities. J. Chem. Soc. Perkin Trans. 2 1999, 1771–1778. [Google Scholar]
- D’Auria, M.; Emanuele, L.; Racioppi, R. On the Paternò–Büchi reaction of chiral phenylglyoxylate esters with furan derivatives. Photochem. Photobiol. Sci. 2003, 2, 904–913. [Google Scholar] [CrossRef]
- Buschmann, H.; Scharf, H.-D.; Hoffmann, N.; Plath, M.W.; Runsink, J. Chiral induction in photochemical reactions. 10. The principle of isoinversion: A model of stereoselection developed from the diastereoselectivity of the Paterno-Buechi reaction. J. Am. Chem. Soc. 1989, 111, 5367–5373. [Google Scholar] [CrossRef]
- Buschmann, H.; Scharf, H.-D.; Hoffmann, N.; Esser, P. The isoinversion principle—A general model of chemical selectivity. Angew. Chem. Int. Ed. Engl. 1991, 30, 477–515. [Google Scholar] [CrossRef]
- Hu, S.; Neckers, D.C. Rapid regio- and diastereoselective Paternò−Büchi reaction of alkyl phenylglyoxylates. J. Org. Chem. 1997, 62, 564–567. [Google Scholar] [CrossRef]
- Kaneko, Y.; Hu, S.; Neckers, D.C. Photochemical reactivities of alkyl thiopheneglyoxylates and alkyl furanylglyoxylates. J. Photochem. Photobiol. A 1998, 114, 173–179. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R. Stereoselectivity in the reaction of chiral phenylglyoxylate esters with furan within zeolites and cyclodextrin. Lett. Org. Chem. 2008, 5, 249–256. [Google Scholar] [CrossRef]
- Arimura, J.; Mizuta, T.; Hiraga, Y.; Abe, M. Formation of macrocyclic lactones in the Paternò-Büchi dimerization reaction. Beilstein J. Org. Chem. 2011, 7, 265–269. [Google Scholar] [CrossRef]
- Žagar, C.; Scharf, H.-D. Chiral induction in photochemical reactions, XIII. The Paternó-Büchi reaction of achiral and chiral acyl cyanides with furan. Chem. Ber. 1991, 124, 967–969. [Google Scholar] [CrossRef]
- Jarosz, S.; Zamojski, A. Asymmetric photocycloaddition between furan and optically active ketones. Tetrahedron 1982, 38, 1453–1456. [Google Scholar] [CrossRef]
- Tronchet, J.M.J.; Baehler, B. Derives C-glycosyliques. XXII. Glycosyl-6-dioxa-2,7-bicyclo[3,2,0]heptenes-3. J. Carbohydrates Nucleosides Nucleotides 1974, 1, 449–459. [Google Scholar]
- Schreiber, S.L. [2 + 2] photocycloadditions in the synthesis of chiral molecules. Science 1985, 227, 857–863. [Google Scholar]
- Schreiber, S.L.; Satake, K. Studies of the furan-carbonyl photocycloaddition reaction: The determination of the absolute stereostructure of asteltoxin. Tetrahedron Lett. 1986, 27, 2575–2578. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R. Diastereoselectivity in the Paternò–Büchi reaction on furan derivatives. Tetrahedron Lett. 2004, 45, 3877–3880. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, l.; Pace, V.; Racioppi, R. Diastereoselective Paternò-Büchi reaction on furan derivatives - reaction with asymmetric ketones. Lett. Org. Chem. 2006, 3, 350–355. [Google Scholar] [CrossRef]
- Adam, W.; Peters, K.; Peters, E.M.; Stegmann, V.R. Hydroxy-directed regio- and diastereoselective [2+2] photocycloaddition (Paternò−Büchi reaction) of benzophenone to chiral allylic alcohols. J. Am. Chem. Soc. 2000, 122, 2958–2959. [Google Scholar] [CrossRef]
- Adam, W.; Stegmann, V.R. Hydroxy-group directivity in the regioselective and diastereoselective [2+2] photocycloaddition (Paternò–Büchi reaction) of aromatic carbonyl compounds to chiral and achiral allylic substrates: The preparation of oxetanes with up to three stereogenic centers as synthetic building blocks. Synthesis 2001, 2001, 1203–1214. [Google Scholar] [CrossRef]
- Adam, W.; Prein, M. The Schenck ene reaction: Diastereoselective oxyfunctionalization with singlet oxygen in synthetic applications. Angew. Chem. Int. Ed. Engl. 1996, 35, 477–494. [Google Scholar]
- Griesbeck, A.G.; Bondock, S. Paternò−Büchi reactions of allylic alcohols and acetates with aldehydes: Hydrogen-bond interaction in the excited singlet and triplet states? J. Am. Chem. Soc. 2001, 123, 6191–6192. [Google Scholar] [CrossRef]
- Bach, T.; Schröder, J.; Harms, K. Diastereoselective photocycloaddition of an axial chiral enamide. Tetrahedron Lett. 1999, 40, 9003–9004. [Google Scholar]
- Bach, T.; Bergmann, H.; Harms, K. High facial diastereoselectivity in the photocycloaddition of a chiral aromatic aldehyde and an enamide induced by intermolecular hydrogen bonding. J. Am Chem. Soc. 1999, 121, 10650–10651. [Google Scholar] [CrossRef]
- Yokohama, A.; Mizuno, K. Stereoselective photocycloaddition of alkenes to naphthalene rings assisted by hydrogen bonding. Org. Lett. 2000, 2, 3457–3459. [Google Scholar] [CrossRef]
- Abe, M.; Terazawa, M.; Nozaki, K.; Masuyama, A.; Hayashi, T. Notable temperature effect on the stereoselectivity in the photochemical [2+2] cycloaddition reaction (Paternò–Büchi reaction) of 2,3-dihydrofuran-3-ol derivatives with benzophenone. Tetrahedron Lett. 2006, 47, 2527–2530. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R.; Romaniello, G. The Paternò-Büchi reaction of 2-furylmethanols. Eur. J. Org. Chem. 2000, 3265–3272. [Google Scholar]
- D’Auria, M.; Racioppi, R. Paterno-Büchi reaction on 5-methyl-2-furylmethanol derivatives. Arkivoc 2000, 1, 133–140. [Google Scholar]
- Hisamoto, K.; Hiraga, Y.; Abe, M. Hydroxy-group effect on the regioselectivity in a photochemical oxetane formation reaction (the Paternò-Büchi Reaction) of geraniol derivatives. Photochem. Photobiol. Sci. 2011, 10, 1469–1473. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Poggi, G.; Racioppi, R.; Romaniello, G. On the stereoselectivity of the Paternò–Büchi reaction between carbonyl compounds and 2-furylmethanol derivatives. The case of aliphatic aldehydes and ketones. Tetrahedron 2002, 58, 5045–5051. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R. The stereoselectivity of the Paternò–Büchi reaction between tertiary 2-furylmethanol derivatives and aromatic carbonyl compounds: On the nature of the hydroxy directing effect. Photochem. Photobiol. Sci. 2004, 3, 927–932. [Google Scholar] [CrossRef]
- Yabuno, Y.; Hiraga, Y.; Abe, M. Site- and stereoselectivity in the photochemical oxetane formation reaction (Paternò–Büchi reaction) of tetrahydrobenzofuranols with benzophenone: Hydroxy-directed diastereoselectivity? Chem. Lett. 2008, 37, 822–823. [Google Scholar] [CrossRef]
- Yabuno, Y.; Hiraga, Y.; Takagi, R.; Abe, M. Concentration and temperature dependency of regio- and stereoselectivity in a photochemical [2 + 2] cycloaddition reaction (the Paternò−Büchi Reaction): Origin of the hydroxy-group directivity. J. Am. Chem. Soc. 2011, 133, 2592–2604. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R. Stereoselectivity in the Paterno-Buchi reaction on chiral allylic alcohols, for a discussion of the hydroxy directing effect. Lett. Org. Chem. 2005, 2, 132–135. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R.; Valente, A. Paternò–Büchi reaction between aromatic carbonyl compounds and 1-(3-furyl)alkanols. Photochem. Photobiol. Sci. 2008, 7, 98–103. [Google Scholar] [CrossRef]
- Bolivar, R.A.; Rivas, C. Quencher effect of thiophene and its derivatives on the photoreduction of carbonyl compounds. J. Photochem. 1981, 17, 91. [Google Scholar] [CrossRef]
- Bolivar, R.A.; Rivas, C. Quencher effect of thiophene and its monomethyl derivatives on photo-reduction and photocycloaddition reactions of ketones. J. Photochem. 1982, 19, 95–99. [Google Scholar] [CrossRef]
- Bolivar, R.A.; Machado, R.; Montero, L.; Vargas, F.; Rivas, C. Quenching effect of five-membered ring heterocycles on the photoreduction and photocycloaddition reactions of benzophenone: II. J. Photochem. 1983, 22, 91–95. [Google Scholar]
- Vargas, F.; Rivas, C. Photochemical formation of oxetanes derived from aromatic ketones and substituted thiophenes and selenophenes. Int. J. Photoenergy 2000, 2, 97–101. [Google Scholar] [CrossRef]
- Von Wilucki, I.; Matthaus, H.; Krauch, C.H. Photosensibilisierte Cyclodimerisation von Thymin in Lösung. Photochem. Photobiol. 1967, 6, 497–500. [Google Scholar] [CrossRef]
- Prakash, G.; Falvey, D.E. Model studies of the (6–4) photoproduct DNA photolyase: Synthesis and photosensitized splitting of a thymine-5,6-oxetane. J. Am. Chem. Soc. 1995, 117, 11375–11376. [Google Scholar] [CrossRef]
- Joseph, A.; Prakash, G.; Falvey, D.E. Model studies of the (6−4) photoproduct photolyase enzyme: Laser flash photolysis experiments confirm radical ion intermediates in the sensitized repair of thymine oxetane adducts. J. Am. Chem. Soc. 2000, 122, 11219–11225. [Google Scholar] [CrossRef]
- Lhiaubet-Vallet, V.; Belmadoui, N.; Climent, M.J.; Miranda, M.A. The long-lived triplet excited state of an elongated ketoprofen derivative and its interactions with amino acids and nucleosides. J. Phys. Chem. B 2007, 111, 8277–8282. [Google Scholar]
- Nakatani, K.; Yoshida, T.; Saito, I. Photochemistry of benzophenone immobilized in a major groove of DNA: Formation of thermally reversible interstrand cross-link. J. Am. Chem. Soc. 2002, 124, 2118–2119. [Google Scholar] [CrossRef]
- Hei, X.-M.; Song, Q.-H.; Li, X.-B.; Tang, W.-J.; Wang, H.-B.; Guo, Q.-X. Origin of a large temperature dependence of regioselectivity observed for [2+2] photocycloaddition (Paternò-Büchi reaction) of 1,3-dimethylthymine with benzophenone and its derivatives: Conformational property of the intermediary triplet 1,4-diradicals. J. Org. Chem. 2005, 70, 2522–2527. [Google Scholar] [CrossRef]
- Zhai, B.-C.; Luo, S.-W.; Kong, F.-F.; Song, Q.-H. Notable solvent effects on the regioselectivity in the Paternò–Büchi reaction of 1,3-dimethylthymine with 4-methoxybenzophenone. J. Photochem. Photobiol. A 2007, 187, 406–409. [Google Scholar] [CrossRef]
- Song, Q.-H; Wang, H.-B.; Li, X.-B.; Hei, X.-M.; Guo, Q.-X.; Yu, S.-Q. Notable substituent and temperature effects on the regioselectivity and the efficiency in Paternò–Büchi reaction of 4,4′-disubstituted benzophenones with 1,3-dimethyluracil and 1,3-dimethylthymine. J. Photochem. Photobiol. A 2006, 183, 198–204. [Google Scholar] [CrossRef]
- Song, Q.-H.; Zhai, B.-C.; Hei, X.-M.; Guo, Q.-X. The Paternò–Büchi reaction of 1,3-dimethyluracil and 1,3-dimethylthymine with 4,4′-disubstituted benzophenones. Eur. J. Org. Chem. 2006, 1790–1800. [Google Scholar]
- Kong, F.-F.; Wang, J.-B.; Song, Q.-H. Heavy atom effects in the Paternò-Büchi reaction of pyrimidine derivatives with 4,4’-disubstituted benzophenones. Beilstein J. Org. Chem. 2011, 7, 113–118. [Google Scholar] [CrossRef]
- Kong, F.-F.; Zhai, B.-C.; Song, Q.-H. Substituent effects on the regioselectivity of the Paternò-Büchi reaction of 5- or/and 6-methyl substituted uracils with 4,4′-disubstituted benzophenones. Photochem. Photobiol. Sci. 2008, 7, 1332–1336. [Google Scholar] [CrossRef]
- Encinas, S.; Belmadoui, N.; Climent, M.J.; Gil, S.; Miranda, M.A. Photosensitization of thymine nucleobase by benzophenone derivatives as models for photoinduced DNA damage: Paterno-Büchi vs energy and electron transfer processes. Chem. Res. Toxicol. 2004, 17, 857–858. [Google Scholar] [CrossRef]
- Belmadoui, N.; Encinas, S.; Climent, M.J.; Gil, S.; Miranda, M.A. Intramolecular interactions in the triplet excited states of benzophenone-thymine dyads. Chem. Eur. J. 2006, 12, 553–561. [Google Scholar] [CrossRef]
- Thomas, M.; Guillaume, D.; Fourrey, J.-L.; Clivio, P. Further insight in the photochemistry of DNA: Structure of a 2-imidazolone (5−4) pyrimidone adduct derived from the mutagenic pyrimidine (6−4) pyrimidone photolesion by UV irradiation. J. Am. Chem. Soc. 2002, 124, 2400–2401. [Google Scholar]
- Seki, K.-I.; Aizawa, K.; Sugaoi, T.; Kimura, M.; Ohkura, K. Synthesis of highly conjugated arylpropenylidene-1,3-diazin-2-ones via Paterno-Büchi reaction by photoreaction of 5-fluoro-1,3-dimethyluracil with 1-methoxynaphthalenes. Chem. Lett. 2008, 37, 872–873. [Google Scholar] [CrossRef]
- Chung, W.-S.; Turro, N.J.; Srivastava, S.; Li, H.; le Noble, W.J. Hyperconjugation as a factor in face selectivity during cycloaddition. J. Am. Chem. Soc. 1988, 110, 7882–7883. [Google Scholar] [CrossRef]
- Chung, W.-S.; Turro, N.J.; Srivastava, S.; le Noble, W.J. Stereochemistry of photocycloaddition of (E)-1,2-dicyano- and (Z)-1,2-diethoxyethylene to 5-substituted adamantanones. J. Org. Chem. 1991, 56, 5020–5025. [Google Scholar] [CrossRef]
- Chung, W.-S.; Wang, N.-J.; Liu, Y.-D.; Leu, Y.-J.; Chiang, M.Y. Photocycloaddition of fumaronitrile to adamantan-2-ones and modification of face selectivity by inclusion in β-cyclodextrin and its derivatives. J. Chem. Soc. Perkin Trans. 2 1995, 307–313. [Google Scholar]
- Chung, W.-S.; Liu, Y.-D.; Wang, N.-. J Face selectivity in the Paterno-Büchi reactions of methacrylonitrile to 5-substituted adamantan-2-ones. J. Chem. Soc. Perkin Trans. 2 1995, 581–586. [Google Scholar] [CrossRef]
- Kossanyi, J.; Jost, P.; Furth, B.; Daccord, G.; Chaquin, P. Intramolecular photoaddition of the excited carbonyl group of cycloakanones to a non-conjugated ethylene double bond. J. Chem. Res. Synop. 1980, 368–369. [Google Scholar]
- Matsumura, K.; Mori, T.; Inoue, Y. Wavelength control of diastereodifferentiating Paternò–Büchi reaction of chiral cyanobenzoates with diphenylethene through direct versus charge-transfer excitation. J. Am. Chem. Soc. 2009, 131, 17076–17077. [Google Scholar] [CrossRef]
- Matsumura, K.; Mori, T.; Inoue, Y. Solvent and temperature effects on diastereodifferentiating Paternó-Büchi reaction of chiral alkyl cyanobenzoates with diphenylethene upon direct versus charge-transfer excitation. J. Org. Chem. 2010, 75, 5461–5469. [Google Scholar] [CrossRef]
- Xue, J. Photoinduced [2+2] cycloadditions (the Paterno-Büchi reaction) of 1H-1-acetylindole-2,3-dione with alkenes. J. Chem. Soc. Perkin Trans. 1 2001, 2001, 183–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, J.; Gao, Y.; Fun, H.-K.; Xu, J.-H. Photoinduced [2+2] cycloadditions (the Paterno–Büchi reaction) of 1-acetylisatin with enol ethers—regioselectivity, diastereoselectivity and acid catalysed transformations of the spirooxetane products. J. Chem. Soc. Perkin Trans. 1 2002, 2002, 345–353. [Google Scholar]
- Bosch, E.; Hubig, S.M.; Kochi, J.K. Paterno-Büchi coupling of (diaryl)acetylenes and quinone via photoinduced electron transfer. J. Am. Chem. Soc. 1998, 120, 386–395. [Google Scholar] [CrossRef]
- Christl, M.; Braun, M. [2+2] Photocycloadditions of homobenzvalene. Liebigs Ann. Recueil 1997, 1135–1141. [Google Scholar] [CrossRef]
- Kang, T.; Scheffer, J.R. An Unexpected Paternò-Büchi reaction in the crystalline state. Org. Lett. 2001, 3, 3361–3364. [Google Scholar] [CrossRef]
- Adam, W.; Stegmann, V.R. Unusual temperature dependence in the cis/trans-oxetane formation discloses competitive syn versus anti attack for the Paternò−Büchi reaction of triplet-excited ketones with cis- and trans-cylooctenes. Conformational control of diastereoselectivity in the cyclization and cleavage of preoxetane diradicals. J. Am. Chem. Soc. 2002, 124, 3600–3607. [Google Scholar] [CrossRef]
- Horspool, W.; Armesto, D. Organic Photochemistry: A Comprehensive Treatment; Prentice Hall: London, UK, 1992. [Google Scholar]
- Büchi, G.; Kofron, J.T.; Koller, E.; Rosenthal, D. Light catalyzed organic reactions. V. 1 The addition of aromatic carbonyl compounds to a disubstituted acetylene. J. Am. Chem. Soc. 1956, 78, 876–877. [Google Scholar]
- Arnold, D.; Glick, A. The photocycloaddition of carbonyl compounds to allenes. Chem. Commun. 1966, 813–814. [Google Scholar]
- Gotthardt, J.K.; Steinmetz, R.; Hammond, G.S. Mechanisms of photochemical reactions in solution. LIII. Cycloaddition of carbonyl compounds to allenes. J. Org. Chem. 1968, 33, 2774–2780. [Google Scholar] [CrossRef]
- Howell, A.R.; Fan, R.; Truong, A. Preparation of 2-alkylidene oxetanes: An investigation of the Paterno-Büchi reaction between aliphatic aldehydes and allenes. Tetrahedron Lett. 1996, 37, 8651–8654. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Kou, Z.; Du, X.; Wei, Y.; Fun, H.-K.; Xu, J.; Zhang, Y. Photoinduced tandem reactions of isoquinoline-1,3,4-trione with alkynes to build aza-polycycles. J. Org. Chem. 2010, 75, 2989–3001. [Google Scholar] [CrossRef]
- Gleiter, R.; Herb, T.; Borzyk, O.; Hyla-Kryspin, I. 2,7,9-Trimethylenetricyclo[4.3.0.03,8]nonane and 2,9-dimethylenetricyclo[4.3.0.03,8]non-4-ene. Synthesis and properties. Liebigs Ann. 1995, 357–364. [Google Scholar]
- Herb, T.; Gleiter, R. 2,7,9-Trimethylenetricyclo[4.3.0.03,8]-non-4-ene. Angew. Chem. Int. Ed. Engl. 1996, 35, 2368–2369. [Google Scholar] [CrossRef]
- Gleiter, R.; Gaa, B.; Sigwart, C.; Lange, H.; Borzyk, O.; Rominger, F.; Irngartinger, H.; Oeser, T. Preparation and Properties of Stelladiones. Eur. J. Org. Chem. 1998, 171–176. [Google Scholar]
- Rawal, V.H.; Dufour, C. A general strategy for increasing molecular complexity: Photocycloaddition-fragmentation route to functionalized di- and triquinanes. J. Am. Chem. Soc. 1994, 116, 2613–2614. [Google Scholar] [CrossRef]
- Dvorak, C.A.; Dufour, C.; Iwasa, S.; Rawal, V.H. Rapid synthesis of di- and triquinanes by direct reductive fragmentation of Paterno−Büchi-derived oxetanes. J. Org. Chem. 1998, 63, 5302–5303. [Google Scholar] [CrossRef]
- Reddy, T.J.; Rawal, V.H. Expeditious syntheses of (±)-5-oxosilphiperfol-6-ene and (±)-silphiperfol-6-ene. Org. Lett. 2000, 2, 2711–2712. [Google Scholar] [CrossRef]
- Boxall, R.J.; Ferris, L.; Grainger, R.S. Synthesis of C-13 oxidised cuparene and herbertane sesquiterpenes via a Paternò–Büchi photocyclisation–oxetane fragmentation strategy: Total synthesis of 1,13-herbertenediol. Synlett 2004, 2379–2381. [Google Scholar]
- De la Torre, M.; Garca, I.; Sierra, M.A. Photochemical access to tetra- and pentacyclic terpene-like products from R-(+)-sclareolide. J. Org. Chem. 2003, 68, 6611–6618. [Google Scholar] [CrossRef]
- Iriondo-Alberdi, J.; Perea-Buceta, J.; Greaney, M.F. A Paternò-Büchi approach to the synthesis of merrilactone A. Org. Lett. 2005, 7, 3969–3971. [Google Scholar] [CrossRef]
- Carless, H.A. J.; Fekarurhobo, G.K. A photochemical route to a thromboxane A2 ring analogue. J. Chem. Soc. Chem. Commun. 1984, 667–668. [Google Scholar] [CrossRef]
- Furth, B.; Daccord, G.; Kossanyi, J. Photochimie en solution. IX. Reactivite des allyl-2 cyclanones. Tetrahedron Lett. 1975, 4259–4262. [Google Scholar] [CrossRef]
- Kirschberg, T.; Mattay, J. Photoinduced electron transfer reactions of α-cyclopropyl- and α-epoxy ketones. Tandem fragmentation−cyclization to bi-, tri-, and spirocyclic ketones. J. Org. Chem. 1996, 61, 8885–8896. [Google Scholar] [CrossRef]
- Gescheidt, G.; Neshchadin, D.; Rist, G.; Borer, A.; Dietliker, K.; Misteli, K. Stereocontrolled photo-reaction pathways of endo/exo-2-benzoyl-substituted bicyclo[2.2.2]oct-5-en-2-ol: Paternò-Büchi reaction versus α-cleavage. Phys. Chem. Chem. Phys. 2003, 5, 1071–1077. [Google Scholar] [CrossRef]
- Hammaecher, C.; Portella, C. New 6-oxa-2-silabicyclo[2.2.0]hexanes by photochemical conversion of acyl(allyl)(dimethyl)silanes. Chem. Commun. 2008, 5833–5835. [Google Scholar] [CrossRef]
- Hu, S.; Neckers, D.C. Photochemical reactions of alkenyl phenylglyoxylates. J. Org. Chem. 1997, 62, 6820–6826. [Google Scholar] [CrossRef]
- Rochat, S.; Minardi, C.; de Saint Laumer, J.-Y.; Herrmann, A. Controlled release of perfumery aldehydes and ketones by Norrish type-II photofragmentation of α-keto esters in undegassed solution. Helv. Chim. Acta 2000, 83, 1645–1671. [Google Scholar] [CrossRef]
- Sakamoto, M.; Aoyama, H.; Omota, Y. Photochemical reactions of N-benzoylformyl α,β-unsaturated amides. J. Chem. Soc. Perkin 1 1986, 1759–1762. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takahashi, M.; Fujita, T.; Watanabe, S.; Nishio, T.; Iida, I.; Aoyama, H. Solid state photochemical reaction of N-(α,β-unsaturated carbonyl)benzoylformamides. J. Org. Chem. 1997, 62, 6298–6308. [Google Scholar] [CrossRef]
- Shvydkiv, O.; Yavorskyy; Nolan, A.K.; Oelgemöller, M. Microflow photochemistry—An advantageous combination of synthetic photochemistry and microreactor technology. EPA Newsletter 2012, 83, 65–69. [Google Scholar]
- Fukuyama, T.; Kajihara, Y.; Hino, Y.; Ryu, I. Continuous microflow [2+2] photocycloaddition reactions using energy-saving compact light sources. J. Flow Chem. 2011, 1, 40–45. [Google Scholar] [CrossRef]
- Conradi, M.; Junkers, T. Photoinduced conjugation of aldehyde functional polymers with olefins via [2+2]-cycloaddition. Macromolecules 2011, 44, 7969–7976. [Google Scholar] [CrossRef]
- Ethirajan, A.; Baeten, L.; Conradi, M.; Ranieri, K.; Conings, B.; Boyen, H.-G.; Junkers, T. UV-induced functionalization of poly(divinylbenzene) nanoparticles via efficient [2+2]-photocycloadditions. Polym. Chem. 2013, 4, 4010–4016. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
D'Auria, M.; Racioppi, R. Oxetane Synthesis through the Paternò-Büchi Reaction. Molecules 2013, 18, 11384-11428. https://doi.org/10.3390/molecules180911384
D'Auria M, Racioppi R. Oxetane Synthesis through the Paternò-Büchi Reaction. Molecules. 2013; 18(9):11384-11428. https://doi.org/10.3390/molecules180911384
Chicago/Turabian StyleD'Auria, Maurizio, and Rocco Racioppi. 2013. "Oxetane Synthesis through the Paternò-Büchi Reaction" Molecules 18, no. 9: 11384-11428. https://doi.org/10.3390/molecules180911384



