Thymoquinone Induces Mitochondria-Mediated Apoptosis in Acute Lymphoblastic Leukaemia in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Cell Culture and Viability Assay
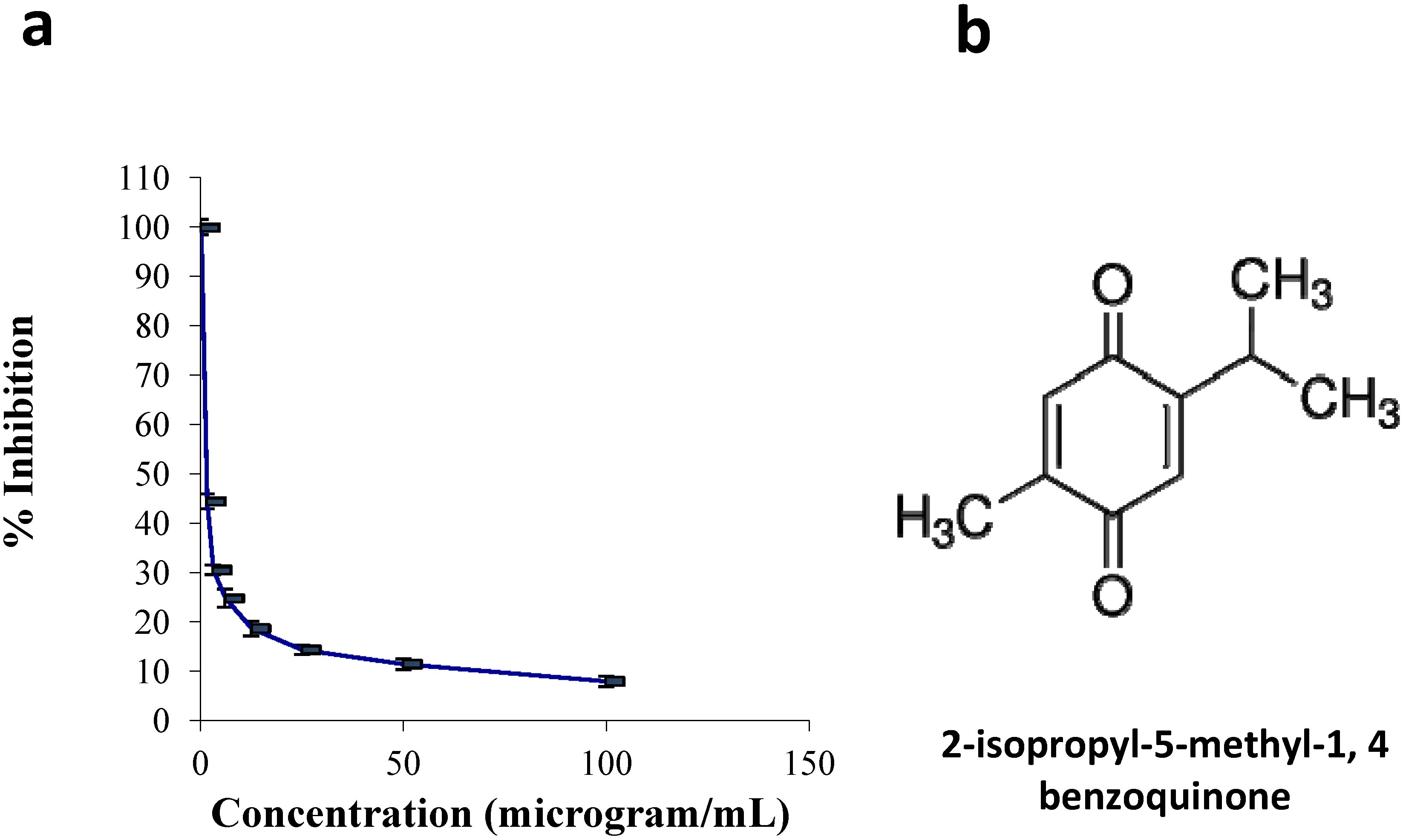
2.1.2. Quantification of Apoptosis Using Propidium Iodide and Acridine Orange Double-Staining


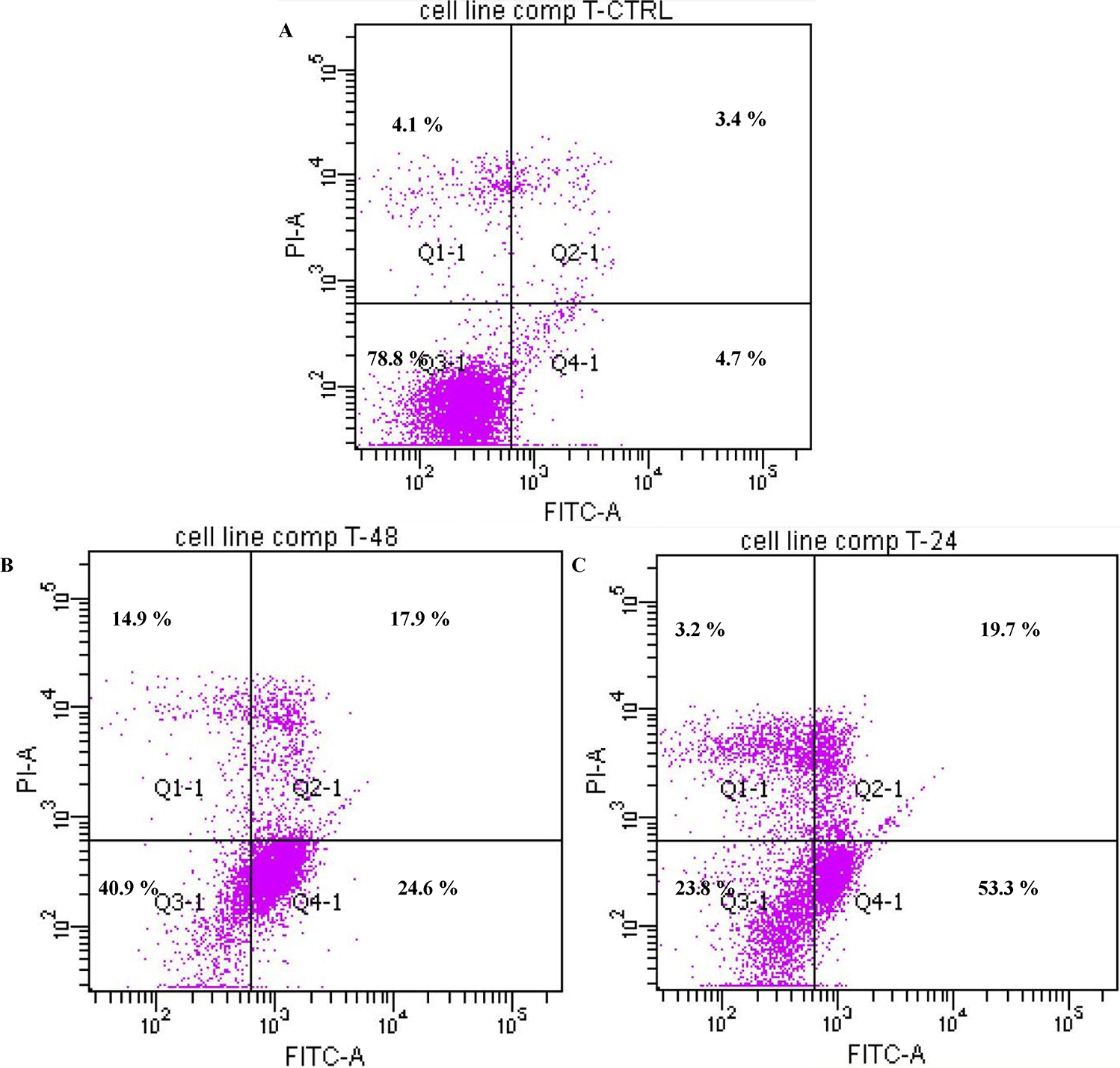
2.1.3. Annexin V
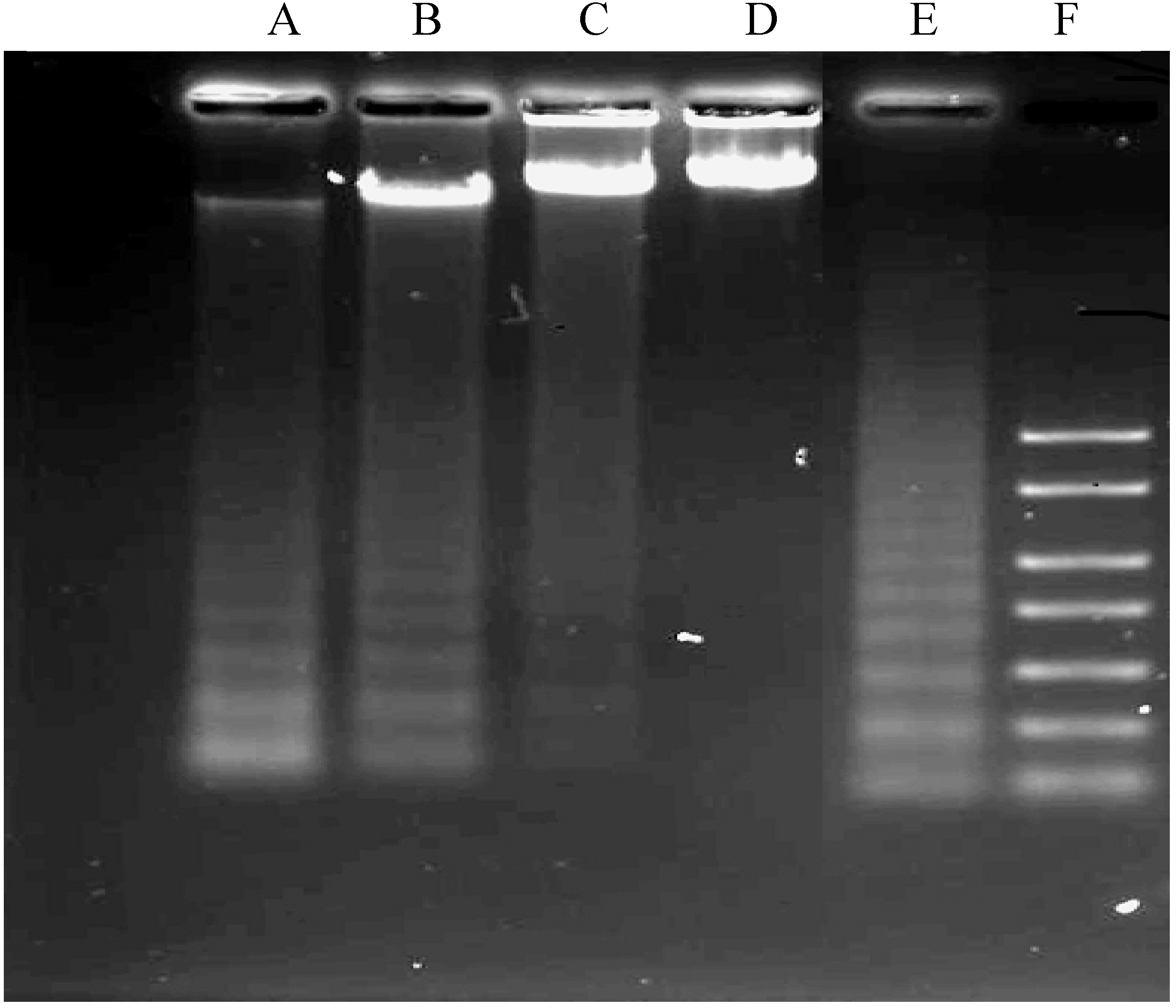
2.1.4. DNA Fragmentation Assay
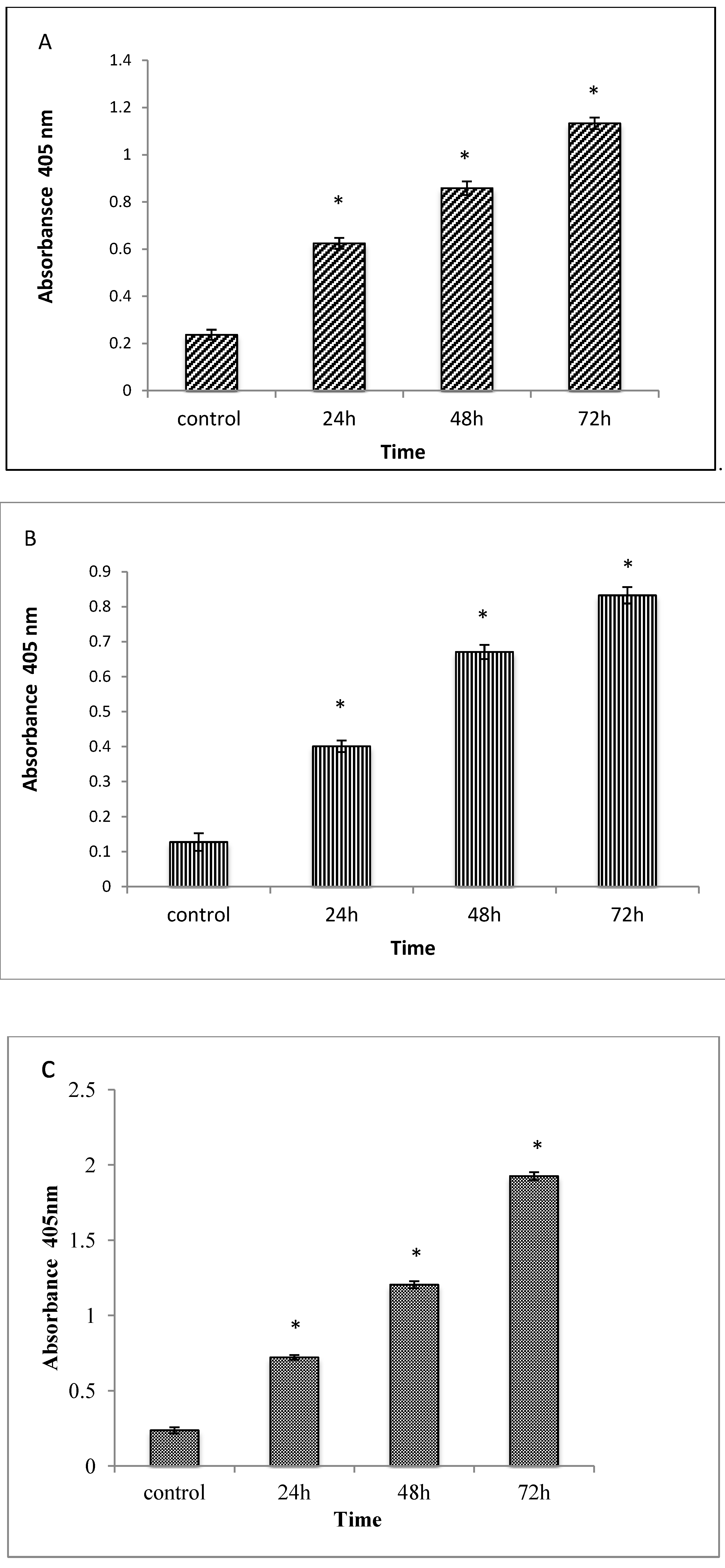
2.1.5. Caspases 3, 8 and 9 Analyses
2.1.6. Cell Cycle Analysis
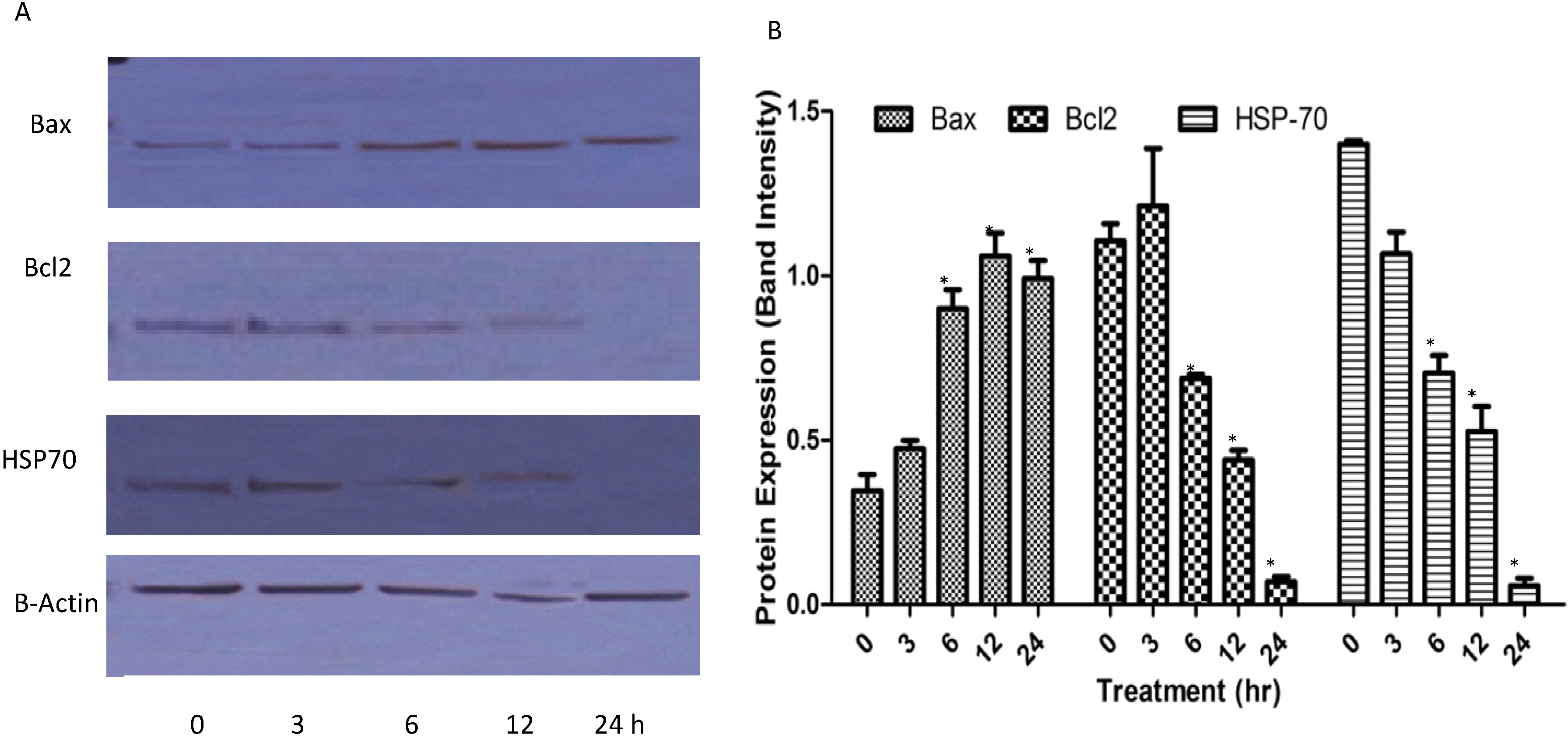
2.1.7. Western Blots

2.1.8. Reactive Oxygen Species (ROS)

2.2. Discussion
3. Experimental
3.1. Chemicals and Reagents
3.2. Cell Culture and Viability Assay
3.3. Quantification of Apoptosis Using Propidium Iodide and Acridine Orange Double-Staining
3.4. Annexin V Assay
3.5. DNA Fragmentation Assay
3.6. Caspase 3, 8 and 9 Activities
3.7. Cell Cycle Analysis
3.8. Protein Detection by Western Blotting
3.8.1. Extraction of Whole Protein from the Cells
3.8.2. Western Blotting Analysis
3.9. Determination of Reactive Oxygen Species (ROS)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yaman, İ.; Balikci, E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp. Toxicol. Pathol. 2010, 62, 183–190. [Google Scholar] [CrossRef]
- Hawsawi, Z.A.; Ali, B.A.; Bamosa, A.O. Effect of Nigella sativa (black seed) and thymoquinone on blood glucose in albino rats. Ann. Saudi. Med. 2001, 21, 242–244. [Google Scholar]
- Ahmed, W.A.; Hassan, S.A.; Galeb, F.M.; El-Taweel, M.A.; Abu-Bedair, F.A. The in vitro promising therapeutic activity of thymoquinone on hepatocellular carcinoma (HepG2) cell line. Global Vet. 2008, 2, 233–241. [Google Scholar]
- Abu-Irmaileh, B.E.; Afifi, F.U. Herbal medicine in Jordan with special emphasis on commonly used herbs. J. Ethnopharmacol. 2003, 89, 193–197. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, Functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An Overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Shah, S.; Kasturi, S.R. Study on antioxidant and antimicrobial properties of black cumin (Nigella sativa Linn). J. Food Sci. Technol. 2003, 40, 70–73. [Google Scholar]
- Ghannadi, A.; Hajhashemi, V.; Jafarabadi, H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J. Med. Food. 2005, 8, 488–493. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C. Chemical constituents and antimicrobial and antioxidant potentials of essential oil and acetone extract of Nigella sativa seeds. J. Sci. Food Agric. 2005, 85, 2297–2306. [Google Scholar] [CrossRef]
- Sayed-Ahmed, M.M.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Yahya, A.A.; Al-Shabanah, O.A.; Hafez, M.M.; Nagi, M.N. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid. Med. Cell. Longev. 2010, 3, 254–261. [Google Scholar] [CrossRef]
- Nasaruddin, N. Extraction of pharmacologically active thymoquinone in Nigella sativa L. Master’s Thesis, Universiti Malaysia Pahang, Pahang, Malaysia, 2006. [Google Scholar]
- Arafa, E.-S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. 2011, 706, 128–135. [Google Scholar]
- Banerjee, S.; Azmi, A.S.; Padhye, S.; Singh, M.W.; Baruah, J.B.; Philip, P.A.; Sarkar, F.H.; Mohammad, R.M. Structure-activity studies on therapeutic potential of Thymoquinone analogs in pancreatic cancer. Pharmaceut. Res. 2010, 27, 1146–1158. [Google Scholar] [CrossRef]
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 2285. [Google Scholar]
- Woo, C.C.; Loo, S.Y.; Gee, V.; Yap, C.W.; Sethi, G.; Kumar, A.P.; Benny Tan, K.H. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem. Pharmacol. 2011, 82, 464–475. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Kuester, D.; Mawrin, C.; Bajbouj, K.; Diestel, A.; Ocker, M.; Habold, C.; Foltzer-Jourdainne, C.; Schoenfeld, P.; Peters, B. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008, 68, 5609–5618. [Google Scholar] [CrossRef]
- Amin, A.; Gali-Muhtasib, H.; Ocker, M.; Schneider-Stock, R. Overview of major classes of plant-derived anticancer drugs. Int. J. Biomed. Sci. 2009, 5, 1–11. [Google Scholar]
- Lim, G.C.C. Overview of cancer in Malaysia. Jpn. J. Clin. Oncol. 2002, 32, S37–S42. [Google Scholar] [CrossRef]
- Trigg, M.E.; Sather, H.N.; Reaman, G.H.; Tubergen, D.G.; Steinherz, P.G.; Gaynon, P.S.; Uckun, F.M.; Hammond, G.D. Ten-year survival of children with acute lymphoblastic leukemia: A Report from the Children’s Oncology Group. Leuk. Lymphoma. 2008, 49, 1142–1154. [Google Scholar] [CrossRef]
- Wagiman, S.N.; Husin, N.H.; Latiff, Z.A.; Alias, H.; Jamal, R.; Zakaria, S.Z.S. Folate-related genes polymorphisms associated with the risk of childhood leukaemia in the Malaysian population. Asia. Pac. J. Mole. Med. 2011, 1, 1–10. [Google Scholar]
- Zhu, J.Y.; Lavrik, I.N.; Mahlknecht, U.; Giaisi, M.; Proksch, P.; Krammer, P.H.; Li-Weber, M. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int. J. Cancer 2007, 121, 1839–1846. [Google Scholar] [CrossRef]
- Sosne, G.; Siddiqi, A.; Kurpakus-Wheater, M. Thymosin-β4 inhibits corneal epithelial cell apoptosis after ethanol exposure in vitro. Invest. Ophthalmol. Visual. Sci. 2004, 45, 1095–1100. [Google Scholar] [CrossRef]
- Chen, H.W.; Huang, H.C. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Brit. J. Pharmacol. 1998, 124, 1029–1040. [Google Scholar] [CrossRef]
- Hsu, Y.-T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc. Nat. Acad. Sci. Usa 1997, 94, 3668–3672. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.-K.; Lu, Z.-X.; Zhao, Y.; Liu, S.-F.; Li, L.-L.; Tan, M.; Weng, X.-X.; Li, W.; Cao, Y. Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, inhibits tumor cell growth by inducing apoptosis in vitro. Febs. Lett. 2005, 579, 3437–3443. [Google Scholar] [CrossRef]
- Jia, N.; Xiong, Y.L.; Kong, B.; Liu, Q.; Xia, X. Radical scavenging activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on gastric cancer cell proliferation via induction of apoptosis. J. Funct. Foods 2012, 4, 382–390. [Google Scholar] [CrossRef]
- Goldsworthy, T.L.; Conolly, R.B.; Fransson-Steen, R. Apoptosis and cancer risk assessment. Mutat. Res-Rev. Genet. Toxicol. 1996, 365, 71–90. [Google Scholar] [CrossRef]
- Taraphdar, A.K.; Roy, M.; Bhattacharya, R. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr. Sci. 2001, 80, 1387–1396. [Google Scholar]
- Edris, A.E. Anti-cancer properties of nigella spp. essential oils and their major constituents, Thymoquinone and-Elemene. Curr. Clin. Pharmacol. 2009, 4, 43–46. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Chinnakannu, K.; Chen, D.; Sivanandam, A.; Tejwani, S.; Menon, M.; Dou, Q.P.; Reddy, G.P.-V. Androgen Receptor–and E2F-1–Targeted Thymoquinone Therapy for Hormone-Refractory Prostate Cancer. Cancer Res. 2007, 67, 7782–7788. [Google Scholar] [CrossRef]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar] [CrossRef]
- Yi, T.; Cho, S.-G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef]
- Al-Johar, D.; Shinwari, N.; Arif, J.; Al-Sanea, N.; Jabbar, A.A.; El-Sayed, R.A.; Mashhour, A.; Billedo, G.; El-Doush, I.; Al-Saleh, I. Role of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in rats. Phytother. Res. 2008, 22, 1311–1323. [Google Scholar] [CrossRef]
- Ivankovic, S.; Stojkovic, R.; Jukic, M.; Milos, M.; Milos, M.; Jurin, M. The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp. Oncol. 2006, 28, 220–224. [Google Scholar]
- Rooney, S.; Ryan, M. Modes of action of alpha-hederin and thymoquinone, active constituents of Nigella sativa, against HEp-2 cancer cells. Anticancer Res. 2005, 25, 4255–4259. [Google Scholar]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A Review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Nagata, S. Apoptotic DNA fragmentation. Exp. Cell. Res. 2000, 256, 12–18. [Google Scholar] [CrossRef]
- Faheina-Martins, G.V.; Silveira, A.L.D.; Cavalcanti, B.C.; Ramos, M.V.; Moraes, M.O.; Pessoa, C.; M Araújo, D.A. Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines. Toxicol. Vitro. 2012, 26, 1161–1169. [Google Scholar] [CrossRef]
- Sari, N.K. DNA Fragmentation on HCT-116 Colon Cancer Cell in the Presence of Short Chain Fatty Acid (SCFA) Extract. Available online: http://repository.ipb.ac.id/handle/123456789/55761 (accessed on 18 January 2013).
- Kizhakkayil, J.; Thayyullathil, F.; Chathoth, S.; Hago, A.; Patel, M.; Galadari, S. Modulation of curcumin-induced Akt phosphorylation and apoptosis by PI3K inhibitor in MCF-7 cells. Biochem. Biophys. Res. Commun. 2010, 394, 476–481. [Google Scholar] [CrossRef]
- Holmgren, A. Biochemistry: SNO removal. Science 2008, 320, 1019–1020. [Google Scholar] [CrossRef]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef]
- Hsuuw, Y.D.; Chang, C.K.; Chan, W.H.; Yu, J.S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar] [CrossRef]
- Kruidering, M.; Evan, G.I. Caspase–8 in Apoptosis: The Beginning of “The End”? Iubmb Life 2000, 50, 85–90. [Google Scholar]
- Lin, S.-S.; Huang, H.-P.; Yang, J.-S.; Wu, J.-Y.; Hsai, T.-C.; Lin, C.-C.; Lin, C.-W.; Kuo, C.-L.; Gibson Wood, W.; Chung, J.-G. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade-and mitochondrial-dependent pathway. Cancer Lett. 2008, 272, 77–90. [Google Scholar] [CrossRef]
- Motaghed, M.; Al-Hassan, F.; Hamid, S. Cellular responses with thymoquinone treatment in human breast cancer cell line MCF-7. Pharmacog. Res. 2013, 5, 200–206. [Google Scholar] [CrossRef]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef]
- Yu, S.-M.; Kim, S.-J. Thymoquinone-induced reactive oxygen species causes apoptosis of chondrocytes via PI3K/Akt and p38kinase pathway. Exp. Biol. Med. 2013, in press. [Google Scholar]
- Salvesen, G.S.; Riedl, S.J. Caspase mechanisms. In Programmed cell death in cancer progression and therapy; Springer: La Jolla, CA, USA, 2007; pp. 13–23. [Google Scholar]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3,-6, and-7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differentiation. 1999, 6, 99–104. [Google Scholar]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Elhassan Taha, M.M.; Ibrahim, M.Y.; Syam, S. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J. Ethnopharmacol. 2010, 131, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Sample Availability: Samples of the compounds Thymoquinone are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salim, L.Z.A.; Mohan, S.; Othman, R.; Abdelwahab, S.I.; Kamalidehghan, B.; Sheikh, B.Y.; Ibrahim, M.Y. Thymoquinone Induces Mitochondria-Mediated Apoptosis in Acute Lymphoblastic Leukaemia in Vitro. Molecules 2013, 18, 11219-11240. https://doi.org/10.3390/molecules180911219
Salim LZA, Mohan S, Othman R, Abdelwahab SI, Kamalidehghan B, Sheikh BY, Ibrahim MY. Thymoquinone Induces Mitochondria-Mediated Apoptosis in Acute Lymphoblastic Leukaemia in Vitro. Molecules. 2013; 18(9):11219-11240. https://doi.org/10.3390/molecules180911219
Chicago/Turabian StyleSalim, Landa Zeenelabdin Ali, Syam Mohan, Rozana Othman, Siddig Ibrahim Abdelwahab, Behnam Kamalidehghan, Bassem Y. Sheikh, and Mohamed Yousif Ibrahim. 2013. "Thymoquinone Induces Mitochondria-Mediated Apoptosis in Acute Lymphoblastic Leukaemia in Vitro" Molecules 18, no. 9: 11219-11240. https://doi.org/10.3390/molecules180911219




