Purification, Partial Characterization and Immobilization of a Mannose-Specific Lectin from Seeds of Dioclea lasiophylla Mart.
Abstract
:1. Introduction
2. Results and Discussion
| Carbohydrate | MIC * |
|---|---|
| d-Glucose | NI ** |
| d-Mannose | 25 mM |
| d-Galactose | NI ** |
| N-acetyl-d-glucosamine | NI ** |
| α-methyl-d-mannoside | 6.25 mM |
| α-methyl-d-galactoside | NI ** |
| α-Lactose | NI ** |
| β-Lactose | NI ** |
| Glycoprotein | |
| Ovalbumin | 0.0625 mg/mL |
| Fetuin | 0.125 mg/mL |

| Fraction | a Total protein (mg/mL) | b Total HU | c Specific activity (HU/mg) | Purification (fold) |
|---|---|---|---|---|
| Crude extract | 5.2 | 210 | 196 | 1 |
| PII (Sephadex® G-50) | 0.76 | 212 | 5390 | 27.5 |
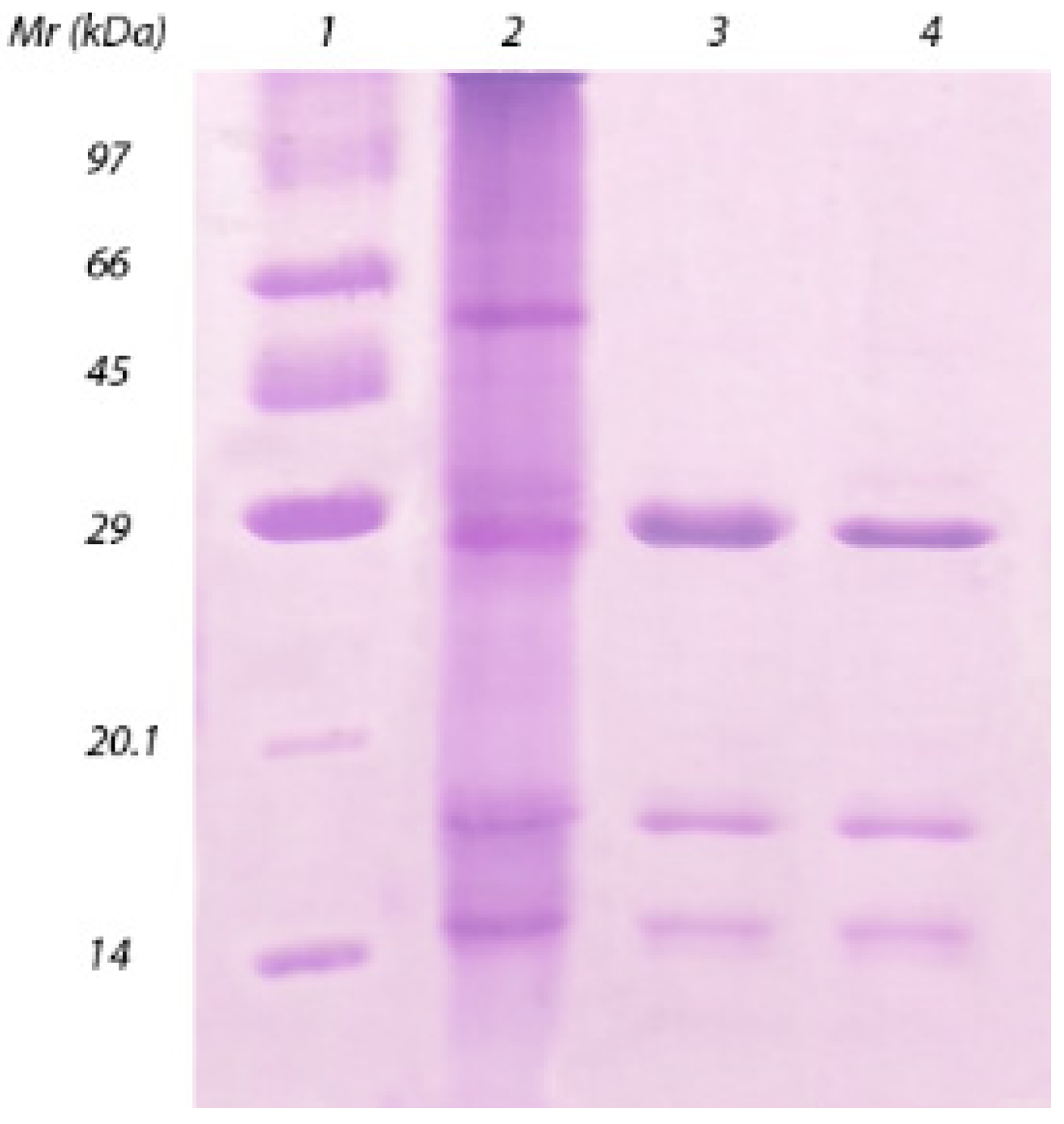

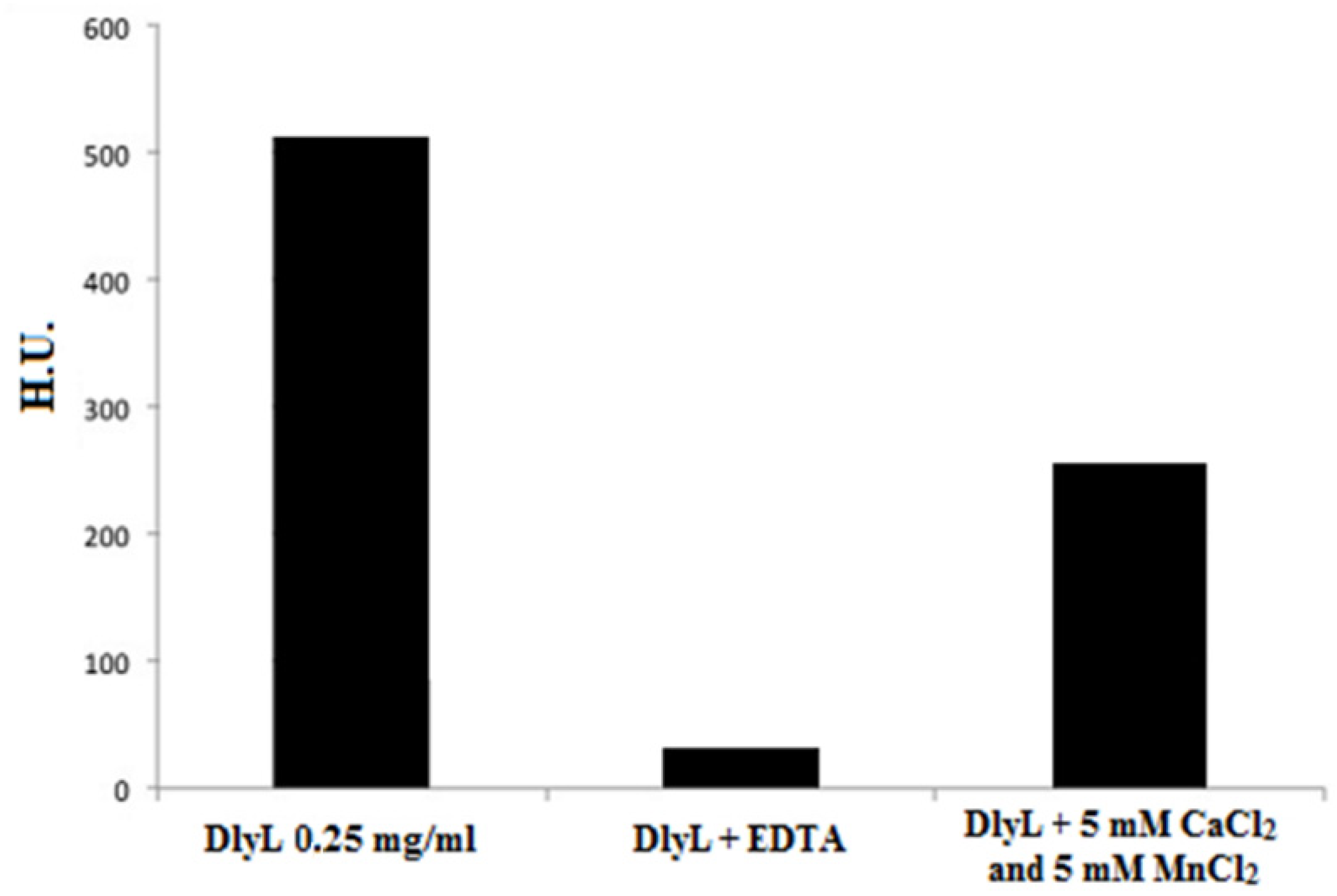
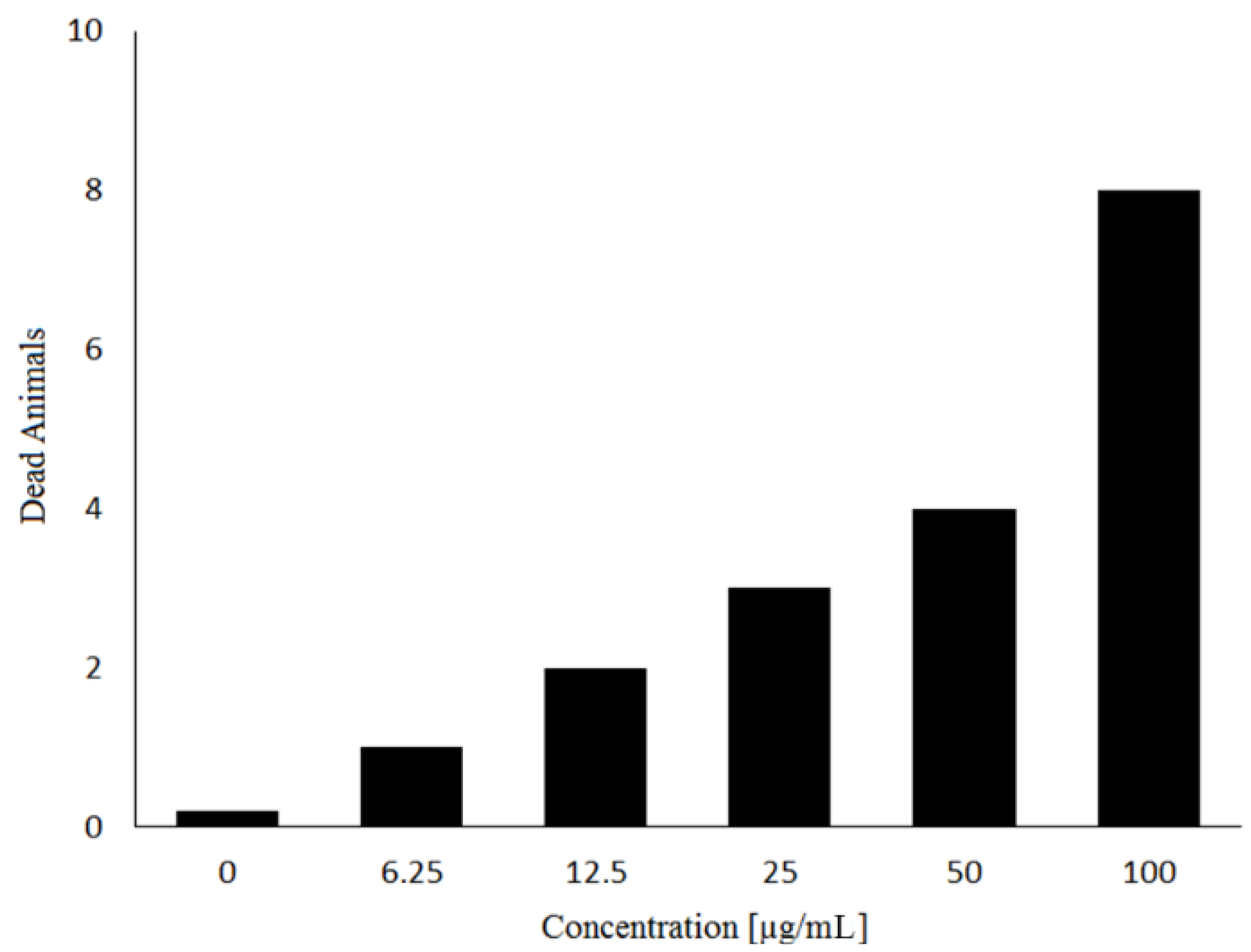
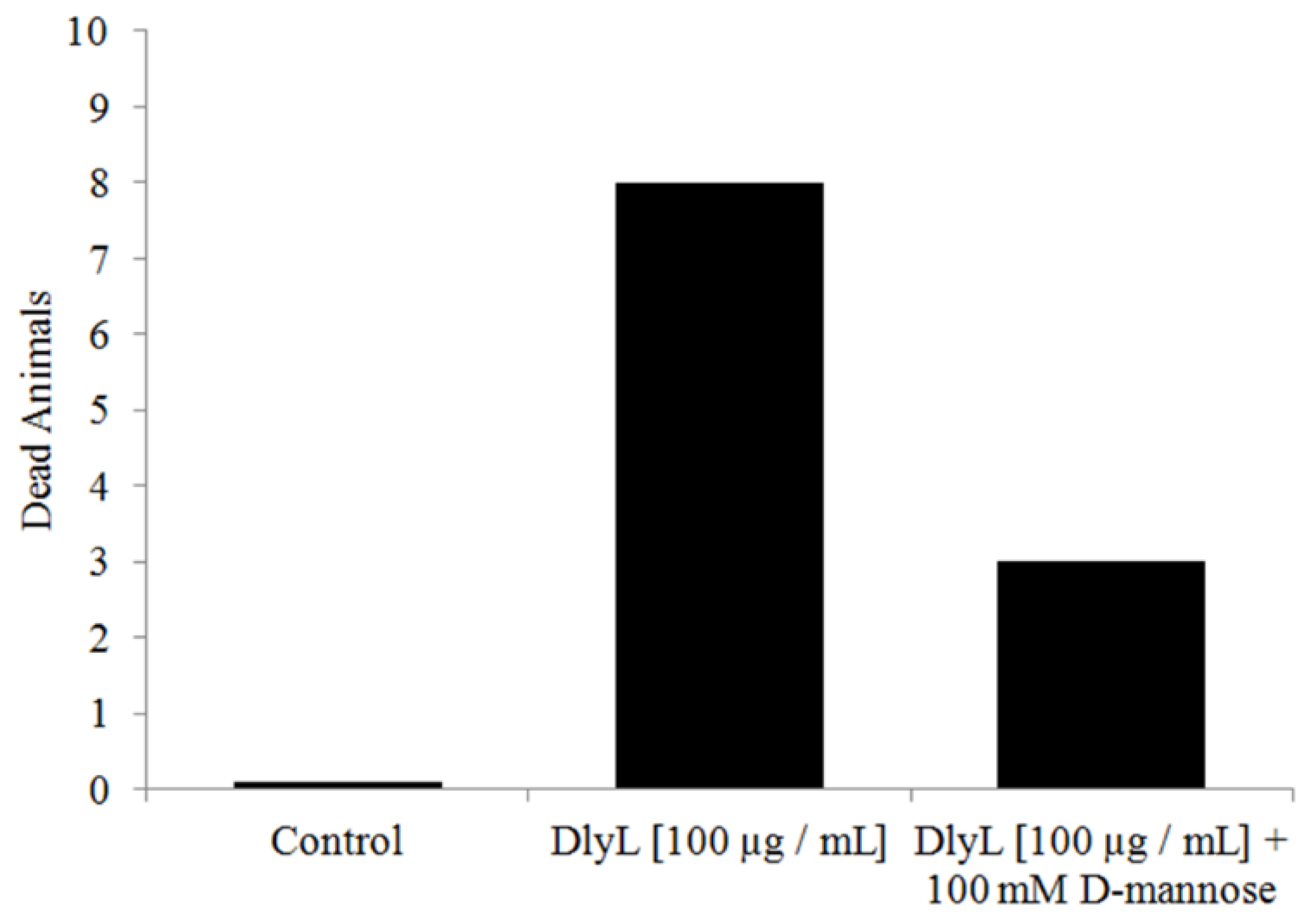

3. Experimental Section
3.1. Plant Material
3.2. Purification of DlyL
3.3. Hemagglutination Tests
3.4. Inhibition Assay
3.5. SDS-PAGE
3.6. Mass Spectrometry
3.7. Effect of pH and Temperature on Lectin Activity
3.8. Effect of Divalent Cations
3.9. Artemia Toxicity Tests
3.10. Determination of the LC50 Value
3.11. DlyL Immobilization on Sepharose 4B
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rougé, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. CRC Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar]
- Gabius, H.J.; Gabius, S. Glycoscience. Status and Perspectives; Chapman & Hall: New York, NY, USA, 1997. [Google Scholar]
- Regnier, F.E.; Jung, K.; Hooser, S.B.; Wilson, C.R. Glycoproteomics based on lectin affinity chromatographic selection of glycoforms. Lectins: Anal. Technol. 2007, 8, 193–212. [Google Scholar]
- Vandenborre, G.; Smagghe, G.; van Damme, E.J.M. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 2011, 72, 1538–1550. [Google Scholar]
- Bies, C.; Lehr, C.M.; Woodley, J.F. Lectin-mediated drug targeting history and applications. Adv. Drug Deliver. Rev. 2004, 56, 425–435. [Google Scholar] [CrossRef]
- Gemeiner, P.; Mislovicová, D.; Tkác, J.; Svitel, J.; Pätoprstý, V.; Hrabárová, E.; Kogan, G.; Kozár, T. Lectinomics II. A highway to biomedical/clinical diagnostics. Biotechnol. Adv. 2009, 27, 1–5. [Google Scholar]
- Brinda, K.V.; Mitra, N.; Surolia, A.; Vishveshwara, S. Determinants of quaternary association in legume lectins. Protein Sci. 2004, 13, 1735–1749. [Google Scholar]
- Ferreira, R.R.; Cavada, B.S.; Moreira, R.A.; Oliveira, J.T.A.; Gomes, J.C. Characteristics of the histamine release from hamster cheek pouch mast cells stimulated by lectins from Brazilian beans And Concanavalin A. Inflamm. Res. 1996, 45, 442–447. [Google Scholar]
- Alencar, N.M.N.; Teixeira, E.H.; Asseury, A.M.; Cavada, B.S.; Flores, C.A.; Ribreiro, R.A. Leguminous lectins as tools for studying the role of sugar residues in leukocyte recruitment. Mediat. Inflamm. 1998, 8, 107–113. [Google Scholar]
- Asseury, A.M.S.; Shimbuya, M.D.; Martins, G.J.; Souza, M.L.P.; Cavada, B.S.; Moreira, R.A.; Oliveira, J.T.A.; Ribeiro, R.A.; Flores, C.A. Antiinflammatory effect of glucose-mannose binding lectins isolated from brazilian beans. Mediat. Inflamm. 1997, 6, 201–210. [Google Scholar]
- Cavada, B.S.; Grangeiro, T.B.; Ramos, M.V.; Crisostomo, C.V.; Silva, L.M.; Moreira, R.A.; Oliveira, J.T.A. Lectin from Dioclea guianensis var. lasiophylla Duke seeds mobilization during germination and seedlings growth in the dark. Rev. Bras. Fisiol. Veg. 1994, 6, 21–25. [Google Scholar]
- Sanz-Aparicio, J.; Hermoso, J.; Grangeiro, T.B.; Calvette, J.J.; Cavada, B.S. The crystal structure of Canavalia brasiliensis lectin suggests a correlation between its quaternary conformation and its distinct biological properties from Concanavalin A. FEBS Lett. 1997, 405, 114–118. [Google Scholar]
- Moreira, R.A.; Monteiro, A.C.O.; Horta, A.C.G.; Oliveira, J.T.A.; Cavada, B.S. Isolation and characterization of Dioclea altissima Var. Megacarpa seed lectin. Phytochemstry 1997, 46, 139–144. [Google Scholar]
- Cavada, B.S.; Grangeiro, T.B.; Ramos, M.V.; Cordeiro, E.F.; Oliveira, J.T.A.; Moreira, R.A. Isolation and partial characterization of a lectin from Dioclea rostrata Benth seeds. Rev. Bras. Fisiol. Veg. 1996, 8, 31–36. [Google Scholar]
- Moreira, R.A.; Cordeiro, E.F.; Ramos, M.V.; Grangeiro, T.B.; Martins, J.L.; Oliveira, J.T.A.; Cavada, B.S. Isolation and partial characterization of a lectin from seeds of Dioclea violacea. Rev. Bras. Fisiol. Veg. 1996, 8, 23–29. [Google Scholar]
- Moreira, R.A.; Barros, A.C.H.; Stewart, J.C.; Pusztai, A. Isolation and characterization of a lectin from the seeds of Dioclea grandiflora (Mart.). Planta 1983, 158, 63–69. [Google Scholar]
- Vasconcellos, I.M.; Cavada, B.S.; Moreira, R.A.; Oliveira, J.T.A. Purification and partial characterization of a lectin from the seed of Dioclea guianensis. J. Food Biochem. 1991, 15, 137–154. [Google Scholar]
- Correia, J.L.A.; Nascimento, A.S.F.; Cajazeiras, J.B.; Gondim, A.C.S.; Pereira, R.I.; Sousa, B.L.; Silva, A.L.C.; Garcia, W.; Teixeira, E.H.; Nascimento, K.S.; et al. Molecular characterization and tandem mass spectrometry of the lectin extracted from the seeds of Dioclea sclerocarpa Ducke. Molecules 2011, 16, 9077–9089. [Google Scholar] [CrossRef]
- Nascimento, A.S.F.; Gondim, A.C.S.; Cajazeiras, J.B.; Correia, J.L.A.; Pires, A.F.; Nascimento, K.S.; Silva, A.L.C.; Nagano, C.S.; Assreuy, A.M.S.; Cavada, B.S. Purification and partial characterization of a novel lectin from Dioclea lasiocarpa Mart seeds with vasodilator effects. J. Mol. Recognit. 2012, 25, 1099–1352. [Google Scholar]
- Moreira, R.A.; Cordeiro, E.F.; Cavada, B.S.; Nunes, E.P.; Fernandes, A.G.; Oliveira, J.T.A. Lectins and the chemotaxonomy of the genus Diocleinae (Leguminosae-Phaseoleae). R. Bras. Fis. Veg. 1995, 7, 7–14. [Google Scholar]
- Rangel, T.B.A.; Assreuy, A.M.S.; Pires, A.F.; Carvalho, A.U.; Benevides, R.G.; Simões, R.C.; Silva, H.C.; Bezerra, M.J.B.; Nascimento, A.S.F.; Nascimento, K.S.; et al. Crystallization and Characterization of an inflammatory lectin purified from the seeds of Dioclea wilsonii. Molecules 2011, 16, 5087–5103. [Google Scholar] [CrossRef]
- Cavada, B.S.; Ramos, M.V.; Cordeiro, E.F.; Grangeiro, T.B.; Oliveira, J.T.A.; Carvalho, A.F.F.U.; Moreira, R.A. Purification and partial characterization of a lectin from Dioclea virgata Benth seeds. Braz. J. Veg. Fis. 1996, 8, 37–42. [Google Scholar]
- Persoone, G. The Brine Shrimp Artemia: Proceedings of the International Symposium on the Brine Shrimp Artemia Salina; Universa Press: Corpus Christi, TX, USA, 1979.
- Sorgeloos, P.; van Der Wielen, C.R.; Persoone, G. The use of artemia nauplii for toxicity tests-A critical analysis. Ecotoxicol. Environ. Saf. 1978, 2, 249–255. [Google Scholar] [CrossRef]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17–21. [Google Scholar]
- Pervin, F.; Hossain, M.M.; Khatun, S.; Siddique, S.P.; Salam, K.A.; Karim, M.R.; Absar, N. Comparative citotoxicity study of six bioactive lectins purified from pondweed (Potamogeton nodosus Poir) rootstock on Brine Shrimp. J. Med. Sci. 2006, 6, 999–1002. [Google Scholar]
- Ho, J.C.; Chen, C.M.; Row, L.C. Oleanane-type triterpenes from the flowers, pith, leaves, and fruit of Tetrapanax papyriferus. Phytochemistry 2007, 68, 631–635. [Google Scholar]
- Santos, A.F.; Cavada, B.S.; Rocha, B.A.M.; Nascimento, K.S.; Sant’Ana, A.E.G. Toxicity of some glucose/mannose-binding lectins to Biomphalaria glabrata and Artemia salina. Bioresour. Technol. 2010, 101, 794–798. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Biochemistry 1976, 72, 248–254. [Google Scholar]
- Ainouz, I.L.; Sampaio, A.H.; Benevides, N.M.B.; Freitas, A.L.P.; Costa, F.H.F.; Carvalho, M.R.; Joventino, F.P. Agglutination of enzyme treated erythrocytes by Brazilian marine algae extracts. Botanica Mar. 1992, 35, 447–479. [Google Scholar]
- Ramos, M.V.; Moreira, R.A.; Cavada, B.S.; Oliveira, J.T.A.; Rouge, P. Interaction of lectins from the sub tribe Diocleinae with specific ligands. R. Bras. Fisiol. Veg. 1996, 8, 193–199. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zacharius, R.M.; Zell, T.E.; Morrison, J.H.; Woodlock, J.J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal. Biochem. 1969, 30, 148–152. [Google Scholar]
- Ferrige, A.G.; Seddon, M.J.; Green, B.N.; Jarvis, S.A.; Skilling, J.; Staunton, J. Disentangling electrospray spectra with maximum entropy. Rapid Commun. Mass Spectrom. 1992, 6, 707–711. [Google Scholar] [CrossRef]
- Paiva, P.M.G.; Souza, A.F.; Oliva, M.L.V.; Kennedy, J.F.; Cavalcanti, M.S.M.; Coelho, L.C.B.B.; Sampaio, C.A.M. Isolation of a trypsin inhibitor from Echinodorus paniculatus seeds by affinity chromatography on immobilized Cratylia mollis isolectins. Bioresour. Technol. 2003, 88, 75–79. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pinto-Júnior, V.R.; De Santiago, M.Q.; Osterne, V.J.d.S.; Correia, J.L.A.; Pereira-Júnior, F.N.; Cajazeiras, J.B.; De Vasconcelos, M.A.; Teixeira, E.H.; Do Nascimento, A.S.F.; Miguel, T.B.A.R.; et al. Purification, Partial Characterization and Immobilization of a Mannose-Specific Lectin from Seeds of Dioclea lasiophylla Mart. Molecules 2013, 18, 10857-10869. https://doi.org/10.3390/molecules180910857
Pinto-Júnior VR, De Santiago MQ, Osterne VJdS, Correia JLA, Pereira-Júnior FN, Cajazeiras JB, De Vasconcelos MA, Teixeira EH, Do Nascimento ASF, Miguel TBAR, et al. Purification, Partial Characterization and Immobilization of a Mannose-Specific Lectin from Seeds of Dioclea lasiophylla Mart. Molecules. 2013; 18(9):10857-10869. https://doi.org/10.3390/molecules180910857
Chicago/Turabian StylePinto-Júnior, Vanir Reis, Mayara Queiroz De Santiago, Vinícius José da Silva Osterne, Jorge Luis Almeida Correia, Francisco Nascimento Pereira-Júnior, João Batista Cajazeiras, Mayron Alves De Vasconcelos, Edson Holanda Teixeira, Antônia Sâmia Fernandes Do Nascimento, Thaiz Batista Azevedo Rangel Miguel, and et al. 2013. "Purification, Partial Characterization and Immobilization of a Mannose-Specific Lectin from Seeds of Dioclea lasiophylla Mart." Molecules 18, no. 9: 10857-10869. https://doi.org/10.3390/molecules180910857




