Synthesis and Biological Evaluation of a New Acyclic Pyrimidine Derivative as a Probe for Imaging Herpes Simplex Virus Type 1 Thymidine Kinase Gene Expression
Abstract
:1. Introduction
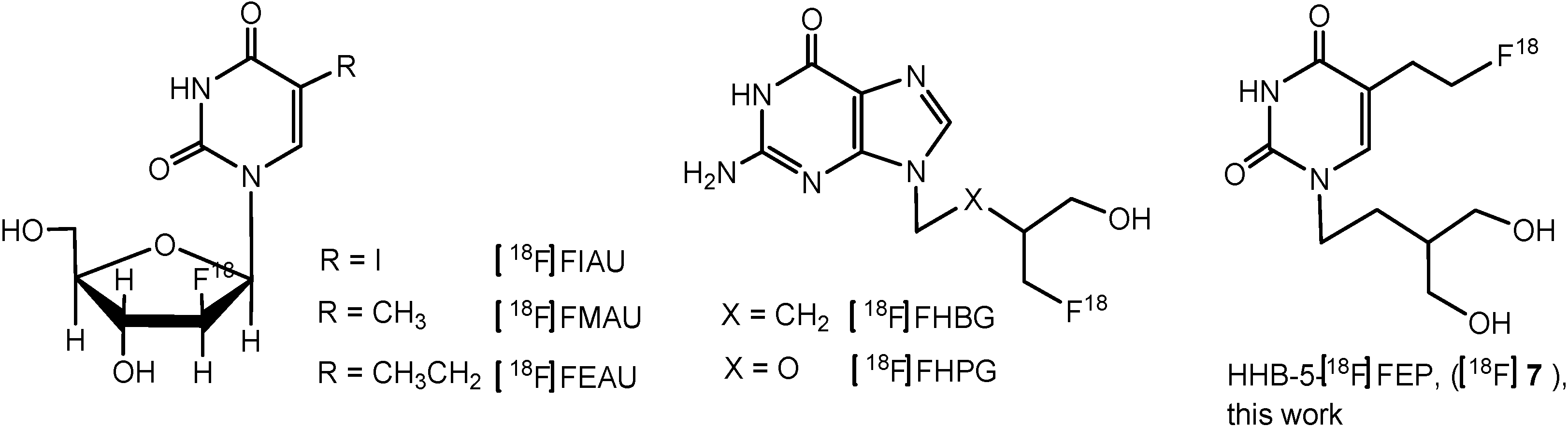
2. Results and Discussion
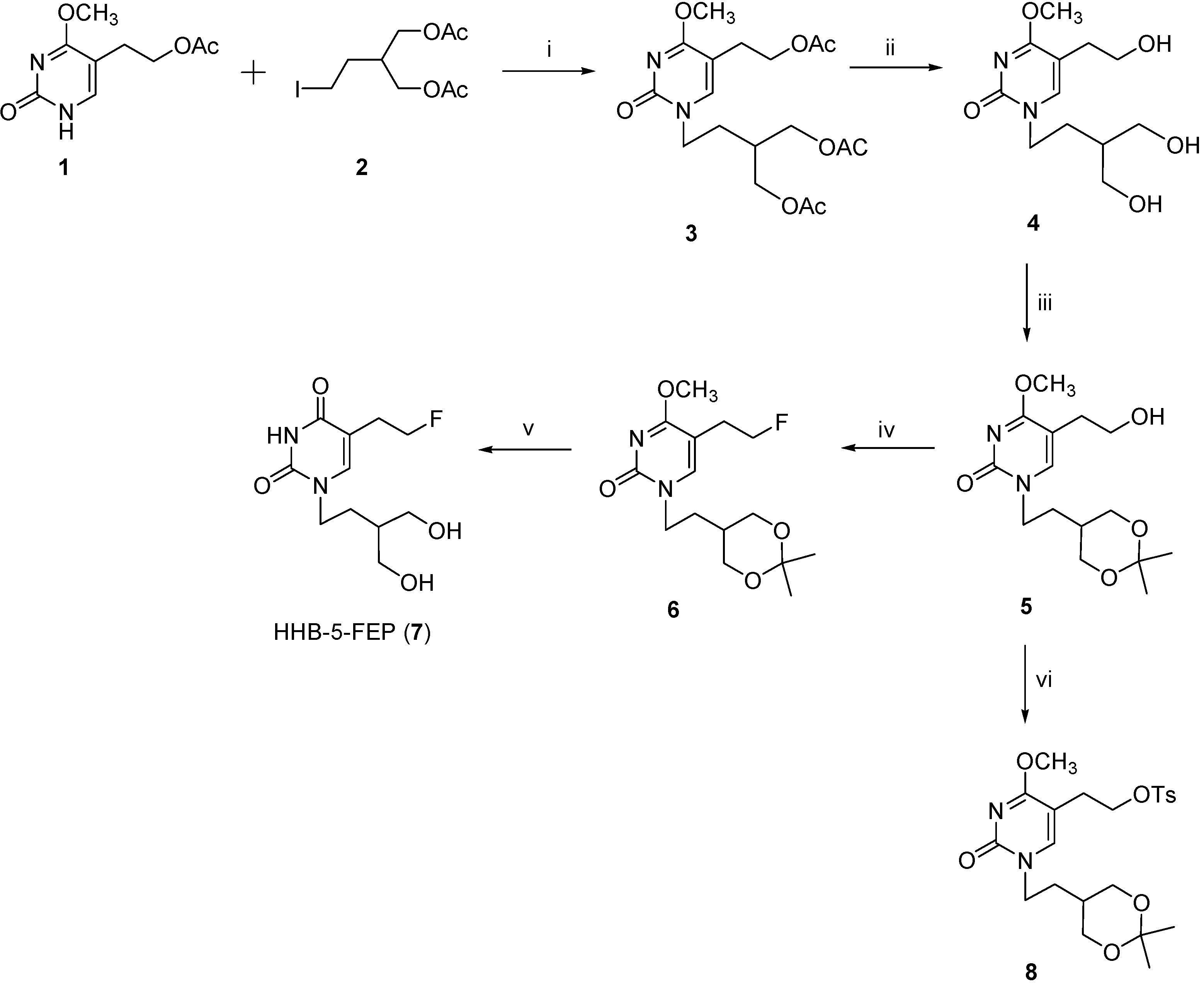
2.1. Synthesis of the Reference HHB-5-FEP (7) and Precursor 8
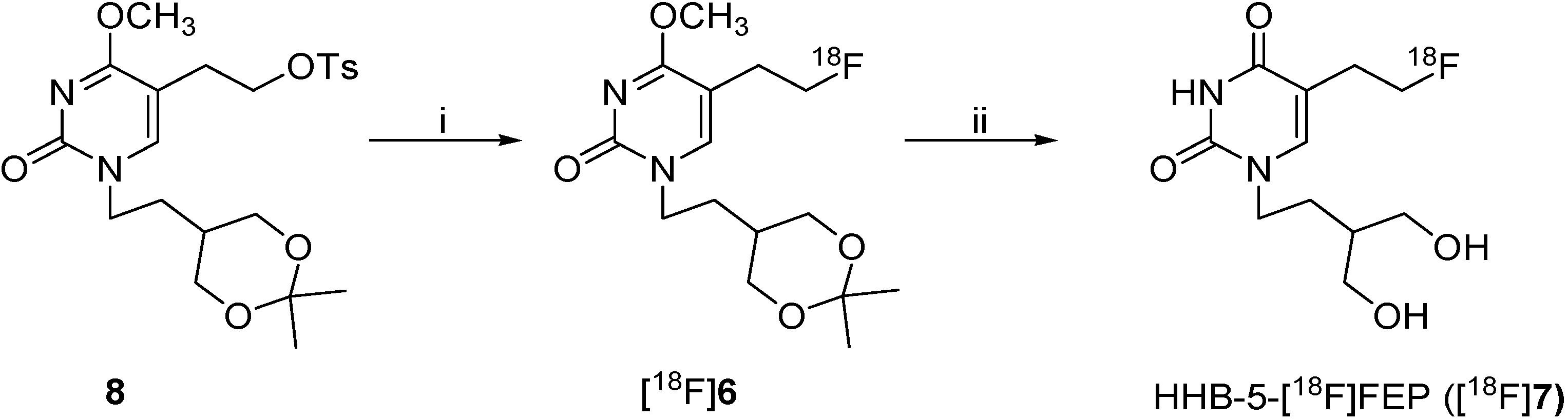
2.2. Radiosynthesis of HHB-5-[18F]FEP ([18F]7)
2.3. Cell Uptake Studies

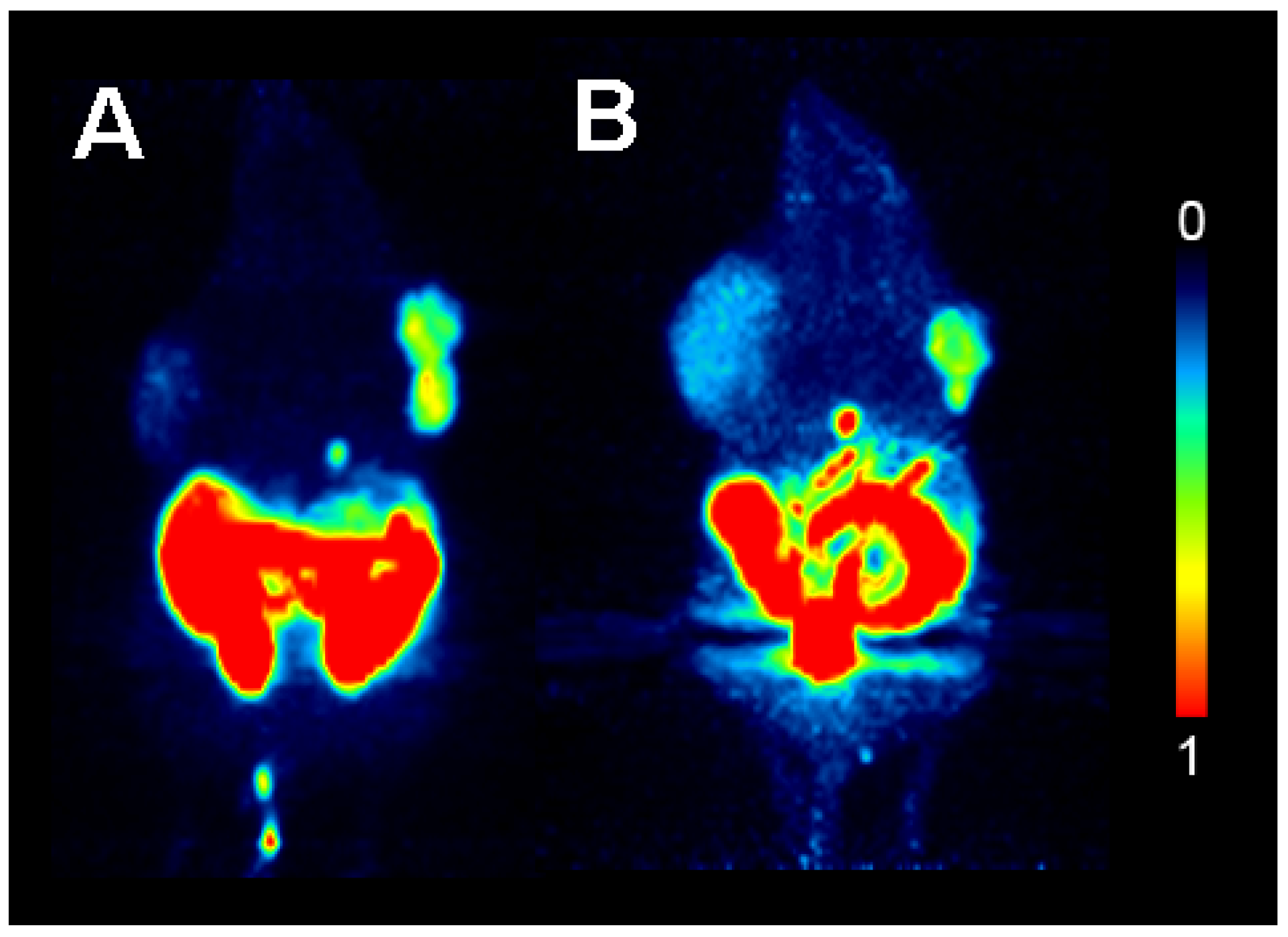
2.4. Small Animal PET Imaging with HHB-5-[18F]FEP and [18F]FHBG
2.5. Biodistribution
| Tissue | [18F]FHBG | HHB-5-[18F]FEP | ||||
|---|---|---|---|---|---|---|
| SUVbiodis | SUVPET | Ratio SUVbiodis | SUVbiodis | SUVPET | Ratio SUVbiodis | |
| Xenograft TK+ | 0.24 ± 0.07 | 0.22 ± 0.02 | TK+/contro l5.7 ± 1.9 | 0.37 ± 0.15 | 0.32 ± 0.06 | TK+/control 3.4 ± 1.1 |
| Xenograft control | 0.04 ± 0.00 | 0.09 ± 0.004 | 0.11 ± 0.01 | 0.17 ± 0.02 | ||
| Blood | 0.03 ± 0.02 | 0.07 ± 0.04 | ||||
| Spleen | 0.08 ± 0.08 | 0.05 ± 0.00 | ||||
| Liver | 0.16 ± 0.19 | 0.06 ± 0.00 | ||||
| Kidney | 0.12 ± 0.06 | 0.25 ± 0.09 | ||||
| Lung | 0.03 ± 0.01 | 0.05 ± 0.00 | ||||
| Bone | 0.04 ± 0.03 | 0.05 ± 0.01 | ||||
| Heart | 0.04 ± 0.05 | 0.04 ± 0.01 | ||||
| Brain | 0.002 ± 0.00 | 0.01 ± 0.00 | ||||
| Stomach w. cont. | 0.02 ± 0.01 | 0.05 ± 0.01 | ||||
| Intestine w. cont. | 1.78 ± 0.04 | 0.78 ± 0.05 | ||||
| Pancreas | 0.03 ± 0.01 | 0.06 ± 0.02 | ||||
| Muscle | 0.02 ± 0.00 | 0.04 ± 0.01 | 0.08 ± 0.02 | 0.08 ± 0.002 | ||
| Thyroid | 0.02 ± 0.01 | 0.12 ± 0.11 | ||||
| Gallbladder | 0.01 to 3.6 | 1.5 to 3.5 | ||||
| Urine | 15 to 29 | 13 to 182 | ||||
3. Experimental
3.1. General
3.2. Cell Lines
3.2.1. Cell Culture
3.2.2. Cell Uptake
3.3. Animals
3.3.1. In Vivo PET Scan
3.3.2. Ex Vivo Biodistribution Studies of [18F]FHBG and of HHB-5-[18F]FEP
3.4. Procedures for the Preparation of Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Rosé, C.; Dose, J.; Avril, N. Positron emission tomography for the diagnosis of breast cancer. Nucl. Med. Commun. 2002, 23, 613–618. [Google Scholar] [CrossRef]
- Vasselle, H.; Grierson, J.; Muzi, M.; Pugsley, J.M.; Schmidt, R.A.; Rabinowitz, P.; Peterson, L.M.; Vallie`res, E.; Wood, D.E. In vivo validation of 3'-deoxy-3'-[18F]fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: co-relation of 18F-FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin. Cancer Res. 2002, 8, 3315–3323. [Google Scholar]
- Soghomonyan, S.; Hajitou, A.; Rangel, R.; Trepel, M.; Pasqualini, R.; Arap, W.; Gelovani, J.G.; Alauddin, M.M. Molecular PET imaging of HSV1-tk reporter gene expression using [18F]FEAU. Nat. Protoc. 2007, 2, 416–423. [Google Scholar] [CrossRef]
- Yeh, H.H.; Ogawa, K.; Balatoni, J.; Mukhapadhyay, U.; Pal, A.; Gonzales-Lepera, C.; Shavrina, A.; Soghomonyana, S.; Flores II, L.; Younga, D.; et al. Molecular imaging of active mutant L858R EGFR kinase expressing non small cell lung carcinomas using PET/CT with [18F]F-PEG6-IPQA. PNAS 2002, 108, 1603–1608. [Google Scholar]
- Kostakoglu, L.; Goldsmith, S.J. [18F]-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J. Nucl. Med. 2003, 44, 224–239. [Google Scholar]
- Paolillo, V.; Yeh, H.H.; Mukhopadhyay, U.; Gelovani, J.G.; Alauddin, M.M. Improved detection and measurement of low levels of [18F]fluoride metabolized from [18F]-labeled pyrimidine nucleoside analogues in biological samples. Nucl. Med. Biol. 2011, 38, 1129–1134. [Google Scholar] [CrossRef]
- Brader, P.; Wong, R.J.; Horowitz, G.; Gil, Z. Combination of PET imaging with viral vectors for identification of cancer metastases. Adv. Drug Deliv. Rev. 2012, 64, 749–755. [Google Scholar] [CrossRef]
- Alauddin, M.M.; Conti, P.S.; Fissekis, J.D. Synthesis of [18F]-labeled 2'-deoxy-2'-fluoro-5-methyl-1-b-D-arabinofuranosyluracil) [18F]-FMAU. J. Labelled Compd. Radiopharm. 2002, 45, 583–590. [Google Scholar] [CrossRef]
- Mangner, T.J.; Klecker, R.W.; Anderson, L.; Shields, A.F. Synthesis of 2'-deoxy-2'-[18F]fluoro-beta-D-arabinofuranosyl nucleosides, [18F]FAU, [18F]FMAU, [18F]FBAU and[18F]FIAU, as potential PET agents for imaging cellular proliferation. Synthesis of [18F]labelled FAU, FMAU, FBAU, FIAU. Nucl. Med. Biol. 2003, 30, 215–224. [Google Scholar] [CrossRef]
- Buursma, A.R.; Rutgers, V.; Hospers, G.A.; Mulder, N.H.; Vaalburg, W.; de Vries, E.F.J. 18FFEAU as a radiotracer for herpes simplex virus thymidine kinase gene expression: in vitro comparison with other PET tracers. Nucl. Med. Commun. 2006, 27, 25–30. [Google Scholar] [CrossRef]
- Yaghoubi, S.S.; Couto, M.A.; Chen, C.C.; Polavaram, L.; Cui, G.; Sen, L.; Gambhir, S.S. Preclinical safety evaluation of 18F-FHBG: a PET reporter probe for imaging herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk’s expression. J. Nucl. Med. 2006, 47, 706–715. [Google Scholar]
- Brust, P.; Haubner, R.; Friedrich, A.; Scheunemann, M.; Anton, M.; Koufaki, O.N.; Hauses, M.; Noll, S.; Noll, B.; Haberkorn, U.; et al. Comparison of [18F]FHPG and [124/125I]FIAU for imaging herpes simplex virus type1 thymidine kinase gene expression. Eur. J. Nucl. Med. 2001, 28, 721–729. [Google Scholar] [CrossRef]
- Alauddin, M.M.; Shahinian, A.; Park, R.; Tohme, M.; Fissekis, J.D.; Conti, P.S. In vivo evaluation of 2'-deoxy-2'-[(18)F]fluoro-5-iodo-1-beta-D-arabinofuranosyluracil ([18F]FIAU) and 2'-deoxy-2'-[18F]fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil ([18F]FEAU) as markers for suicide gene expression. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 822–829. [Google Scholar] [CrossRef]
- Miyagawa, T.; Gogiberidze, G.; Serganova, I.; Cai, S.; Balatoni, J.A.; Thaler, H.T.; Ageyeva, L.; Pillarsetty, N.; Finn, R.D.; Blasberg, R.G. Imaging of HSV-tk Reporter gene expression: comparison between[18F]FEAU, [18F]FFEAU, and other imaging probes. J. Nucl. Med. 2008, 49, 637–648. [Google Scholar] [CrossRef]
- Gambhir, S.S.; Herschman, H.R.; Cherry, S.R.; Barrio, J.R.; Satyamurthy, N.; Toyokuni, T.; Phelps, M.E.; Larson, S.M.; Balatoni, J.; Finn, R.; et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia 2000, 2, 118–138. [Google Scholar] [CrossRef]
- De Clercq, E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004, 2, 704–720. [Google Scholar] [CrossRef]
- Huang, H.-L; Chiang, L.-W.; Chen, J.-R.; Yang, W.K.; Jeng, K.-C.; Chen, J.-T.; Duh, T.-S.; Lin, W.-J.; Farn, S.-S.; Chiang, C.-S.; et al. Study of [18F]FLT and [123I]IaraU for cellular imaging in HSV1 tk-transfected murine fibrosarcoma cells: evaluation of the tracer uptake using 5-fluoro, 5-iodo and 5-iodovinyl arabinosyl uridines as competitive probes. Nucl. Med. Biol. 2012, 39, 371–376. [Google Scholar] [CrossRef]
- Yaghoubi, S.S.; Jensen, M.C.; Satyamurthy, N.; Budhiraja, S.; Paik, D.; Czernin, J.; Gambhir, S.S. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat. Clin. Pract. Oncol. 2009, 6, 53–58. [Google Scholar] [CrossRef]
- Yaghoubi, S.S.; Gambhir, S.S. PET imaging of herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk reporter gene expression in mice and humans using [18F]FHBG. Nat. Protoc. 2006, 1, 3069–3074. [Google Scholar] [CrossRef]
- Tjuvajev, J.G.; Doubrovin, M.; Akhurst, T.; Cai, S.; Balatoni, J.; Alauddin, M.M.; Finn, R.; Bornmann, W.; Thaler, H.; Conti, P.S.; et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J. Nucl. Med. 2002, 43, 1072–1083. [Google Scholar]
- Yaghoubi, S.; Barrio, J.R.; Dahlbom, M.; Iyer, M.; Namavari, M.; Satyamurthy, N.; Goldman, R.; Herschman, H.R.; Phelps, M.E.; Gambhir, S.S. Human pharmacokinetic and dosimetry studies of [18F]FHBG: a reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J. Nucl. Med. 2001, 42, 1225–1234. [Google Scholar]
- Raić-Malić, S.; Johayem, A.; Ametamey, S.M.; Batinac, S.; De Clercq, E.; Folkers, G.; Scapozza, L. Synthesis, 18F-radiolabelling and biological evaluations of C-6 alkylated pyrimidine nucleoside analogues. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1707–1721. [Google Scholar] [CrossRef]
- Johayem, A.; Raić-Malić, S.; Lazzati, K.; Schubiger, P.A.; Scapozza, L.; Ametamey, S.M. Synthesis and characterization of a C(6) nucleoside analogue for the in vivo imaging of the gene expression of herpes simplex virus type-1 thymidine kinase (HSV1 TK). Chem. Biodiv. 2006, 3, 274–283. [Google Scholar] [CrossRef]
- Krištafor, S.; Novaković, I.; Gazivoda Kraljević, T.; Kraljević Pavelić, S.; Lučin, P.; Westermaier, Y.; Pernot, L.; Scapozza, L.; Ametamey, S.M.; Raić-Malić, S. Synthetic Approach to New N-methyl Thymine Derivative Comprising Dihydroxyisobutenyl Unit as Ligand for Thymidine Kinase of Herpes Simplex Virus Type 1 (HSV1-TK). Bioorg. Med. Chem. Lett. 2011, 21, 6161–6165. [Google Scholar] [CrossRef]
- Müller, U.; Martić, M.; Gazivoda Kraljević, T.; Krištafor, S.; Ross, T.L.; Ranadheera, C.; Müller, A.; Born, M.; Krämer, S.D.; Raić-Malić, S.; et al. Synthesis and evaluation of a C-6 alkylated pyrimidine derivative for the in vivo imaging of HSV1-TK gene expression. Nucl. Med. Biol. 2012, 39, 235–246. [Google Scholar] [CrossRef]
- Meščić, A.; Krištafor, S.; Novaković, I.; Osmanović, A.; Müller, U.; Završnik, D.; Ametamey, S.M.; Scapozza, L.; Raić-Malić, S. C-5 Hydroxyethyl and Hydroxypropyl Acyclonucleosides as Substrates for Thymidine Kinase of Herpes Simplex Virus Type 1 (HSV-1 TK): Syntheses and Biological Evaluation. Molecules 2013, 18, 5104–5124. [Google Scholar] [CrossRef]
- Meščić, A.; Glavač, D.; Osmanović, A.; Završnik, D.; Cetina, M.; Makuc, D.; Plavec, J.; Ametamey, S.M.; Raić-Malić, S. N -alkylated and O -alkylated regioisomers of 5-(hydroxyalkyl)pyrimidines: Synthesis and structural study. J. Mol. Struct. 2013, 1039, 160–166. [Google Scholar] [CrossRef]
- Müller, U.; Ross, T.L; Ranadheera, C.; Slavik, R.; Müller, A.; Born, M.; Trauffer, E.; Miličević Sephton, S.; Scapozza, L.; Krämer, S.D.; Ametamey, S.M. Synthesis and preclinical evaluation of a new C-6 alkylated pyrimidine derivative as a PET imaging agent for HSV1-tk gene expression. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 71–84. [Google Scholar]
- Yaghoubi, S.S; Gambhir, S.S. Measuring herpes simplex virus thymidine kinase reporter gene expression in vitro. Nat. Protoc. 2006, 1, 2137–2142. [Google Scholar] [CrossRef]
- Honer, M.; Brühlmeier, M.; Missimer, J.; Schubiger, A.P.; Ametamey, S.M. Dynamic imaging of striatal D2 receptors in mice using quad-HIDAC PET. J. Nucl. Med. 2004, 45, 464–470. [Google Scholar]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meščić, A.; Betzel, T.; Müller, A.; Slavik, R.; Čermak, S.; Raić-Malić, S.; Ametamey, S.M. Synthesis and Biological Evaluation of a New Acyclic Pyrimidine Derivative as a Probe for Imaging Herpes Simplex Virus Type 1 Thymidine Kinase Gene Expression. Molecules 2013, 18, 8535-8549. https://doi.org/10.3390/molecules18078535
Meščić A, Betzel T, Müller A, Slavik R, Čermak S, Raić-Malić S, Ametamey SM. Synthesis and Biological Evaluation of a New Acyclic Pyrimidine Derivative as a Probe for Imaging Herpes Simplex Virus Type 1 Thymidine Kinase Gene Expression. Molecules. 2013; 18(7):8535-8549. https://doi.org/10.3390/molecules18078535
Chicago/Turabian StyleMeščić, Andrijana, Thomas Betzel, Adrienne Müller, Roger Slavik, Stjepko Čermak, Silvana Raić-Malić, and Simon M. Ametamey. 2013. "Synthesis and Biological Evaluation of a New Acyclic Pyrimidine Derivative as a Probe for Imaging Herpes Simplex Virus Type 1 Thymidine Kinase Gene Expression" Molecules 18, no. 7: 8535-8549. https://doi.org/10.3390/molecules18078535




