Characterization of Water Extractable Arabinoxylans from a Spring Wheat Flour: Rheological Properties and Microstructure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Characterization of WEAX
| Arabinose a | 23.50 ± 1.1 |
| Xylose a | 35.30 ± 0.4 |
| Glucose a | 4.80 ± 0.4 |
| Protein a | 4.20 ± 0.01 |
| Ferulic acid b | 0.526 ± 0.001 |
| Diferulic acids b | 0.036 ± 0.001 |
| Triferulic acid b | traces |
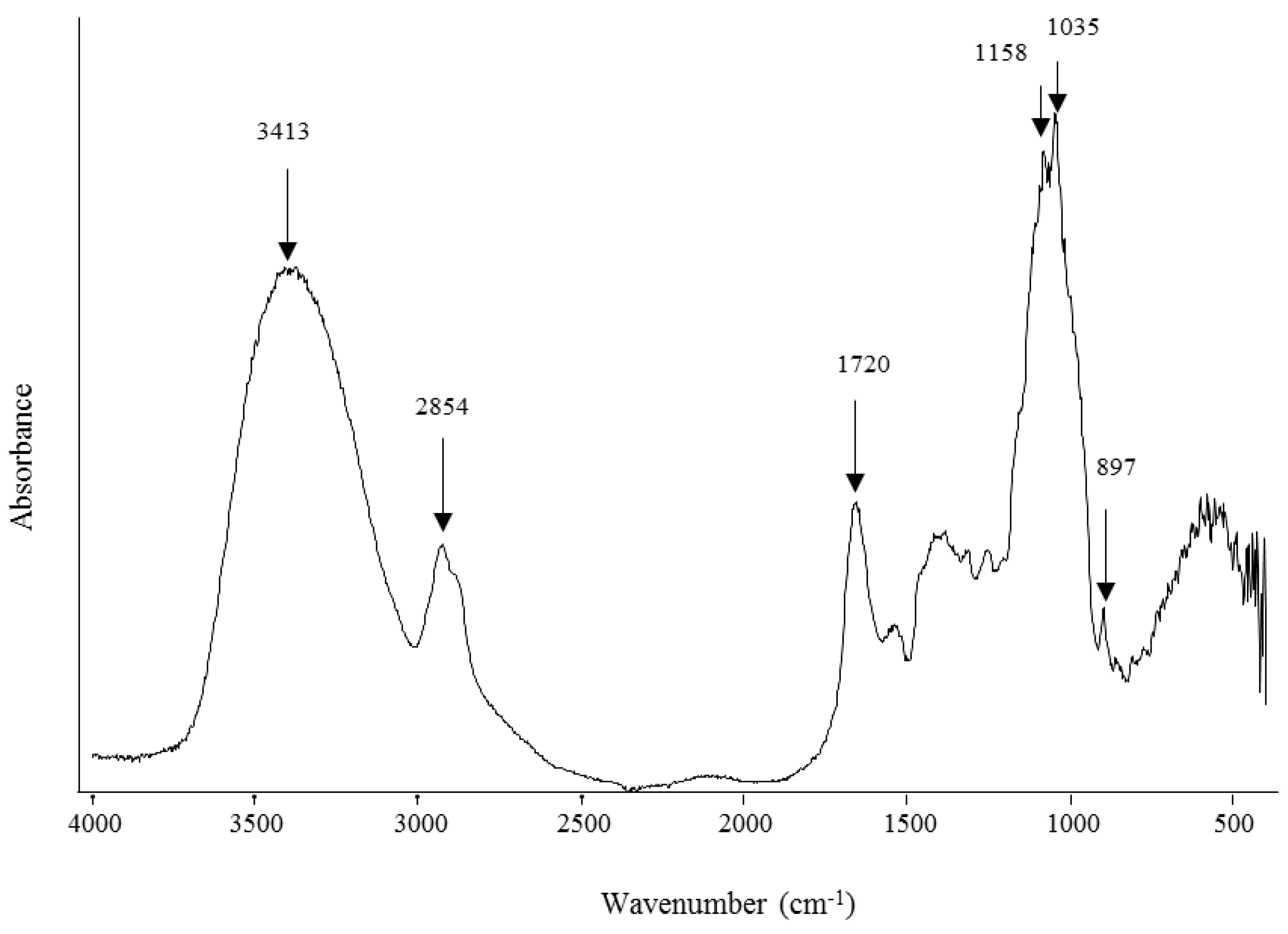
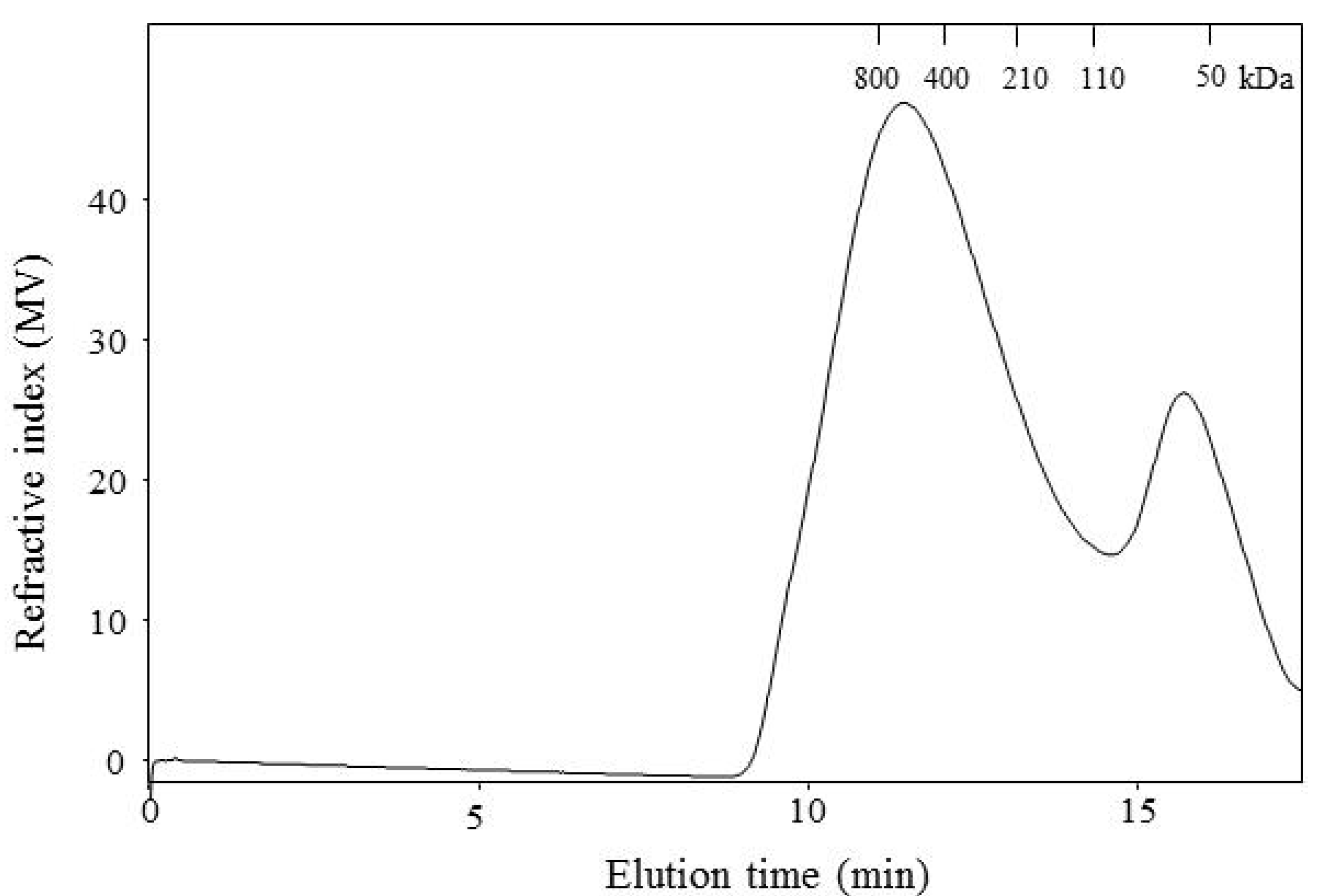
2.2. WEAX Gelation
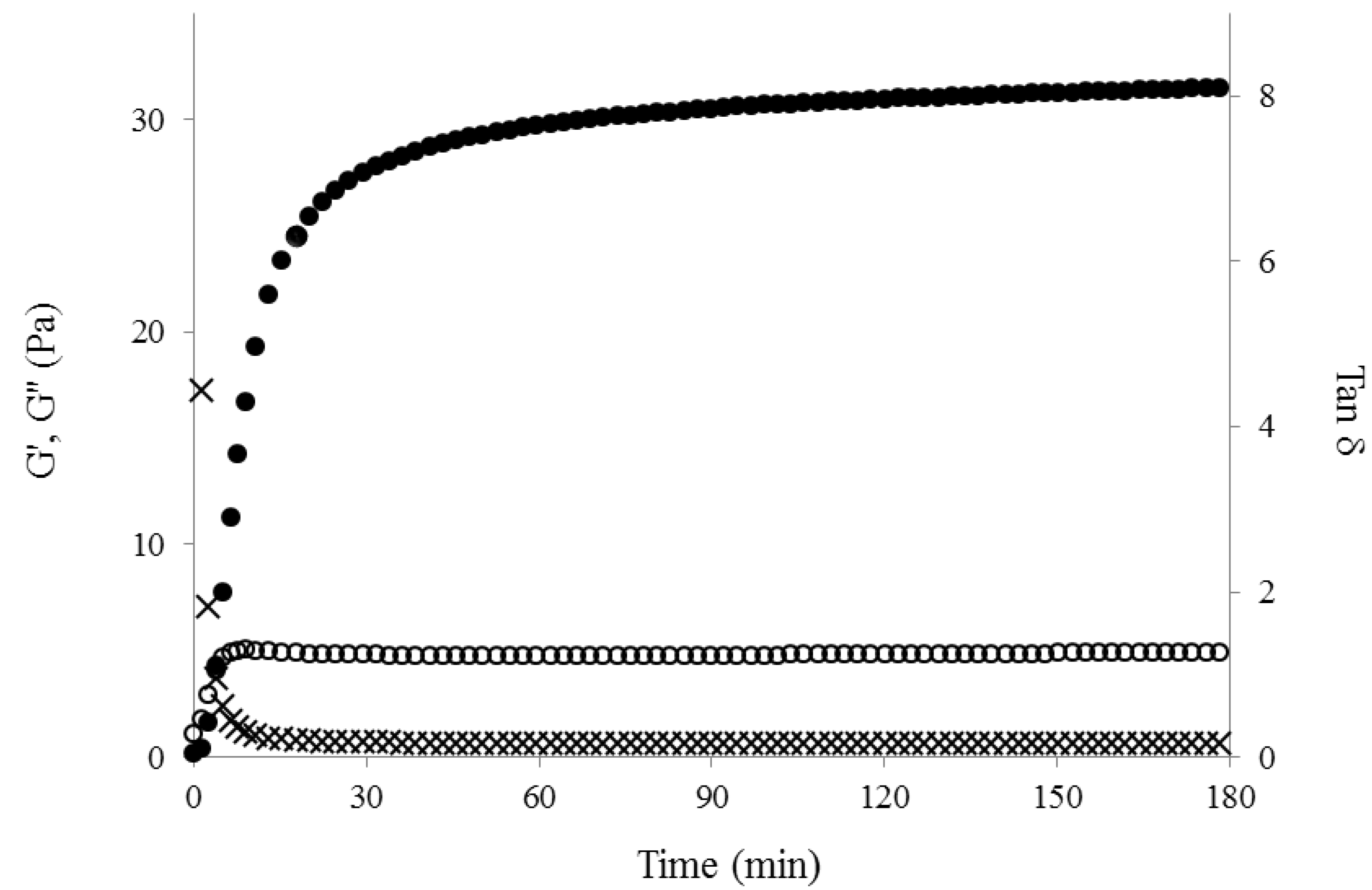

2.3. Freeze Dried WEAX Gel
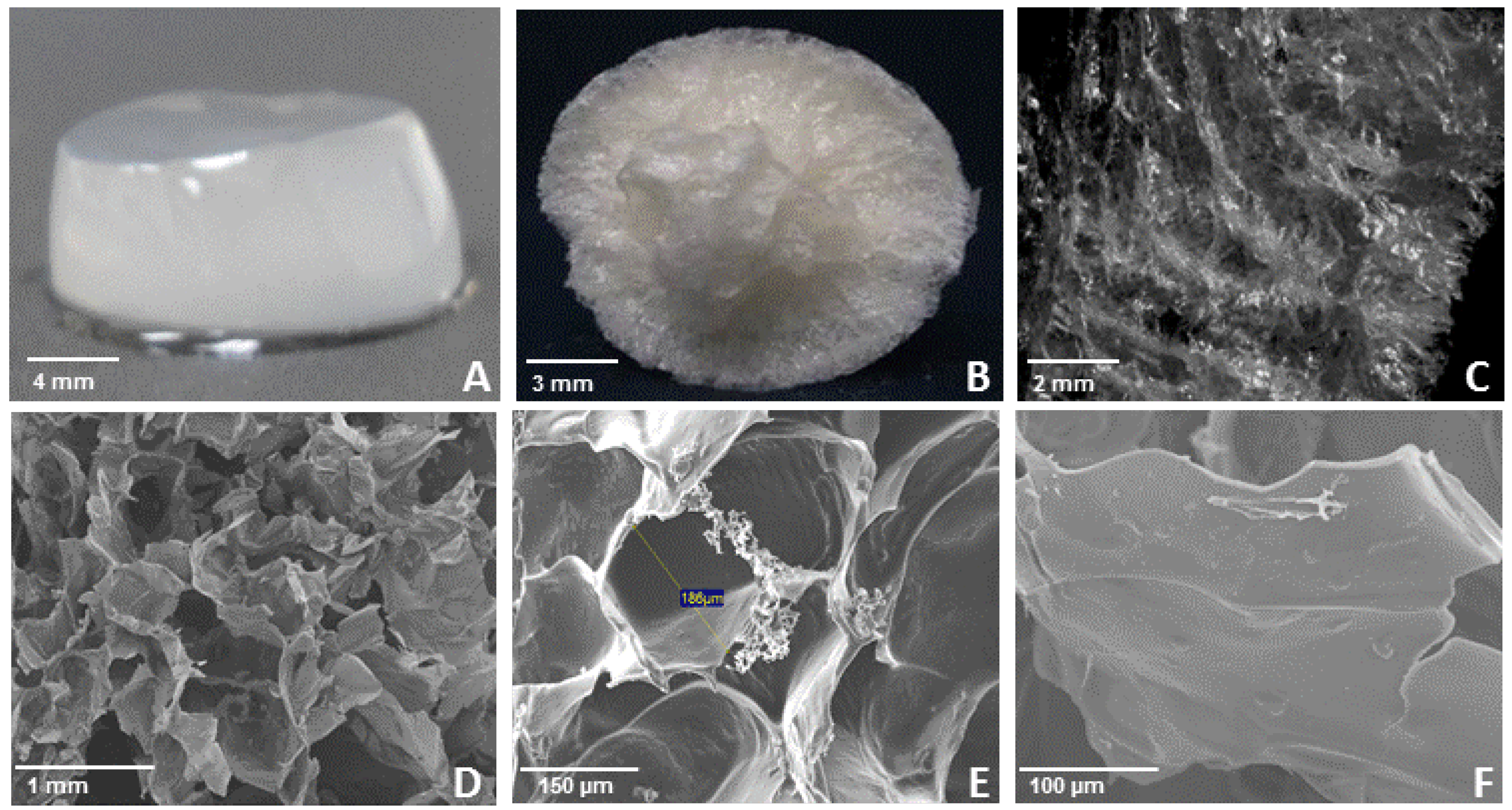
3. Experimental
3.1. Materials
3.2. Isolation of WEAX
3.3. Chemical and Physicochemical Analyses
3.3.1. Laccase Activity
3.3.2. Neutral Sugar
3.3.3. Phenolic Acids
3.3.4. Protein Content
3.3.5. Intrinsic Viscosity and Viscosimetric Molecular Weight
3.3.6. Molecular Weight Distribution
3.3.7. Fourier Transform Infra-Red (FT-IR) Spectroscopy
3.4. WEAX Gelation
3.5. Rheological Tests
3.6. Structure of Freeze Dried WEAX Gels
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Li, S.; Morris, C.F.; Bettge, A.D. Genotype and environment variation for arabinoxylans in hard winter and spring wheats of the U.S. Pacific Northwest. Cereal Chem. 2009, 86, 88–95. [Google Scholar] [CrossRef]
- Camacho Casas, M.A.; Singh, R.P.; Figueroa López, P.; Huerta Espino, J.; Fuentes Dávila, G.; Ortiz-Monasterio Rosas, I. Tacupeto F2001 Nueva Variedad de Trigo Harinero Para el Noroeste de México. Folleto Técnico No. 50; Talleres Gráficos de CIRNO: Obregón, México, 2003; pp. 1–20. [Google Scholar]
- Li, Y.; Wua, Y.; Hernandez-Espinosa, N.; Peña, R.J. The influence of drought and heat stress on the expression of end-use quality parameters of common wheat. J. Cereal Sci. 2013, 57, 73–78. [Google Scholar] [CrossRef]
- Singh, R.P. Pros and Cons of Utilizing Major, Race-specific Resistance Genes versus Partial Resistance in Breeding Rust Resistant Wheat. In Proceedings of the BGRI Technical Workshop, Beijing, China, 1–4 September 2012; Name ID: 2418. Bakum, J., Ed.;
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Rascon-Chu, A.; Márquez-Escalante, J.A.; Guerrero, V.; Salas-Muñoz, E. Feruloylated arabinoxylans and arabinoxylan gels: Structure, sources and applications. Phytochem. Rev. 2010, 9, 111–120. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Rouau, X. Oxidative crosslinking of pentosans by a fungal laccase and horseradish peroxidase: Mechanism of linkage between feruloylated arabinoxylans. Cereal Chem. 1998, 75, 259–265. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guigliarelli, B.; Belle, V.; Rouau, X.; Micard, V. Storage stability of arabinoxylan gels. Carbohydr. Polym. 2005, 59, 181–188. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: Impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Biliaderis, C.G.; Bushuk, W. Comparison of the structure and composition of water-soluble pentosans from different wheat varieties. Cereal Chem. 1991, 68, 139–144. [Google Scholar]
- Saulnier, L.; Sado, P.E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Dervilly, G.; Saulnier, L.; Roger, P.; Thibault, J.F. Isolation of homogeneous fractions of wheat water-soluble arabinoxylan. Influence of structure on their macromolecular characteristics. J. Agric. Food Chem. 2000, 48, 270–278. [Google Scholar] [CrossRef]
- Fincher, G.B.; Stone, B.A. A water–soluble arabinogalactan-peptide from wheat endosperm. Aust. J. Biol. Sci. 1974, 27, 117–132. [Google Scholar]
- Dervilly-Pinel, G.; Rimsten, L.; Saulnier, L.; Andersson, R.; Aman, P. Water-extractable arabinoxylan from pearled flours of wheat, barley, rye and triticale. Evidence for the presence of ferulic acid dimmers and their involvement in gel formation. J. Cereal Sci. 2001, 34, 207–214. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Oxidative gelation studies of water-soluble pentosans from wheat. J. Cereal Sci. 1990, 11, 153–169. [Google Scholar] [CrossRef]
- Barron, C.; Rouau, X. FTIR and Raman signatures of wheat grain peripheral tissues. Cereal Chem. 2008, 85, 619–625. [Google Scholar] [CrossRef]
- Urias-Orona, V.; Huerta-Oros, J.; Carvajal-Millán, E.; Lizardi-Mendoza, J.; Rascón-Chu, A.; Gardea, A.A. Component analysis and free radicals scavenging activity of Cicer arietinum L. husk pectin. Molecules 2010, 15, 6948–6955. [Google Scholar] [CrossRef]
- Séné, C.F.B.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy. An investigation of five higher plant cell walls and their components. Plant Physiol. 1994, 106, 1623–1631. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Physical-properties of water-soluble pentosans from different wheat- varieties. Cereal Chem. 1991, 68, 145–150. [Google Scholar]
- Skendi, A.; Biliaderis, C.G.; Izydorczyk, M.S.; Zervou, M.; Zoumpoulakis, P. Structural variation and rheological properties of water-extractable arabinoxylans from six Greek wheat cultivars. Food Chem. 2011, 126, 526–536. [Google Scholar] [CrossRef]
- Doublier, J.L.; Cuvelier, G. Gums and Hydrocolloids: Functional Aspects. In Carbohydrates in Food; Eliasson, A.C., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 283–318. [Google Scholar]
- Ross-Murphy, S.B. Rheological Methods. In Biophysical Methods in Food Research; Chan, H.W.S., Ed.; Blackwell Scientific Publications: Oxford, UK, 1984; pp. 138–199. [Google Scholar]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; López-Franco, Y.L.; Rascón-Chu, A.; Salas-Muñoz, E.; Barron, C.; Micard, V. The peroxidase/H2O2 system as a free radical-generating agent for gelling maize bran arabinoxylans: Rheological and structural properties. Molecules 2011, 16, 8410–8418. [Google Scholar] [CrossRef] [Green Version]
- Paës, G.; Chabbert, B. Characterization of Arabinoxylan/cellulose nanocrystals gels to investigate fluorescent probes mobility in bioinspired models of plant secondary cell wall. Biomacromolecules 2012, 13, 206–214. [Google Scholar] [CrossRef]
- Iravani, S.; Fitchett, C.S.; Georget, D.M.R. Physical characterization of arabinoxylan powder and its hydrogel containing a methyl xanthine. Carbohydr. Polym. 2011, 85, 201–207. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Miki-Yoshida, M.; Alvarez-Contreras, L.; Rascón-Chu, A.; Lizardi-Mendoza, J.; López-Franco, Y. Arabinoxylan microspheres: Structural and textural characteristics. Molecules 2013, 18, 4640–4650. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.; Martínez-Robinson, K. Gels of ferulated arabinoxylans extracted from nixtamalized and non-nixtamalized maize bran: Rheological and structural characteristics. CyTA-J Food 2013, 11, 22–28. [Google Scholar] [CrossRef]
- Marquez-Escalante, J.; Carvajal-Millan, E.; Miki-Yoshida, M.; Alvarez-Contreras, L.; Toledo-Guillén, A.R.; Lizardi-Mendoza, J.; Rascón-Chu, A. Water extractable arabinoxylan aerogels prepared by supercritical CO2 drying. Molecules 2013, 18, 5531–5542. [Google Scholar] [CrossRef]
- AACC, Approved Methods of the American Association of Cereal Chemists, 10th ed.AACC: St. Paul, MN, USA, 2000.
- Rouau, X.; Chaynier, V.; Surget, A.; Gloux, D.; Barron, C.; Meudeu, E.; Montero, J.L.; Criton, M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. [Google Scholar] [CrossRef]
- AOAC, Official Method of Analysis of AOAC Intl. Method 991.43, 16th ed.; Association of Official Analytical Communities: Arlington, TX, USA, 1995.
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morales-Ortega, A.; Carvajal-Millan, E.; López-Franco, Y.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Torres-Chavez, P.; Campa-Mada, A. Characterization of Water Extractable Arabinoxylans from a Spring Wheat Flour: Rheological Properties and Microstructure. Molecules 2013, 18, 8417-8428. https://doi.org/10.3390/molecules18078417
Morales-Ortega A, Carvajal-Millan E, López-Franco Y, Rascón-Chu A, Lizardi-Mendoza J, Torres-Chavez P, Campa-Mada A. Characterization of Water Extractable Arabinoxylans from a Spring Wheat Flour: Rheological Properties and Microstructure. Molecules. 2013; 18(7):8417-8428. https://doi.org/10.3390/molecules18078417
Chicago/Turabian StyleMorales-Ortega, Adriana, Elizabeth Carvajal-Millan, Yolanda López-Franco, Agustín Rascón-Chu, Jaime Lizardi-Mendoza, Patricia Torres-Chavez, and Alma Campa-Mada. 2013. "Characterization of Water Extractable Arabinoxylans from a Spring Wheat Flour: Rheological Properties and Microstructure" Molecules 18, no. 7: 8417-8428. https://doi.org/10.3390/molecules18078417





