Synthesis of New Cytotoxic Aminoanthraquinone Derivatives via Nucleophilic Substitution Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reduction, Methylation and Acylation

| Entry | NaBH4 (equiv.) | Reaction Time | Product (% Yield) |
|---|---|---|---|
| I | 1 | 30 min | 2 (69), 3 (21) |
| II | 3 | 30 min | 3 (90) |
| III | 15 | 30 min | 3 (60) |
| IV | - | 3 h | 4 (5), 5 (85) |
| V | - | 4 h | 5 (96) |
| VI | - | 2 h | 6 (26), 7 (60) |
| VII | - | 3 h | 6 (15), 7 (75) |
| VII | - | 9 h | 7 (90) |
2.2. Amination
| Entry | BuNH2 (equiv.) | PhI(OAc)2 (equiv.) | Temperature | Product (% Yield) |
|---|---|---|---|---|
| I | 450 | 1.1 | RT | 1a (40), 1b (38) |
| II | 225 | 1.1 | RT | 1a (90) |
| III | 225 | - | RT | 1a (70) |
| IV | 225 | - | 80 °C | 1a (70), 1b (15) |
| V | 225 | 1.1 | RT | 3a (60) |
| VI | 225 | - | RT | 3a (51) |
| VII | 112 | 1.1 | RT | 3a (46) |
| VIII | 225 | 1.1 | RT | 5a (7), 5b (10), 5 (78) |
| IX | 225 | 1.1 | 80 °C | 5c (10), 5d (73) |
| X | 225 | 1.1 | RT | 1a (83) |
| XI | 225 | - | RT | 1a (55) |
| XII | 225 | - | 80 °C | 1a (46) |

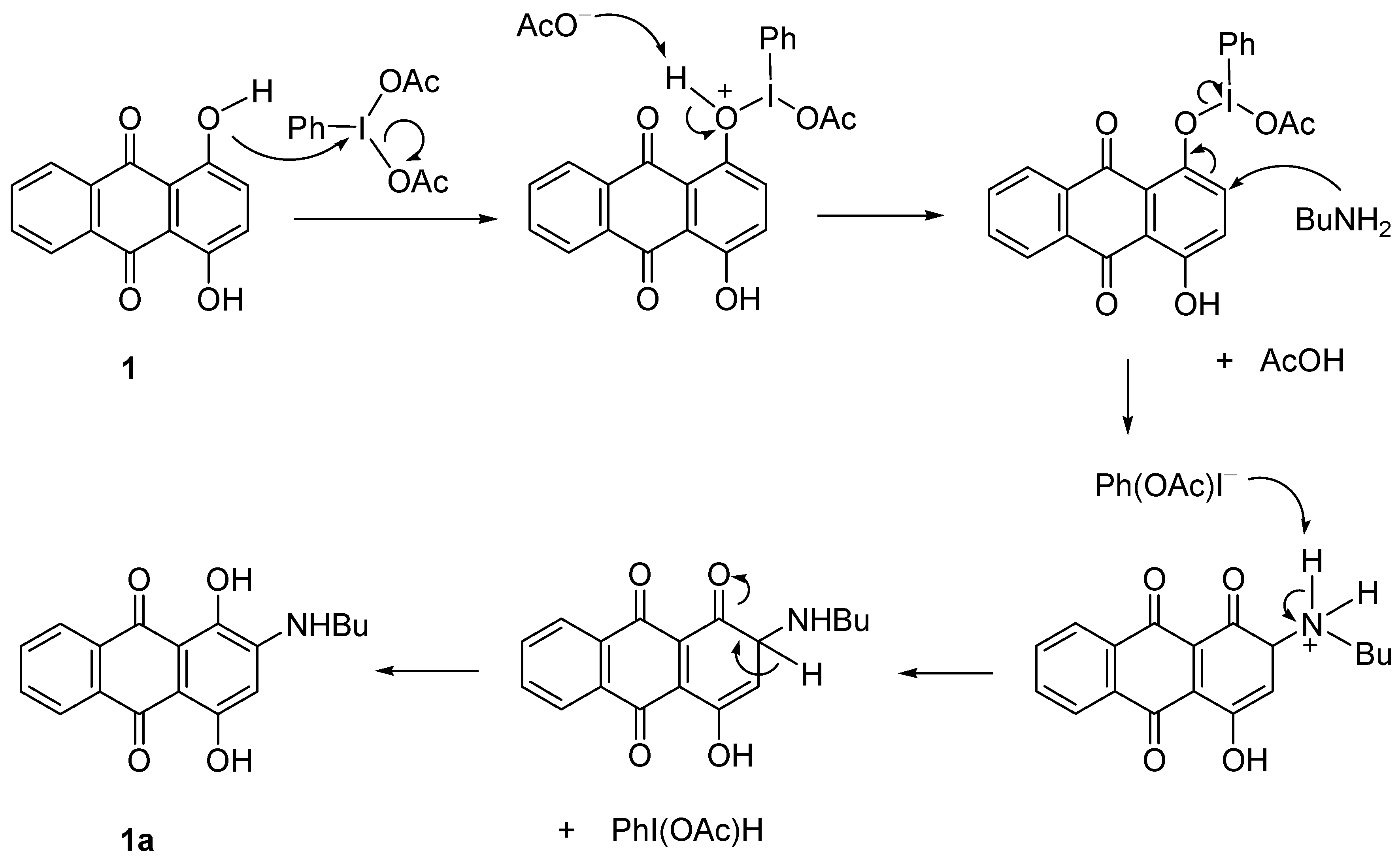
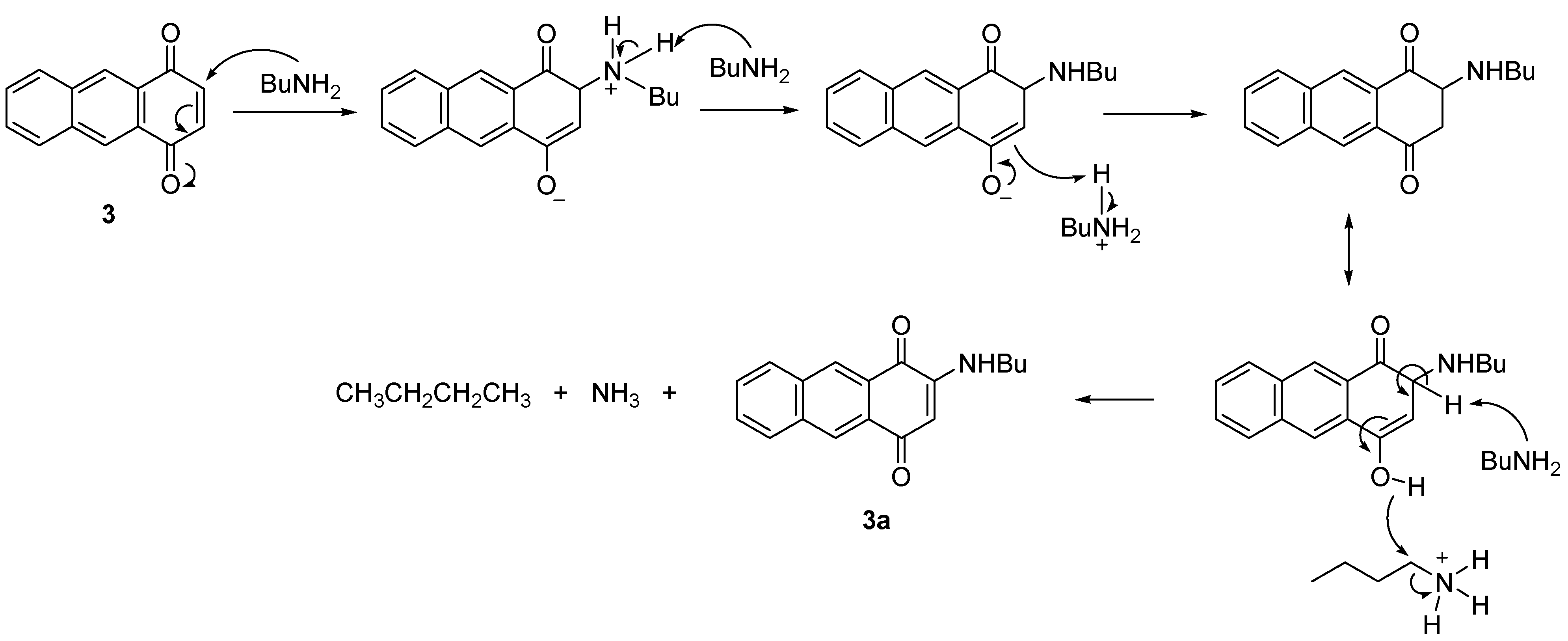
2.2.1. Amination of 3



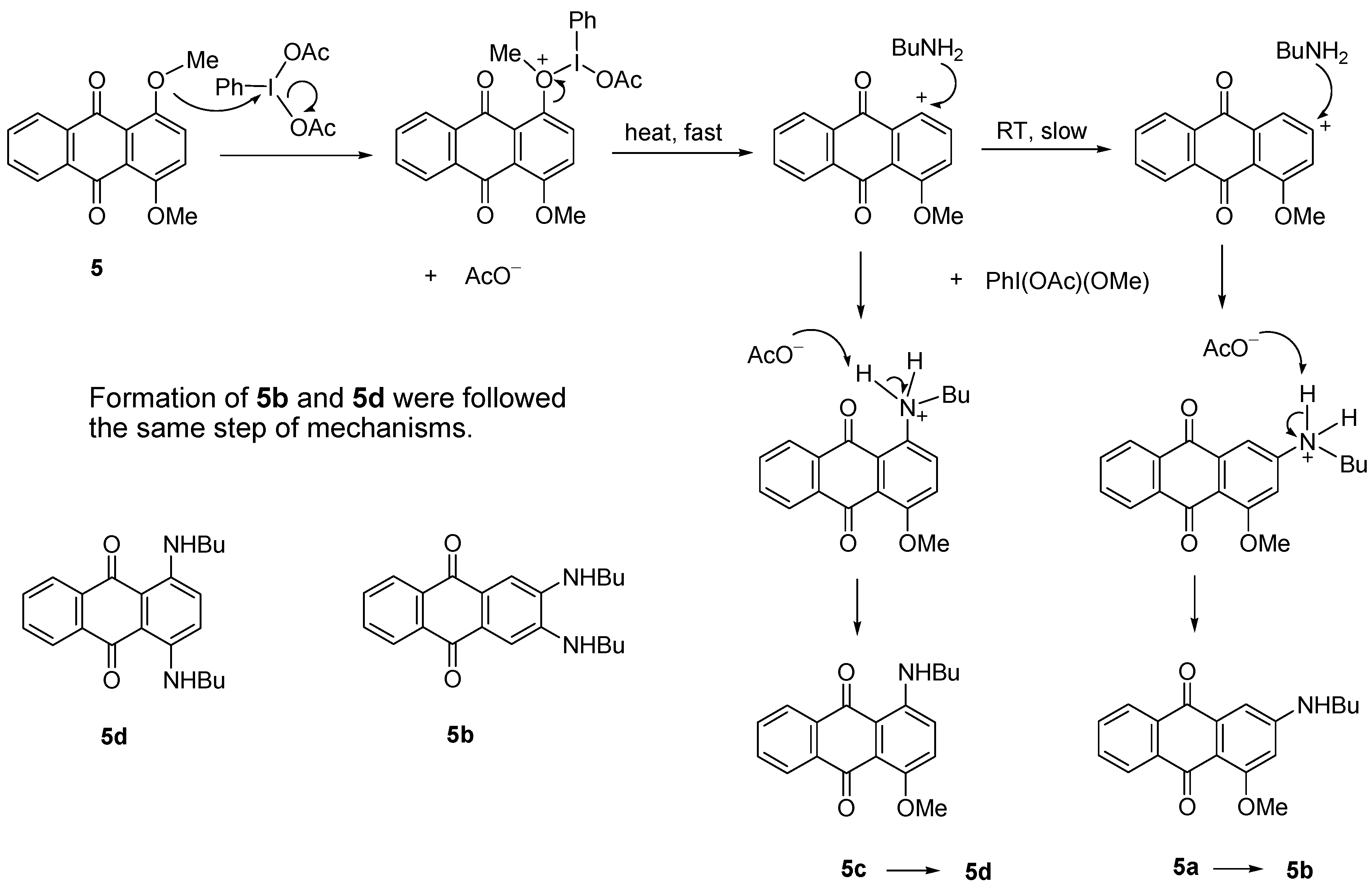
2.2.2. Amination of 5
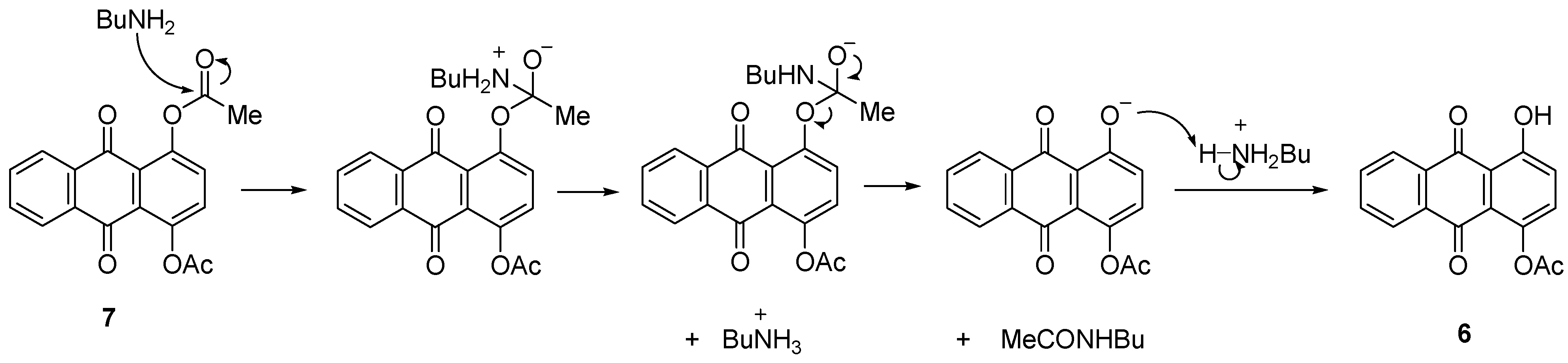
2.2.3. Amination of 7
| Sample | Cytotoxic Activity IC50 (µg/mL) | |
|---|---|---|
| MCF-7 | Hep-G2 | |
| 3a | 1.1 | 1.2 |
| 5a | 1.1 | 3.0 |
| 5b | 3.0 | 13.0 |
3. Experimental
3.1. General
3.2. Reduction
3.3. Methylation
3.4. Acylation
3.5. Amination—General Procedure
3.6. Cytotoxic Assays
3.7. Antimicrobial Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ashnagar, A.; Bruce, M.; Dutton, L.; Prince, R.C. One- and two-electron reduction of hydroxy-1,4-naphthoquinones and hydroxy-9,10-anthraquinones: The role of internal hydrogen bonding and its bearing on the redox chemistry of the anthracycline antitumour quinones. BBA-Gen Subjects 1984, 801, 351–359. [Google Scholar] [CrossRef]
- Ge, P.; Russel, R.A. The synthesis of anthraquinone derivatives as potential anticancer agent. Tetrahedron 1997, 53, 17469–17476. [Google Scholar] [CrossRef]
- Hua, D.H.; Lou, K.; Havens, J.; Perchellet, E.M.; Wang, Y.; Perchellet, J.P.; Iwamotoc, T. Synthesis and in vitro antitumor activity of substituted anthracene-1,4-diones. Tetrahedron 2004, 60, 10155–10163. [Google Scholar] [CrossRef]
- Chang, P.; Chen, C. Isolation and characterisation of antitumour anthraquinones from Morinda umbellata. Chin. Pharm. J. (Taipei) 1995, 47, 347–353. [Google Scholar]
- Yadav, J.P.; Arya, V.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia 2010, 81, 223–230. [Google Scholar] [CrossRef]
- Osman, C.P.; Ismail, N.H.; Ahmad, R.; Ahmat, N.; Awang, K.; Jaafar, F.M. Anthraquinones with antiplasmodial activity from the roots of Rennellia elliptica Korth. (Rubiaceae). Molecules 2010, 15, 7218–7226. [Google Scholar] [CrossRef]
- Sittie, A.A.; Lemmich, E.; Olsen, C.E.; Hviid, L.; Kharazmi, A.; Nkrumah, F.K.; Christensen, S.B. Structure-Activity studies: In vitro antileishmanial and antimalarial activities of anthranones from Morinda lucida. Planta Med. 1999, 65, 259–261. [Google Scholar] [CrossRef]
- Xiang, W.; Song, Q.S.; Zhang, H.J.; Guo, S.P. Antimicrobial anthraquinones from Morinda angustifolia. Fitoterapia 2008, 79, 501–504. [Google Scholar] [CrossRef]
- Rath, G.; Ndonzao, M.; Hostettmann, K. Antifungal anthraquinones from Morinda lucida. Int. J. Pharmacogn. 1995, 33, 107–114. [Google Scholar] [CrossRef]
- Chang, P.; Lee, K.H. Cytotoxic antileukemic anthraquinones from Morinda parvifolia. Phytochemistry 1984, 23, 1733–1736. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Lajis, N.H. Anthraquinone from Morinda ellintica. Phytochem. 1997, 45, 1723–1725. [Google Scholar] [CrossRef]
- Schinazi, R.F.; Chu, C.K.; Babu, J.R.; Oswald, B.J.; Saalmann, V.; Cannon, D.L.; Eriksson, B.F. H.; Nasr, M. Anthraquinones as a new class of antiviral agents against human immunodeficiency virus. Antiviral Res. 1990, 13, 265–272. [Google Scholar] [CrossRef]
- Alves, D.S.; Pérez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef]
- Andersen, D.O.; weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinones derivatives. Antiviral Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Barnard, D.L.; Huffman, J.H.; Morris, J.L.; Wood, S.G.; Hughes, B.G.; Sidwell, R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinones derivatives against human cytomegalovirus. Antiviral Res. 1992, 17, 63–77. [Google Scholar]
- Yen, G.C.; Duh, P.D.; Chuang, D.Y. Antioxidant activity of anthraquinones and anthrones. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Sokolyuk, N.T.; V.V. Romanov, L.P.; Pisulina, L.P. Naphthacenequinones: Synthesis and properties. Russ. Chem. Rev. 1993, 62, 1005–1024. [Google Scholar] [CrossRef]
- Bechtold, T.; Burtscher, E.; Turcanu, A. Anthraquinones as mediators for the indirect cathodic reduction of dispersed organic dyestuffs. J. Electroanal Chem. 1999, 465, 80–87. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, J.; Wang, D.; Tian, C.; Wang, P.; Salah Uddin, M.; Yu, H. Biocatalyst effects of immobilised anthraquinone on the anaerobic reduction of azo dyes by the salt-tolerant bacteria. Water Res. 2007, 41, 426–432. [Google Scholar] [CrossRef]
- Ahn, K.D.; Yoo, K.W.; Soh, J.H.; Kang, J.H. Fluorescent photoimaging with polymers having protected quinizarin dye precursors by a dry process based on chemical amplification. React. Funct. Polym. 2009, 69, 111–116. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Glazunova, V.A.; Dezhenkova, L.G.; Shevtsova, E.K.; Traven, V.F.; Balzarini, J.; Huang, H.S.; Shtil, A.A.; Preobrazhenskaya, M.N. The first series of 4,11-bis[(2-aminoethyl)amino]anthra[2,3-b]furan-5,10-diones: Synthesis and anti-proliferative characteristics. Eur. J. Med. Chem. 2011, 46, 423–428. [Google Scholar] [CrossRef]
- Lee, C.C.; Huang, K.F.; Lin, P.Y.; Huang, F.C.; Chen, C.L.; Chen, T.C.; Guh, J.H.; Lin, J.J.; Huang, H.S. Synthesis, antiproliferative activities and telomerase inhibition evaluation of novel asymmetrical 1,2-disubstituted amidoanthraquinone derivatives. Eur. J. Med. Chem. 2012, 47, 323–336. [Google Scholar] [CrossRef]
- Jin, G.Z.; Song, G.Y.; Zheng, X.G.; Kim, Y.; Sok, D.E.; Ahn, B.Z. 2-(1 -Oxyalkyl)-1,4-dioxy-9,10-anthraquinones: Synthesis and evaluation of antitumor activity. Arch. Pharm. Res. 1998, 21, 198–206. [Google Scholar] [CrossRef]
- Teich, L.; Daub, K.S.; Krugel, V.; Nissler, L.; Gebhardt, R.; and Eger, K. Synthesis and biological evaluation of new derivatives of emodin. Bioorg. Med. Chem. 2004, 12, 5961–5971. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Glazunova, V.A.; Luzikov, Y.N.; Buyanov, V.N.; Susova, O.Y.; Shtil, A.A.; Preobrazhenskaya, M.N. Synthesis and structure-activity relationship studies of 4,11-diaminophtho[2,3-f]indole-5,10-diones. Bioorg. Med. Chem. 2006, 14, 5241–5251. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Shtil, A.A.; Luzikov, Y.N.; Bobrysheva, T.V.; Buyanov, V.N.; Preobrazhenskaya, M.N. 3-Aminomethyl derivatives of 4,11-dihydroxynaphtho[2,3-f]indole-5,10-dione for circumvention of anticancer drug resistance. Bioorg. Med. Chem. 2005, 13, 2285–2291. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Shaw, K.J.; Landi, J.J., Jr.; Phinney, D.J. Synthesis of unsymmetrical 1,4-Bis[(aminoalkyl)amino]anthracene-9,10-diones for Antineoplastic Evaluation. J. Org. Chem. 1984, 49, 5253–5255. [Google Scholar]
- Glänzel, M.; Bültmann, R.; Starke, K.; Frahm, A.W. Structure-activity relationships of novel P2-receptor antagonists structurally related to reactive blue 2. Eur. J. Med. Chem. 2005, 40, 1262–1276. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Efficient and mild deamination procedure for 1-Aminoanthraquinones yielding a diverse library of novel derivatives with potential biological activity. Tetrahedron Lett. 2012, 53, 6739–6742. [Google Scholar] [CrossRef]
- Jin, G.Z.; Jin, H.S.; Jin, L.L. Synthesis and antiproliferative activity of 1,4-Bis(dimethylamino)-9,10-anthraquinone derivatives against P388 mouse leukemic tumor cells. Arch Pharm. Res. 2011, 34, 1071–1076. [Google Scholar] [CrossRef]
- Sugimoto, N.; Kawasaki, Y.; Sato, K.; Aoki, H.; Ichi, T.; Koda, T.; Yamazaki, T.; Maitani, T. Structure of Acid-Stable Carmine. J. Food Hyg. Soc.Jpn. 2002, 43, 18–23. [Google Scholar] [CrossRef]
- Zielske, A.G. (Tosyloxy)anthraquinones: Versatile synthones for the preparation of various aminoanthraquinones. J. Org. Chem. 1987, 52, 1305–1309. [Google Scholar]
- Camara, C.A.; Pinto, A.C.; Rosa, M.A.; Vargas, M.D. Secondary amine and unexpected 1-Aza-anthraquinone from 2-Methoxylapachol. Tetrahedron 2001, 57, 9569–9574. [Google Scholar]
- Saha, K.; Lam, K.W.; Abas, F.; Hamzah, A.S.; Stanslas, J.; Hui, L.S.; Lajis, N.H. Synthesis of damnacanthal, a naturally occurring 9,10-Anthraquinone and its analogues, and its biological evaluation against five cancer cell lines. Med. Chem. Res. 2013, 22, 2093–2104. [Google Scholar] [CrossRef]
- Sukari, M.A.; Tang, S.W.; Neoh, B.K.; Ee, G.C.L.; Rahmani, M. Antileukemic activity and chemical constituents of some Zingiberaceae species. Asian J. Chem. 2010, 22, 7891–7896. [Google Scholar]
- Garba, S.; Okenyi, S.O. Antimicrobial activities of total alkaloids extracted from some nigerian medicinal plants. J. Microbiol. Antimicrob. 2012, 4, 60–63. [Google Scholar]
- Sample Availability: Samples of the compounds 3a and 5d are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nor, S.M.M.; Sukari, M.A.H.M.; Azziz, S.S.S.A.; Fah, W.C.; Alimon, H.; Juhan, S.F. Synthesis of New Cytotoxic Aminoanthraquinone Derivatives via Nucleophilic Substitution Reactions. Molecules 2013, 18, 8046-8062. https://doi.org/10.3390/molecules18078046
Nor SMM, Sukari MAHM, Azziz SSSA, Fah WC, Alimon H, Juhan SF. Synthesis of New Cytotoxic Aminoanthraquinone Derivatives via Nucleophilic Substitution Reactions. Molecules. 2013; 18(7):8046-8062. https://doi.org/10.3390/molecules18078046
Chicago/Turabian StyleNor, Siti Mariam Mohd, Mohd Aspollah Hj Md Sukari, Saripah Salbiah Syed Abdul Azziz, Wong Chee Fah, Hasimah Alimon, and Siti Fadilah Juhan. 2013. "Synthesis of New Cytotoxic Aminoanthraquinone Derivatives via Nucleophilic Substitution Reactions" Molecules 18, no. 7: 8046-8062. https://doi.org/10.3390/molecules18078046




