Mechanistic Studies on Regioselective Dephosphorylation of Phosphate Prodrugs during a Facile Synthesis of Antitumor Phosphorylated 2-Phenyl-6,7-methylenedioxy-1H-quinolin-4-one
Abstract
:1. Introduction
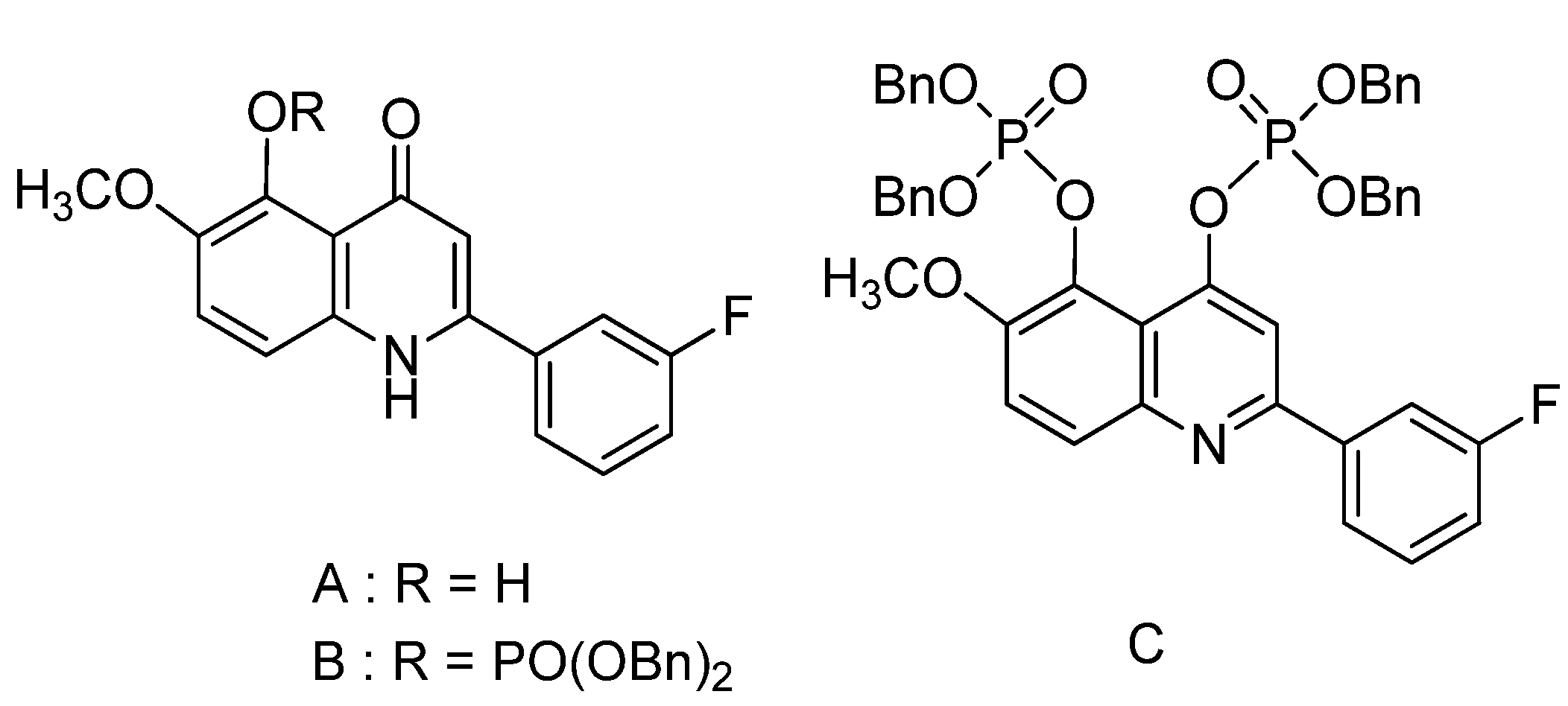
2. Results and Discussion
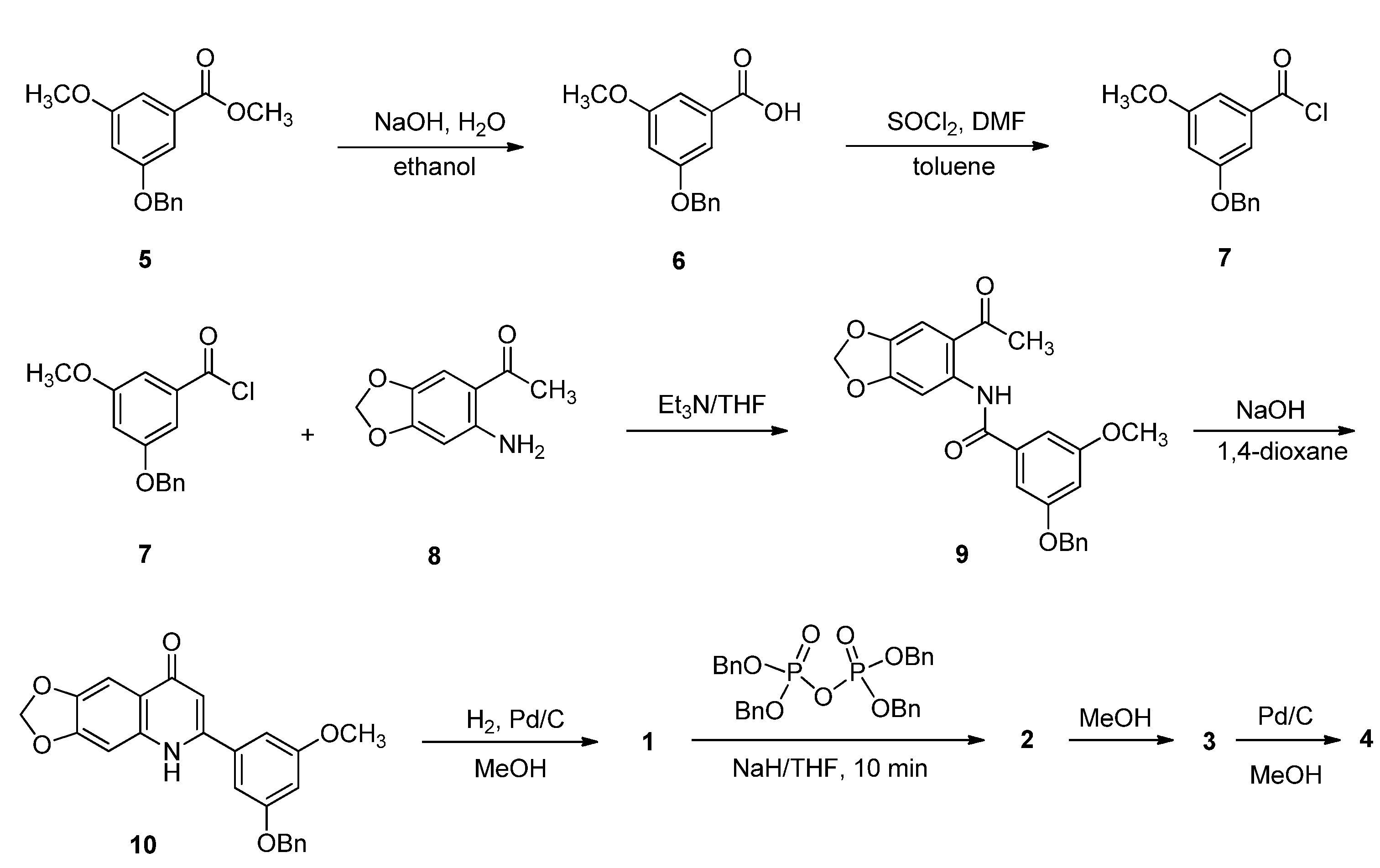
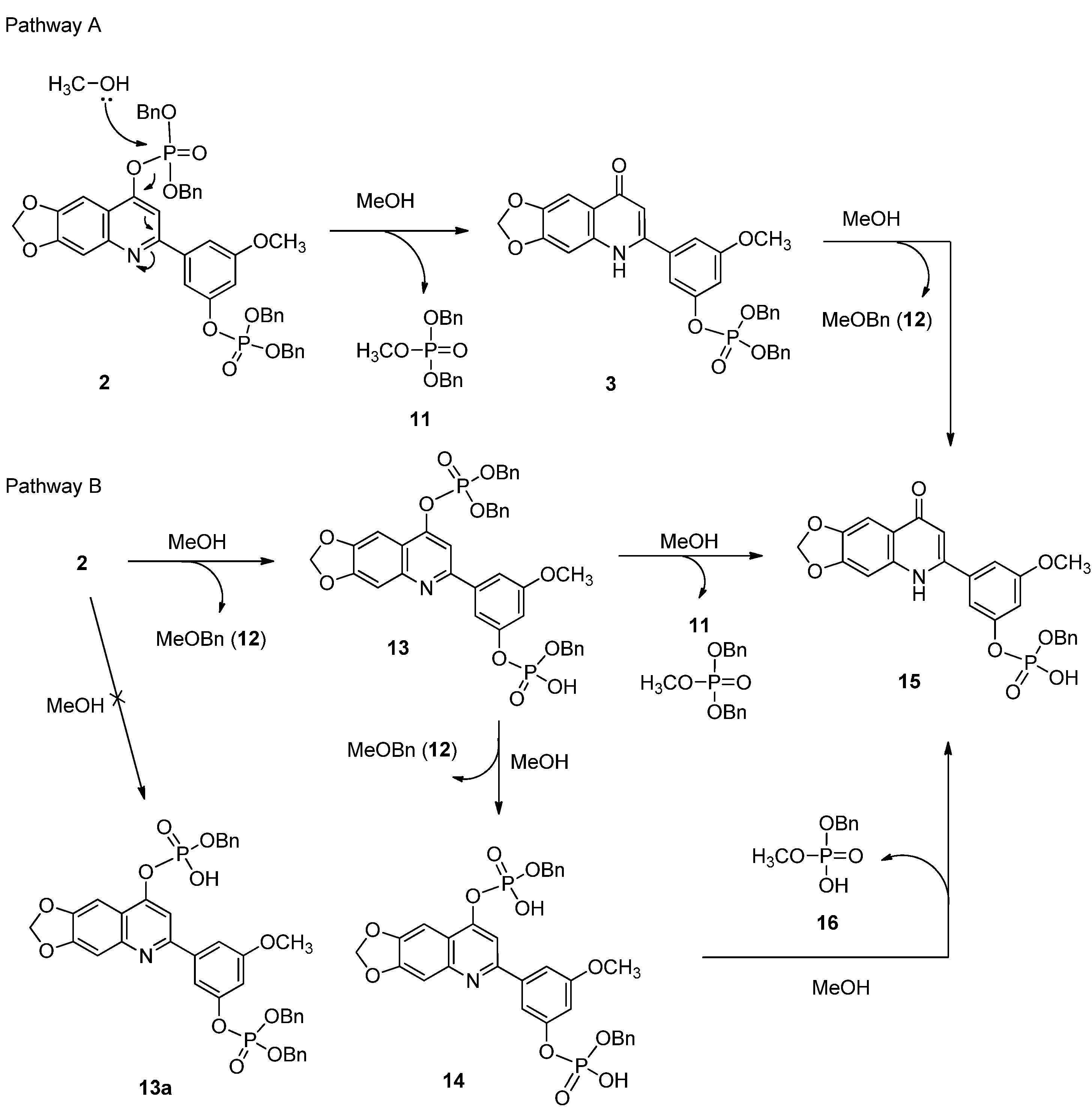
2.1. Chemistry

| Compounds | tR (min) | HRESIMS a (m/z) | ESIMS a (m/z) | Formula |
|---|---|---|---|---|
| 2 | 63.4 | 832.1916 | 832 | C45H40NO11P2 |
| 3 | 46.9 | 572.1449 | 572 | C31H27NO8P |
| 11 | 46.5 | ND b | 293 | C15H17O4P |
| 12 | 49.8 | ND b | 123 | C8H10O |
| 13 | 39.8 | 742.1568 | 742 | C38H34NO11P2 |
| 14 | 36.3 | 652.1158 | 652 | C31H28NO11P2 |
| 15 | 38.3 | 482.0988 | 482 | C24H21NO8P |
2.2. In Vitro Biological Evaluation
| Cell lines | logGI50 | logTGI | logLC50 |
|---|---|---|---|
| K562 | −6.47 | >−4.00 | >−4.00 |
| NCI-H522 | −6.70 | −6.33 | >−4.00 |
| OVCAR-3 | −6.55 | −6.13 | >−4.00 |
| MCF-7 | −6.39 | >−4.00 | >−4.00 |
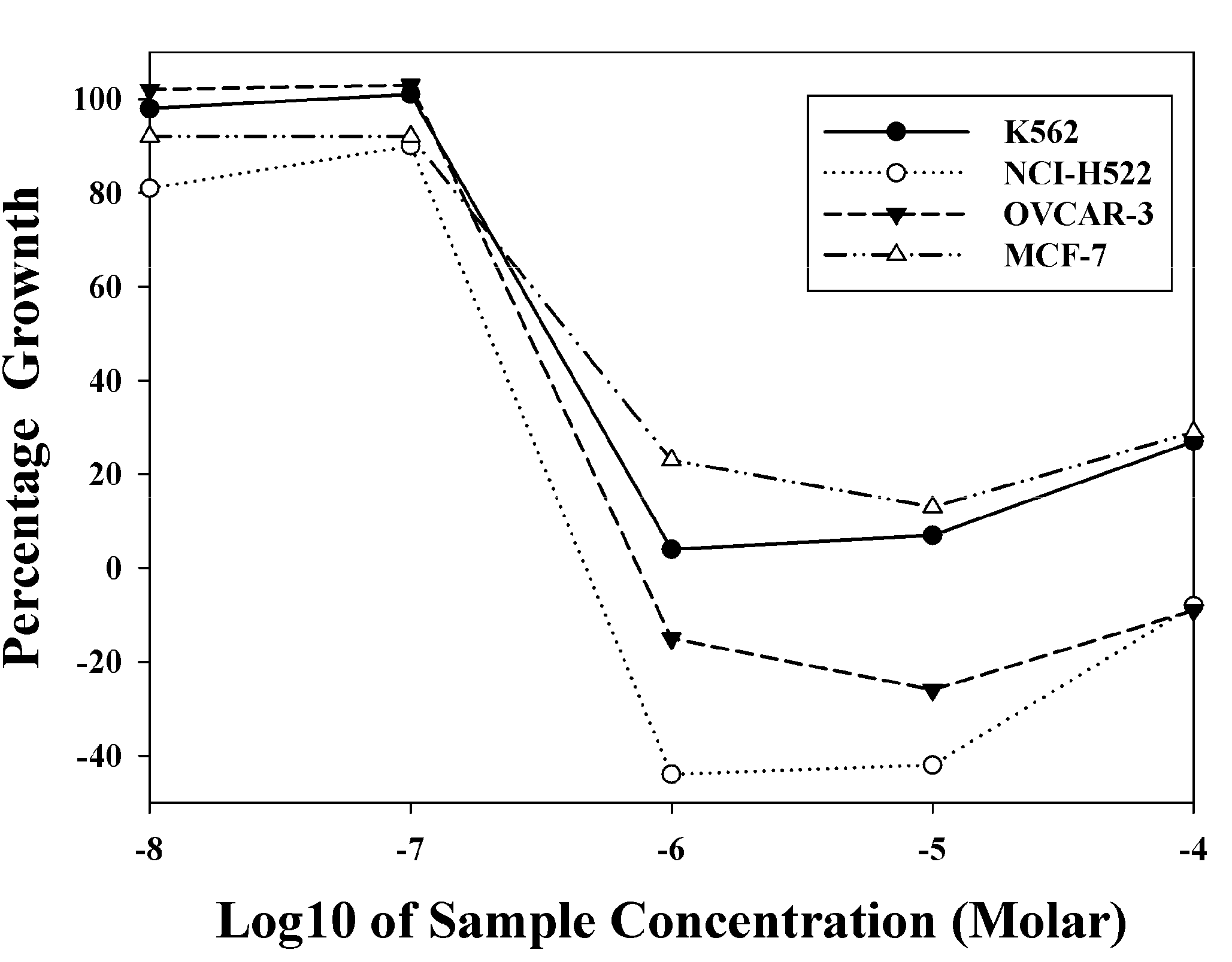
2.3. In Vivo Antitumor Activity
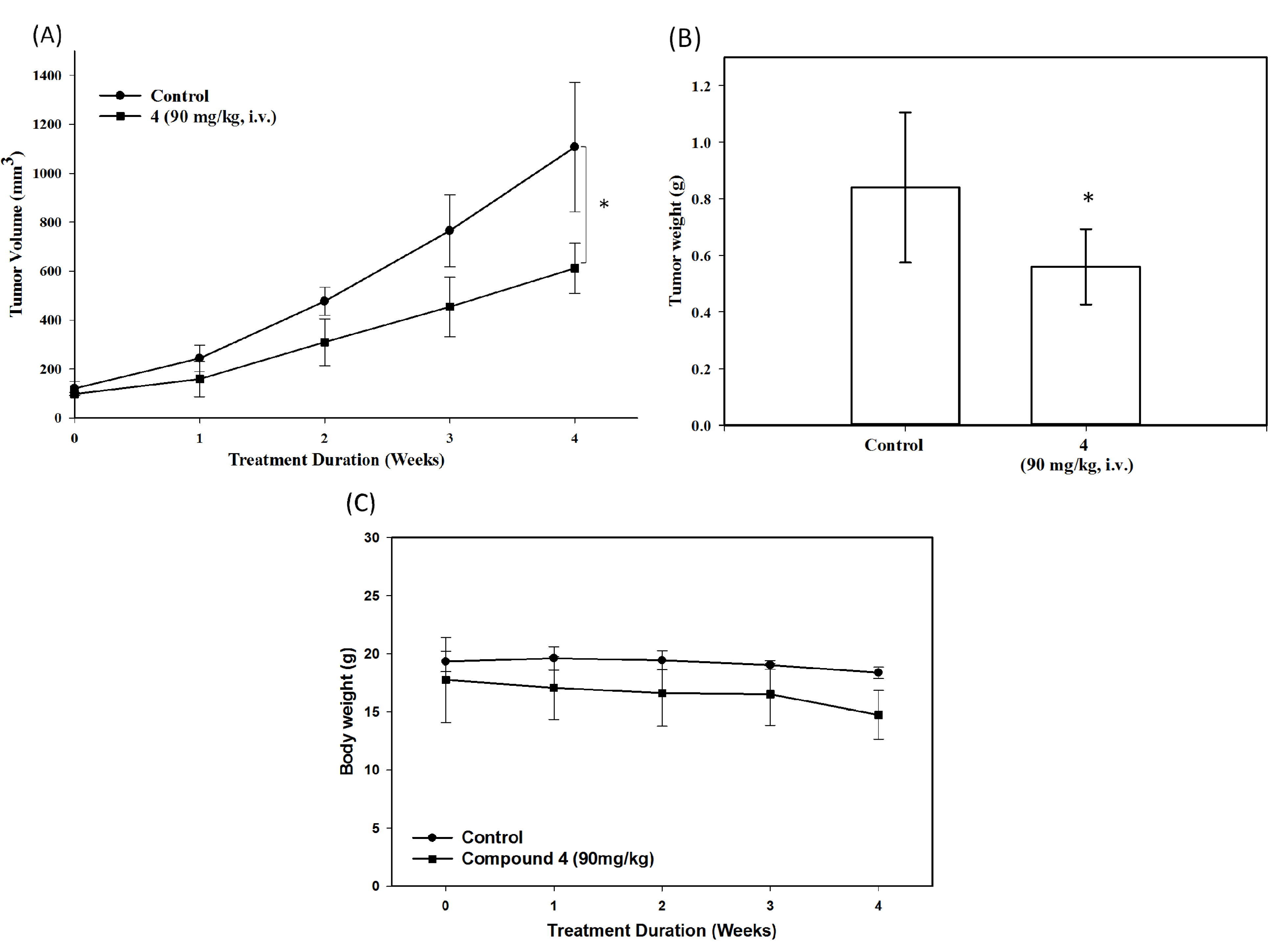
3. Experimental
3.1. Synthesis
3.1.1. General
3.1.2. Synthesis of Compounds 5−7 and 9
3.1.3. Synthesis of Compound 10
3.1.4. Synthesis of Compound 1
3.1.5. Synthesis of Compound 2
3.1.6. Synthesis of Compounds 3 and 11
3.1.7. Synthesis of Compound 15
3.1.8. Synthesis of Compound 4
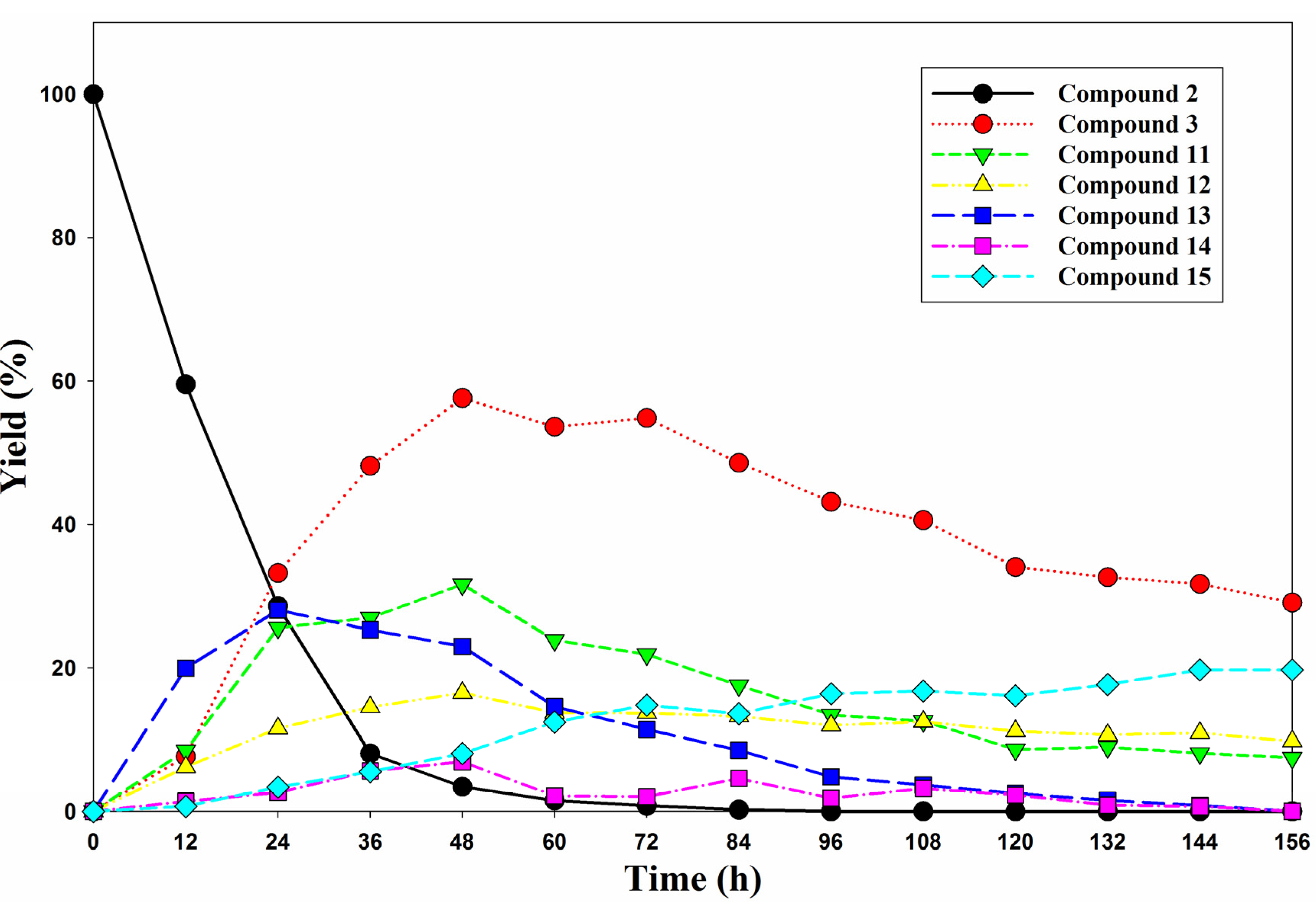
3.2. Isolation Reaction Products from Reaction Mixture
3.3. Reaction Products Analysis by HPLC-ESI-MS
3.4. Bioassay
3.4.1. Cell Culture
3.4.2. In vitro Cell Viability Assay
3.4.3. In Vivo Antitumor Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Heimbach, T.; Oh, D.M.; Li, L.Y.; Forsberg, M.; Savolainen, J.; Leppanen, J.; Matsunaga, Y.; Flynn, G.; Fleisher, D. Absorption rate limit considerations for oral phosphate prodrugs. Pharm. Res. 2003, 20, 848–856. [Google Scholar] [CrossRef]
- Simoni, D.; Romagnoli, R.; Baruchello, R.; Rondanin, R.; Rizzi, M.; Pavani, M.G.; Alloatti, D.; Giannini, G.; Marcellini, M.; Riccioni, T.; et al. Novel combretastatin analogues endowed with antitumor activity. J. Med. Chem. 2006, 49, 3143–3152. [Google Scholar] [CrossRef]
- Fleisher, D.; Bong, R.; Stewartb, B.H. Improved oral drug delivery: solubility limitations overcome by use of prodrugs. Adv. Drug Deliv. Rev. 1996, 19, 115–130. [Google Scholar] [CrossRef]
- Oslob, J.D.; Heumann, S.A.; Yu, C.H.; Allen, D.A.; Baskaran, S.; Bui, M.; Delarosa, E.; Fung, A.D.; Hashash, A.; Hau, J.; et al. Water-soluble prodrugs of an aurora kinase inhibitor. Bioorg. Med. Chem. Lett. 2009, 19, 1409–1412. [Google Scholar] [CrossRef]
- Shaw, J.P.; Sueoko, C.M.; Oliyai, R.; Lee, W.A.; Arimilli, M.N.; Kim, C.U.; Cundy, K.C. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res. 1997, 14, 1824–1829. [Google Scholar] [CrossRef]
- Bedford, S.B.; Quarterman, C.P.; Rathbone, D.L.; Slack, J.A. Synthesis of water-soluble prodrugs of the cytotoxic agent combretastin A4. Bioorg. Med. Chem. Lett. 1996, 6, 157–160. [Google Scholar]
- Kearney, A.S. Prodrugs and targeted drug delivery. Adv. Drug Deliv. Rev. 1996, 19, 225–239. [Google Scholar] [CrossRef]
- Sherwood, R.F. Advanced drug delivery reviews: Enzyme prodrug therapy. Adv. Drug Deliv. Rev. 1996, 22, 269–288. [Google Scholar] [CrossRef]
- Stella, V.J. A case for prodrugs: Fosphenytoin. Adv. Drug Deliv. Rev. 1996, 19, 311–330. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Jarvinen, T.; Savolainen, J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Heimbach, T.; Fleisher, D.; Kaddoumi, A. Overcoming poor aqueous Solubility of drugs for oral delivery. In Prodrugs: Challenges and Rewards Part 1; Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W., Eds.; Springer: New York, NY, USA, 2007; pp. 157–215. [Google Scholar]
- Stella, V.J.; Borchardt, R.T.; Hageman, M.J.; Oliyai, R.; Maag, H.; illey, J.W. A Case for prodrugs. In Prodrugs: Challenges and Rewards Part 1; Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W., Eds.; Springer: New York, NY, USA, 2007; Volume Part 1, pp. 3–33. [Google Scholar]
- Stella, V.J.; Nti-Addae, K.W. Prodrug strategies to overcome poor water solubility. Adv. Drug Deliv. Rev. 2007, 59, 677–694. [Google Scholar] [CrossRef]
- Pettit, G.R.; Grealish, M.P.; Jung, M.K.; Hamel, E.; Pettit, R.K.; Chapuis, J.C.; Schmidt, J.M. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate. J. Med. Chem. 2002, 45, 2534–2542. [Google Scholar] [CrossRef]
- Chou, L.C.; Tsai, M.T.; Hsu, M.H.; Lin, H.Y.; Huang, L.J.; Kuo, S.C.; Wang, S.H.; Way, T.D.; Huang, C.H.; Qian, K.; et al. Design, Synthesis, And preclinical evaluation of new 5,6- (or 6,7-) disubstituted-2-(fluorophenyl)quinolin-4-one derivatives as potent antitumor agents. J. Med. Chem. 2010, 53, 8047–8058. [Google Scholar]
- Chou, L.C.; Chen, C.T.; Lee, J.C.; Huang, S.M.; Huang, L.J.; Kuo, S.C.; Qian, K.; Morris-Natschke, S.L.; Lee, K.H.; Way, T.D.; et al. Synthesis and preclinical evaluations of 2-(2-fluorophenyl)-6,7- methylenedioxyquinolln-4-one monosodium phosphate (CHM-I-P-Na) as a potent antitumor agent. J. Med. Chem. 2010, 53, 1616–1626. [Google Scholar] [CrossRef]
- Chang, Y.H.; Hsu, M.H.; Wang, S.H.; Huang, L.J.; Qian, K.; Morris-Natschke, S.L.; Hamel, E.; Kuo, S.C.; Lee, K.H. Design and synthesis of 2-(3-benzo[b]thienyl)-6,7-methylenedioxyquinolin-4-one analogues as potent antitumor agents that inhibit tubulin assembly. J. Med. Chem. 2009, 52, 4883–4891. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.K.; Kuo, S.C.; Wu, T.S.; Lednicer, D.; Lin, C.M.; Hamel, E.; Lee, K.H. Antitumor agents. 150. 2',3',4',5',5,6,7-Substituted 2-phenyl-4-quinolones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 1994, 37, 1126–1135. [Google Scholar] [CrossRef]
- Kuo, S.C.; Lee, H.Z.; Juang, J.P.; Lin, Y.T.; Wu, T.S.; Chang, J.J.; Lednicer, D.; Paull, K.D.; Lin, C.M.; Hamel, E.; et al. Synthesis and cytotoxicity of 1,6,7,8-substituted 2-(4'-substituted phenyl)-4-quinolones and related compounds: Identification as antimitotic agents interacting with tubulin. J. Med. Chem. 1993, 36, 1146–1156. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Yang, J.S.; Tsai, S.C.; Liaw, C.C.; Chung, J.G.; Huang, L.J.; Lee, K.H.; Lu, C.C.; Chien, H.C.; Tsuzuki, M.; et al. The newly synthesized 2-(3-hydroxy-5-methoxypheny1)-6,7-methylenedioxyquinolin-4-one triggers cell apoptosis through induction of oxidative stress and upregulation of the p38 MAPK signaling pathway in HL-60 human leukemia cells. Oncol. Rep. 2012, 28, 1482–1490. [Google Scholar]
- Perry, C.M.; McTavish, D. Estramustine phosphate sodium. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in prostate cancer. Drugs Aging 1995, 7, 49–74. [Google Scholar] [CrossRef]
- Witterland, A.H.; Koks, C.H.; Beijnen, J.H. Etoposide phosphate, the water soluble prodrug of etoposide. Pharm. World Sci. 1996, 18, 163–170. [Google Scholar] [CrossRef]
- Vincent, L.; Kermani, P.; Young, L.M.; Cheng, J.; Zhang, F.; Shido, K.; Lam, G.; Bompais-Vincent, H.; Zhu, Z.; Hicklin, D.J.; et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J. Clin. Invest. 2005, 115, 2992–3006. [Google Scholar] [CrossRef]
- Brooks, D.J.; Srinivas, N.R.; Alberts, D.S.; Thomas, T.; Igwemzie, L.M.; McKinney, L.M.; Randolph, J.; Schacter, L.; Kaul, S.; Barbhaiya, R.H. Phase I and pharmacokinetic study of etoposide phosphate. Anti-cancer Drugs 1995, 6, 637–644. [Google Scholar] [CrossRef]
- Edsall, A.B.; Agoston, G.E.; Treston, A.M.; Plum, S.M.; McClanahan, R.H.; Lu, T.S.; Song, W.; Cushman, M. Synthesis and in vivo antitumor evaluation of 2-methoxyestradiol 3-phosphate, 17-phosphate, and 3,17-diphosphate. J. Med. Chem. 2007, 50, 6700–6705. [Google Scholar] [CrossRef]
- Mäkelä, T.; Matikainen, J.; Wähälä, K.; Hase, T. Development of a novel hapten for radioimmunoassay of the lignan, enterolactone in plasma (Serum). Total synthesis of (±)-trans-5-carboxymethoxyenterolactone and several analogues. Tetrahedron 2000, 56, 1873–1882. [Google Scholar] [CrossRef]
- Denmark, S.E.; Regens, C.S.; Kobayashi, T. Total synthesis of papulacandin D. J. Am. Chem. Soc. 2007, 129, 2774–2776. [Google Scholar] [CrossRef]
- Sorme, P.; Arnoux, P.; Kahl-Knutsson, B.; Leffler, H.; Rini, J.M.; Nilsson, U.J. Structural and thermodynamic studies on cation-Pi interactions in lectin-ligand complexes: High-affinity galectin-3 inhibitors through fine-tuning of an arginine-arene interaction. J. Am. Chem. Soc. 2005, 127, 1737–1743. [Google Scholar]
- Santos, M.D.; Lopes, N.P. HPLC-ESI-MS/MS analysis of oxidized di-caffeoylquinic acids generated by metalloporphyrin-catalyzed reactions. Quim. Nova 2008, 31, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.X.; Zhang, J.C.; Xu, J.; Liao, X.C.; Zhao, Y.F. Analysis of hydrolysis reaction of N-phosphoryl phenylalanine by HPLC-ESI-MS/MS. Chinese Chem. Lett. 2004, 15, 817–820. [Google Scholar]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemoth. Pharm. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, Y.-Y.; Liu, C.-Y.; Huang, L.-J.; Huang, C.-H.; Lee, K.-H.; Lin, C.-T.; Kuo, S.-C. Mechanistic Studies on Regioselective Dephosphorylation of Phosphate Prodrugs during a Facile Synthesis of Antitumor Phosphorylated 2-Phenyl-6,7-methylenedioxy-1H-quinolin-4-one. Molecules 2013, 18, 8028-8045. https://doi.org/10.3390/molecules18078028
Cheng Y-Y, Liu C-Y, Huang L-J, Huang C-H, Lee K-H, Lin C-T, Kuo S-C. Mechanistic Studies on Regioselective Dephosphorylation of Phosphate Prodrugs during a Facile Synthesis of Antitumor Phosphorylated 2-Phenyl-6,7-methylenedioxy-1H-quinolin-4-one. Molecules. 2013; 18(7):8028-8045. https://doi.org/10.3390/molecules18078028
Chicago/Turabian StyleCheng, Yung-Yi, Chin-Yu Liu, Li-Jiau Huang, Chi-Hung Huang, Kuo-Hsiung Lee, Cheng-Tung Lin, and Sheng-Chu Kuo. 2013. "Mechanistic Studies on Regioselective Dephosphorylation of Phosphate Prodrugs during a Facile Synthesis of Antitumor Phosphorylated 2-Phenyl-6,7-methylenedioxy-1H-quinolin-4-one" Molecules 18, no. 7: 8028-8045. https://doi.org/10.3390/molecules18078028




