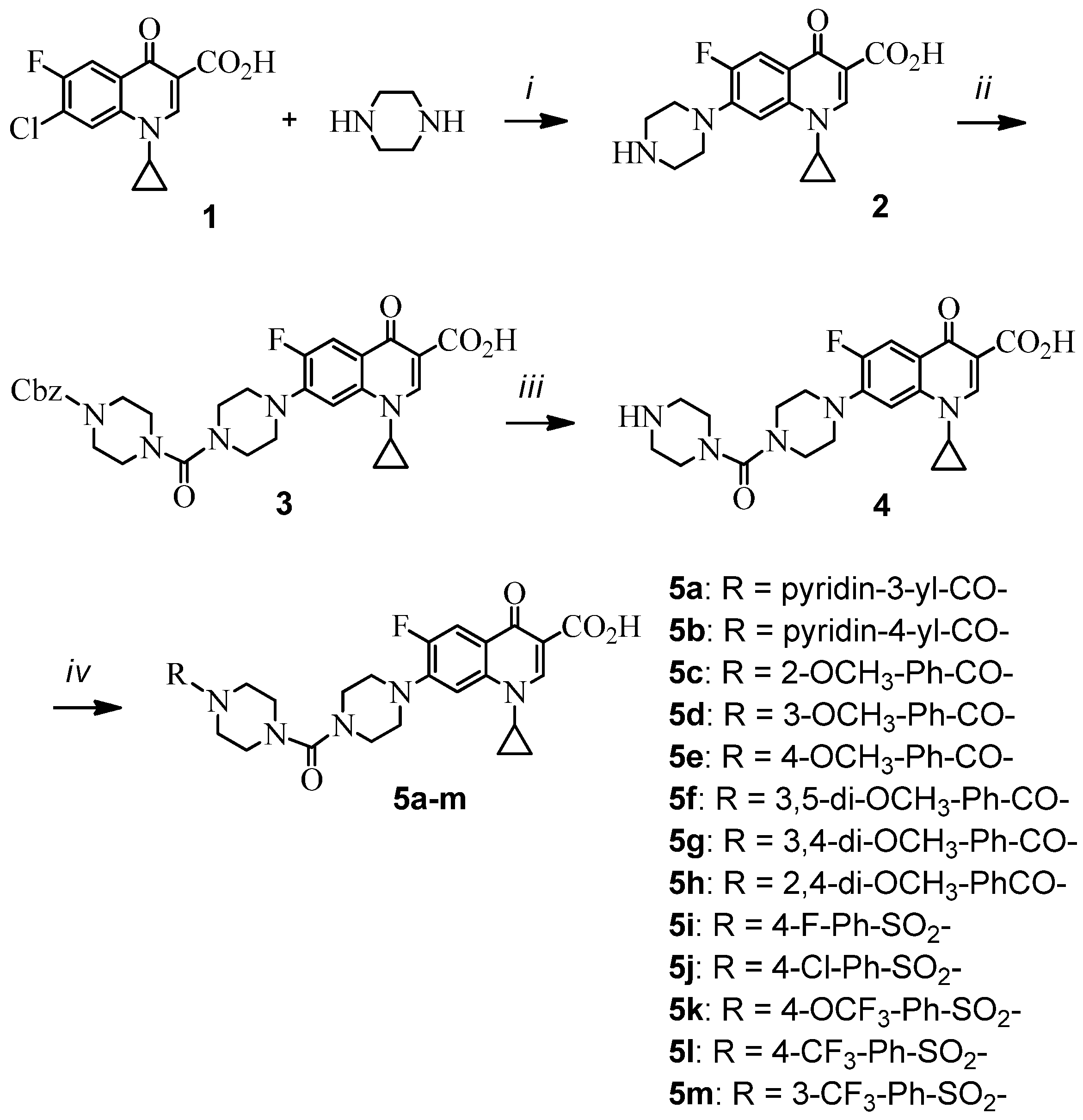
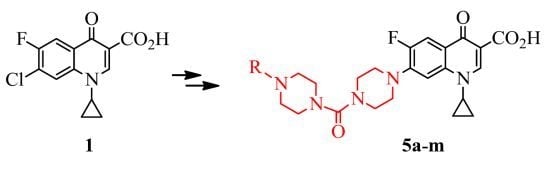
3.2. Synthesis
7-(4-[4-(Benzyloxycarbonyl)piperazino]carbopiperazino)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (3). A solution of N-(benzyloxycarbonyl)piperazine (220 mg, 1 mmol) in dichloromethane (10 mL) was slowly added to the stirred solution of triphosgene (110 g, 0.37 mmol) in dichloromethane (2 mL) over a period of 30 min using a syringe pump. After 30 further min of stirring, a solution of 2 (398 mg, 1.2 mmol) and diisopropylethylamine (DIEA, 0.38 mL, 2.2 mmol) in DCM/EtOH (120 mL, 4:1) was added in one portion. The reaction mixture was stirred for 2 hours at room temperature. After evaporation of solvent under vacuum, the residue was purified by silica gel chromatography to give 3 (318 mg, 55%); tlc Rf = 0.30 (DCM/EtOH = 20:1); mp 215–216 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε) 240.4 (9.22); 1H-NMR (DMSO-d6) δ 8.67 (s, 1H, C2-H), 7.90 (d, J = 13.2 Hz, 1H, C5-H), 7.56 (d, J = 7.5 Hz, 1H, C8-H), 7.37–7.31 (m, 5H, Ar-H), 5.09 (s, 2H, -O-CH2-Ar), 3.78–3.82 (m, 1H, cyclopropyl), 3.15–3.40 (m, 16H, piperazinyl), 1.17–1.32 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.2, 41.4, 41.5, 44.3, 44.6, 48.1, 49.3, 49.6, 50.3, 69.5, 105.4, 108.4, 111.1, 112.8, 118.8, 127.3, 128.2, 128.8, 129.6, 136.9, 137.8, 146.5, 149.8, 153.4, 161.2, 165.3, 168.6, 176,2; FABMS: m/z 578 [M+H]+.
1-Cyclopropyl-6-fluoro-4-oxo-7-[4-(piperazin-1-yl)carbopiperazino]-1,4-dihydro-3-quinolinecarboxylic acid (4). To a solution of 3 (289 mg, 0.5 mmol) and 10% Pd/C in DCM/EtOH (50 mL, 1:1) was charged with H2 at 1 atm and stirred at room temperature for 1 h. The catalyst was filtered off through celite. After evaporation of solvent under vacuum, the residue was recrystallized from DCM/acetone to give 4 (177 mg, 80%); tlc Rf = 0.10 (DCM/EtOH = 9:1); mp 196–197 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε) 238.8 (9.11); 1H-NMR (DMSO-d6) δ 9.10 (s, 1H, br, CH2-NH-CH2), 8.77 (s, 1H, C2-H), 7.91 (d, J = 13.2 Hz, 1H, C5-H), 7.55 (d, J = 7.5 Hz, 1H, C8-H), 3.78–3.82 (m, 1H, cyclopropyl), 3.08–3.40 (m, 16H, piperazinyl), 1.16–1.31 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.2, 40.2, 41.6, 43.6, 44.6, 48.2, 49.2, 49.6, 50.3, 106.2, 108.4, 112.8, 118.8, 137.8, 146.5, 149.8, 153.4, 161.2, 166.6, 176,2; FABMS: m/z 444 [M+H]+.
General procedure for the synthesis of 1-cyclopropyl-6-fluoro-4-oxo-7-(4-[4-(substituted benzoyl or benzenesulfonyl) piperazino]carbopiperazino)-1,4-dihydro-3-quinolinecarboxylic acids 5a–m.
To a solution of amine 4 (0.67 g, 1.5 mmol) in DCM/EtOH (150 mL, 4:1) was added triethylamine (0.4 mL, 3.0 mmol) and the appropriate aroyl or benzenesulfonyl halide (1.2 mmol). The mixture was stirred at room temperature under argon for several hours depending on the completion of the reaction, which was checked by tlc. After evaporation of solvent under vacuum, the residue was purified by silica gel chromatography and recrystallized from an appropriate solvent to give the title products.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-[4-(3-pyridylcarbonyl)piperazino]carbopiperazino)-1,4-dihydro-3-quinolinecarboxylic acid (5a). Amine 4 (0.67 g, 1.5 mmol) was treated with nicotinyl chloride (0.17 g, 1.2 mmol) to give 5a (0.41 g, 62%) as a white solid; tlc Rf = 0.18 (DCM/EtOH = 20 : 1); mp 219–220 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 242.6 (9.20); 1H-NMR (CDCl3) δ 8.63–8.65 (m, 2H, Ar-H), 8.61 (s, 1H, C2-H), 7.84 (d, J = 12.9 Hz, 1H, C5-H), 7.73–7.76 (m, 1H, Ar-H), 7.35–7.39 (m, 1H, Ar-H), 7.32 (d, J = 7.2 Hz, 1H, C8-H), 3.76–3.80 (m, 1H, cyclopropyl), 3.32–3.53 (m, 16H, piperazinyl), 1.17–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.3, 41.3, 41.9, 44.4, 44.8, 48.8, 49.2, 49.6, 50.1, 105.1, 107.2, 109.4, 112.0, 112.3, 119.8, 127.1, 138.0, 144.6, 145.0, 147.2, 149.8, 153.6, 160.6, 166.0, 168.8, 176,6; FABMS: m/z 549 [M+H]+; HRFABMS: calcd for C28H30FN6O5 [M+H]+ 549.2264, found 549.2268.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-[4-(4-pyridylcarbonyl)piperazino]carbopiperazino)-1,4-dihydro-3-quinolinecarboxylic acid (5b). Amine 4 (0.67 g, 1.5 mmol) was treated with isonicotinyl chloride (0.17 g, 1.2 mmol) to give 5b (0.36 g, 55%) as a white solid; tlc Rf = 0.18 (DCM/EtOH = 20:1); mp 217–218 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 239.4 (9.20); 1H-NMR (CDCl3) δ 8.74–8.65 (m, 2H, Ar-H), 8.67 (s, 1H, C2-H), 7.91 (d, J = 12.9 Hz, 1H, C5-H), 7.34 (d, J = 7.2 Hz, 1H, C8-H), 7.30–7.35 (m, 2H, Ar-H), 3.78–3.82 (m, 1H, cyclopropyl), 3.32–3.54 (m, 16H, piperazinyl), 1.18–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.3, 41.6, 41.9, 44.3, 44.5, 47.9, 49.3, 49.6, 50.4, 105.8, 106.9, 108.7, 111.3, 112.2, 118.8, 127.3, 137.8, 144.2, 146.5, 147.4, 149.8, 153.4, 161.2, 165.3, 168.2, 176,3; FABMS: m/z 549 [M+H]+; HRFABMS: calcd for C28H30FN6O5 [M+H]+ 549.2264, found 549.2262.
1-Cyclopropyl-6-fluoro-7-(4-[4-(2-methoxybenzoyl)piperazino]carbopiperazino)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5c). Amine 4 (0.67 g, 1.5 mmol) was treated with 2-methoxybenzoyl chloride (0.2 g, 1.2 mmol) to give 5c (0.46 g, 67%) as a white solid; tlc Rf = 0.33 (DCM/EtOH = 20:1); mp 240–241 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 241.8 (9.27); 1H-NMR (CDCl3) δ 8.66 (s, 1H, C2-H), 7.90 (d, J = 12.9 Hz, 1H, C5-H), 7.34 (d, J = 7.2 Hz, 1H, C8-H), 6.89–7.38 (m, 4H, Ar-H), 3.82 (s, 3H, -OCH3), 3.78–3.85 (m, 1H, cyclopropyl), 3.26–3.53 (m, 16H, piperazinyl), 1.17–1.39 (m, 4H, cyclopropyl); 13C NMR (CDCl3) δ 8.2, 35.3, 41.6, 41.9, 44.3, 44.5, 47.9, 49.3, 49.6, 50.4, 54.2, 107.1, 108.7, 111.3, 112.2, 114.6, 118.8, 120.8, 127.3, 128.2, 129.2, 137.8, 146.5, 149.8, 153.4, 160.2, 161.2, 165.3, 168.6, 176,2; FABMS: m/z 578 [M+H]+; HRFABMS: calcd for C30H33FN5O6 [M+H]+ 578.2417, found 578.2420.
1-Cyclopropyl-6-fluoro-7-(4-[4-(3-methoxybenzoyl)piperazino]carbopiperazino)-4-oxo-1,4-dihydro- 3-quinolinecarboxylic acid (5d). Amine 4 (0.67 g, 1.5 mmol) was treated with 3-methoxybenzoyl chloride (0.2 g, 1.2 mmol) to give 5d (0.57 g, 82%) as a white solid; tlc Rf = 0.34 (DCM/EtOH = 20:1); mp 236–237 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 240.7 (9.27); 1H-NMR (CDCl3) δ 8.70 (s, 1H, C2-H), 7.95 (d, J = 12.9 Hz, 1H, C5-H), 7.35 (d, J = 7.2 Hz, 1H, C8-H), 7.29–7.35 (m, 1H, Ar-H), 6.92–6.98 (m, 3H, Ar-H), 3.82 (s, 3H, -OCH3), 3.78–3.82 (m, 1H, cyclopropyl), 3.26–3.54 (m, 16H, piperazinyl), 1.1–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.1, 41.4, 41.9, 44.3, 44.5, 47.9, 49.2, 49.6, 50.3, 53.7, 107.2, 107.9, 111.5, 112.2, 114.6, 118.8, 127.3, 128.2, 129.2, 137.8, 146.4, 149.8, 153.4, 160.2, 161.2, 165.6, 168.4, 176,2; FABMS: m/z 578 [M+H]+, HRFABMS: calcd for C30H33FN5O6 [M+H]+ 578.2417, found 578.2413.
1-Cyclopropyl-6-fluoro-7-(4-[4-(4-methoxybenzoyl)piperazino]carbopiperazino)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5e). Amine 4 (0.67 g, 1.5 mmol) was treated with 4-methoxybenzoyl chloride (0.2 g, 1.2 mmol) to give 5e (0.59 g, 85%) as a white solid; tlc Rf = 0.34 (DCM/EtOH = 20:1); mp 224–225 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 239.0 (9.23); 1H-NMR (CDCl3) δ 8.60 (s, 1H, C2-H), 7.83 (d, J = 12.9 Hz, 1H, C5-H), 7.35 (dd, J = 6.9, 2.1 Hz, 2H, Ar-H), 7.32 (d, J = 7.2 Hz, 1H, C8-H), 6.88 (dd, J = 6.9, 2.1 Hz, 2H, Ar-H), 3.80 (s, 3H, -OCH3), 3.78–3.82 (m, 1H, cyclopropyl), 3.30–3.62 (m, 16H, piperazinyl), 1.16–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.2, 41.7, 41.2, 44.6, 44.9, 47.3, 49.2, 49.6, 50.3, 53.2, 107.3, 107.9, 110.8, 111.6, 114.7, 118.8, 127.5, 128.2, 129.2, 137.8, 146.4, 148.2, 153.4, 160.2, 161.6, 165.8, 168.3, 176,2; FABMS: m/z 578 [M+H]+, HRFABMS: calcd for C30H33FN5O6 [M+H]+ 578.2417, found 578.2422.
1-Cyclopropyl-7-(4-[4-(3,5-dimethoxybenzoyl)piperazino]carbopiperazino)-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5f). Amine 4 (0.67 g, 1.5 mmol) was treated with 3,5-dimethoxybenzoyl chloride (0.24 g, 1.2 mmol) to give 5f (0.53 g, 73%) as a white solid; tlc Rf = 0.38 (DCM/EtOH = 20:1); mp 233–234 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 240.4 (9.25); 1H-NMR (CDCl3) δ 8.61 (s, 1H, C2-H), 7.84 (d, J = 12.9 Hz, 1H, C5-H), 7.32 (d, J = 6.9 Hz, 1H, C8-H), 6.46–6.48 (m, 3H, Ar-H), 3.77 (s, 6H, -OCH3), 3.78–3.82 (m, 1H, cyclopropyl), 3.30–3.65 (m, 16H, piperazinyl), 1.17–1.39 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.2, 41.6, 41.2, 44.6, 44.8, 47.3, 49.5, 49.2, 50.3, 53.4, 53.8, 106.8, 107.9, 110.8, 112.4, 114.7, 118.9, 127.5, 128.1, 137.8, 146.4, 148.2, 153.4, 159.8, 160.4, 161.4, 165.8, 168.2, 176,2; FABMS: m/z 608 [M+H]+, HRFABMS: calcd for C31H35FN5O7 [M+H]+ 608.2523, found 608.2527.
1-Cyclopropyl-7-(4-[4-(3,4-dimethoxybenzoyl)piperazino]carbopiperazino)-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5g). Amine 4 (0.67 g, 1.5 mmol) was treated with 3,4-dimethoxybenzoyl chloride (0.24 g, 1.2 mmol) to give 5g (0.57 g, 78%) as a white solid; tlc Rf = 0.38 (DCM/EtOH = 20:1); mp 257–258 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 243.8 (9.28); 1H-NMR (CDCl3) δ 8.72 (s, 1H, C2-H), δ 7.98 (d, J = 12.9 Hz, 1H, C5-H), δ 7.36 (d, J = 7.2 Hz, 1H, C8-H), δ 6.99 (d, J = 1.8 Hz, 1H, Ar-H), δ 6.98 (dd, J = 9.9 Hz, 1.8 Hz, 1H, Ar-H), 6.87(d, J = 9.9 Hz, 1H, Ar-H), 3.91 (s, 3H, -OCH3), 3.90 (s, 3H, -OCH3), 3.66–3.68 (m, 1H, cyclopropyl), 3.33–3.55 (m, 16H, piperazinyl), 1.19–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.3, 41.2, 41.2, 44.8, 44.8, 48.1, 49.5, 49.2, 50.3, 53.4, 54.2, 106.8, 107.9, 110.8, 112.4, 114.7, 119.1, 128.5, 128.1, 137.2, 146.3, 149.5, 153.4, 159.1, 160.6, 161.2, 165.8, 168.2, 176,4; FAB MS: m/z 608 [M+H]+; HRFABMS: calcd for C31H35FN5O7 [M+H]+ 608.2523, found 608.2525.
1-Cyclopropyl-7-(4-[4-(2,4-dimethoxybenzoyl)piperazino]carbopiperazino)-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5h). Amine 4 (0.67 g, 1.5 mmol) was treated with 2,4-dimethoxybenzoyl chloride (0.24 g, 1.2 mmol) to give 5h (0.47 g, 65%) as a white solid; tlc Rf = 0.47 (DCM/EtOH = 20:1); mp 244–245 °C; UV λmax (DCM/EtOH = 3 : 2) nm (log ε): 240.4 (9.29); 1H-NMR (CDCl3) δ 8.64 (s, 1H, C2-H), 7.88 (d, J = 12.9 Hz, 1H, C5-H), 7.33 (d, J = 7.2 Hz, 1H, C8-H), 7.17 (d, J = 8.4 Hz, 1H, Ar-H), 6.50 (dd, J = 8.4 Hz, 2.1 Hz, 1H, Ar-H), 6.43 (d, J = 2.1 Hz, 1H, Ar-H), 3.80 (s, 3H, -OCH3), 3.79 (s, 3H, -OCH3), 3.78–3.82 (m, 1H, cyclopropyl), 3.25–3.52 (m, 16H, piperaznyl), 1.18–1.39 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.3, 41.2, 41.1, 44.6, 44.8, 47.3, 49.4, 49.2, 50.3, 53.4, 53.7, 106.8, 107.9, 110.8, 112.4, 114.7, 118.9, 127.2, 128.5, 137.8, 146.4, 148.2, 153.4, 159.3, 160.3, 161.2, 165.7, 168.1, 176,3; FABMS: m/z 608 [M+H]+; HRFABMS: calcd for C31H35FN5O7 [M+H]+ 608.2523, found 608.2519.
1-Cyclopropyl-6-fluoro-7-(4-[4-(4-fluorobenzenesulfonyl)piperazino]carbopiperazino)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5i). Amine 4 (0.67g, 1.5 mmol) was treated with 4-fluoro- benzenesulfonyl chloride (0.23 g, 1.2 mmol) to give 5i (0.55 g, 76%) as a white solid; tlc Rf = 0.42 (DCM/EtOH = 20:1); mp 268–269 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 241.1 (9.24); 1H-NMR (CDCl3) δ 8.76 (s, 1H, C2-H), 8.02 (d, J = 12.9 Hz, 1H, C5-H), 7.77 (dt, J = 4.8 Hz, 2.1 Hz, 2H, Ar-H), 7.34 (d, J = 7.2 Hz, 1H, C8-H), 7.25 (dd, J = 4.8 Hz, 2.1 Hz, 2H, Ar-H), 3.02–3.51 (m, 17H, piperazinyl, cyclopropyl), 1.20–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.3, 41.4, 41.9, 44.3, 44.5, 47.9, 49.3, 49.6, 50.4, 107.1, 108.7, 111.3, 112.2, 112.6, 114.4, 127.3, 128.2, 137.8, 138.6, 146.5, 149.8, 153.4, 158.2, 161.9, 165.8, 176,5; FABMS: m/z 602 [M+H]+; HRFABMS: calcd for C28H30F2N5O6S [M+H]+ 602.1887, found 602.1885.
7-(4-[4-(4-Chlorobenzenesulfonyl)piperazino]carbopiperazino)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid (5j). Amine 4 (0.67 g, 1.5 mmol) was treated with 4-chlorobenzenesulfonyl chloride (0.25 g, 1.2 mmol) to give 5j (0.58 g, 78%) as a white solid; tlc Rf = 0.42 (DCM/EtOH = 20:1); mp 274–275 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 242.5 (9.29); 1H-NMR (CDCl3) δ 8.74 (s, 1H, C2-H), 8.00 (d, J = 12.9 Hz, 1H, C5-H), 7.69 (d, J = 8.4 Hz, 2H, Ar-H), 7.53 (d, J = 8.4 Hz, 2H, Ar-H), 7.33 (d, J = 7.2 Hz, 1H, C8-H), 3.04–3.47 (m, 17H, piperazinyl, cyclopropyl), 1.20–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.3, 41.4, 41.9, 44.3, 44.5, 47.9, 49.3, 49.6, 50.4, 107.1, 108.7, 111.3, 112.6, 127.3, 128.2, 128.9, 129.3, 135.6, 137.8, 138.6, 146.5, 149.8, 153.4, 161.9, 165.8, 176,4; FABMS: m/z 618 [M+H]+; HRFABMS: calcd for C28H30FClN5O6S [M+H]+ 618.1592, found 618.1589.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-[4-(4-trifluoromethoxybenzenesulfonyl)piperazino]carbopiperazino)-1,4-dihydro-3-quinolinecarboxylic acid (5k). Amine 4 (0.67 g, 1.5 mmol) was treated with 4-trifluoromethoxybenzenesulfonyl chloride (0.31 g, 1.2 mmol) to give 5k (0.64 g, 80%) as a white solid; tlc Rf = 0.40 (DCM/EtOH = 20:1); mp 248–249 °C; UV λmax (DCM/EtOH = 3 : 2) nm (log ε): 239.2 (9.33); 1H-NMR (CDCl3) δ 8.77 (s, 1H, C2-H), 8.03 (d, J = 12.9 Hz, 1H, C5-H), 7.81 (d, J = 8.7 Hz, 2H, Ar-H), 7.38 (d, J = 8.7 Hz, 2H, Ar-H), 7.35 (d, J = 7.2 Hz, 1H, C8-H), 3.06–3.46 (m, 17H, piperazinyl, cyclopropyl), 1.19–1.40 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.2, 41.6, 41.9, 44.2, 44.5, 47.9, 49.8, 49.5, 50.4, 107.2, 108.7, 111.3, 112.6, 114.7, 119.3, 127.3, 128.2, 137.8, 138.6, 146.5, 149.2, 151.8, 158.2, 160.4, 161.5, 165.4, 176,2; FABMS: m/z 668 [M+H]+; HRFABMS: calcd for C29H30F4N5O7S [M+H]+ 668.1804, found 668.1802.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-[4-(4-trifluoromethylbenzenesulfonyl)piperazino]carbopiperazino)-1,4-dihydro-3-quinolinecarboxylic acid (5l). Amine 4 (0.67 g, 1.5 mmol) was treated with 4-trifluoromethylbenzenesulfonyl chloride (0.29 g, 1.2 mmol) to give 5l (0.61 g, 78%); tlc Rf = 0.40 (DCM/EtOH = 20:1); mp 167–168 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 241.1 (9.28); 1H-NMR (DMSO-d6 with a drop of D2O) δ 8.64 (s, 1H, C2-H), 8.04 (d, J = 8.4 Hz, 2H, Ar-H), 7.96 (d, J = 8.4 Hz, 2H, Ar-H), 7.87 (d, J = 13.2 Hz, 1H, C5-H), 7.51 (d, J = 7.5 Hz, 1H, C8-H), 3.74–3.80 (m, 1H, cyclopropyl), 2.96–3.30 (m, 16H, piperazinyl), 1.12–1.30 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.2, 35.1, 41.6, 41.9, 44.3, 44.5, 47.9, 49.3, 49.6, 50.4, 107.3, 108.7, 111.3, 112.2, 124.5, 125.6, 126.2, 127.3, 128.2, 130.8, 137.8, 138.6, 145.7, 149.2, 158.7, 161.3, 165.4, 176,2; FABMS: m/z 652 [M+H]+; HRFABMS: calcd for C29H30F4N5O6S [M+H]+ 652.1855, found 652.1853.
1-Cyclopropyl-6-fluoro-4-oxo-7-(4-[4-(3-trifluoromethylbenzenesulfonyl)piperazino]carbopiperazino)-1,4-dihydro-3-quinolinecarboxylic acid (5m). Amine 4 (0.67 g, 1.5 mmol) was treated with 3-trifluoromethylbenzenesulfonyl chloride (0.29 g, 1.2 mmol) to give 5m (0.59 g, 76%) as a white solid; tlc Rf = 0.37 (DCM/EtOH = 20:1); mp 267–268 °C; UV λmax (DCM/EtOH = 3:2) nm (log ε): 239.2 (9.32); 1H-NMR (DMSO-d6 with a drop of D2O) δ 8.64 (s, 1H, C2-H), 8.15 (d, J = 7.5 Hz, 1H, Ar-H), δ 8.07 (d, J = 7.8 Hz, 1H, Ar-H), δ 7.97 (s, 1H, Ar-H), δ 7.92 (dd, J = 7.8 Hz, 7.5 Hz, 1H, Ar-H), 7.89 (d, J = 13.2 Hz, 1H, C5-H), 7.52 (d, J = 7.5 Hz, 1H, C8-H), 3.76 ~ δ 3.81 (m, 1H, cyclopropyl), 2.97–3.30 (m, 16H, piperazinyl), 1.13–1.30 (m, 4H, cyclopropyl); 13C-NMR (CDCl3) δ 8.1, 35.4, 41.2, 41.9, 44.4, 44.9, 47.5, 49.3, 49.6, 50.4, 107.3, 108.1, 111.5, 112.2, 124.1, 125.2, 126.6, 127.4, 128.1, 130.2, 137.8, 138.6, 145.1, 149.6, 158.7, 161.6, 165.2, 176,2; FABMS: m/z 652 [M+H]+; HRFABMS: calcd for C29H30F4N5O6S [M+H]+ 652.1855, found 652.1852.