Effects of Combined Elicitors on Tanshinone Metabolic Profiling and SmCPS Expression in Salvia miltiorrhiza Hairy Root Cultures
Abstract
:1. Introduction
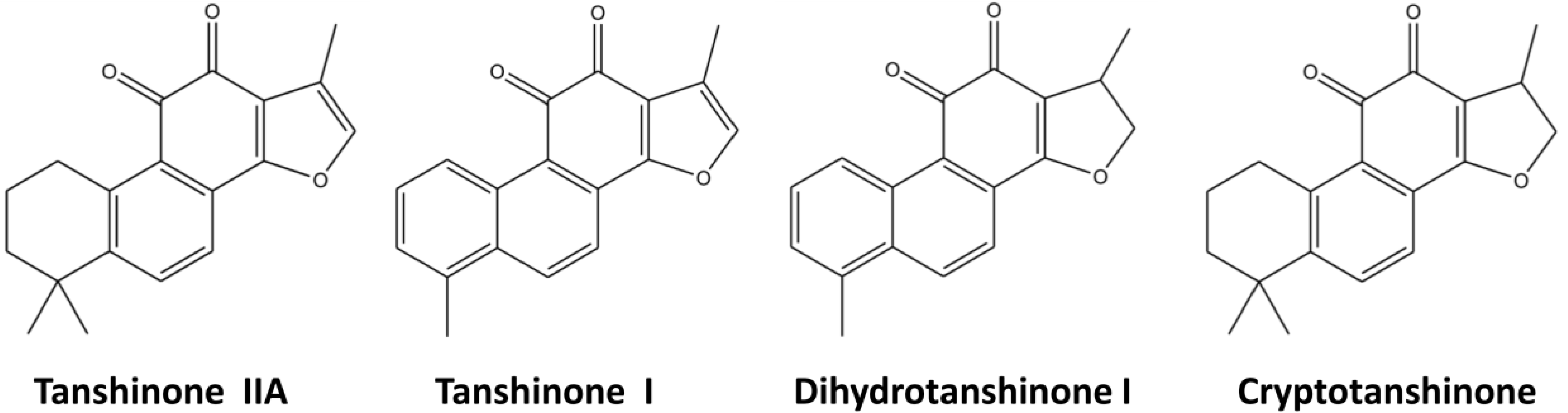
2. Results and Discussion
2.1. Establishment of Salvia miltiorrhiza Hairy Root Cultures
2.2. Effects of Combined Elicitors on SmCPS Expression

2.3. Tanshinone Production in S. miltiorrhiza Hairy Root Cultures
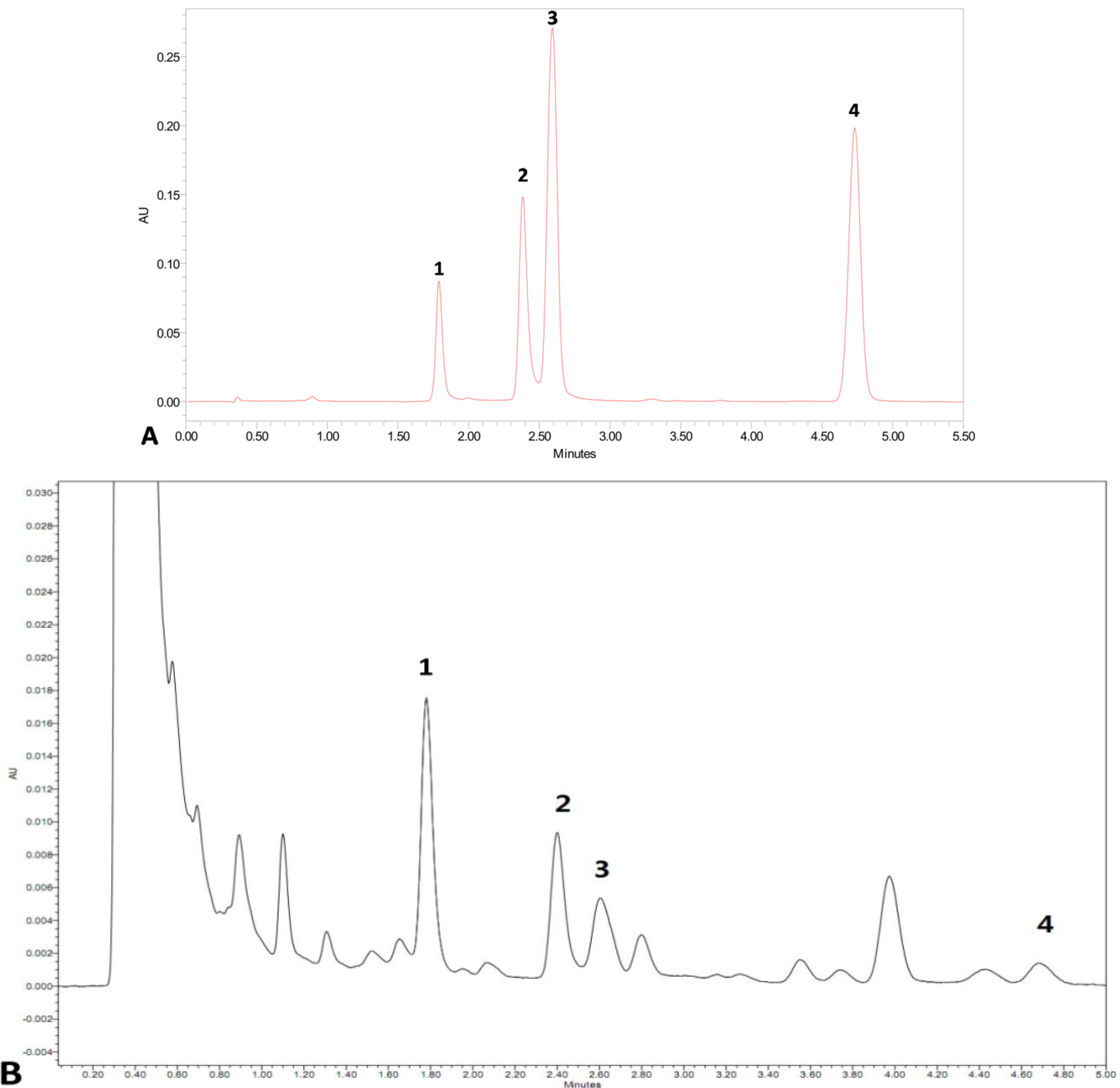

2.4. Effects of Combined Elicitors on the Accumulation of Cryptotanshinone and Dihydrotanshinone I

3. Experimental
3.1. Plant Materials
3.2. Growth of Agrobacterium rhizogenes and Hairy Root Cultures
3.3. Elicitation of Combined Elicitors
3.4. Metabolite Analysis of Hairy Roots
3.5. Quantitative Real-Time PCR
| gene | Sequences of the primers(5′→3′) |
|---|---|
| SmCPS | GAGGGAGAGGTGAGGAAGGAA |
| AGGGAACAAAAGTTGAAAAGG | |
| β-Actin | AGGAACCACCGATCCAGACA |
| GGTGCCCTGAGGTCCTGTT |
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhou, L.M.; Zuo, Z.; Chow, M.S.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 4, 1345–1359. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Zhou, C.C.; Xiao, J.B.; Liao, P.; Kai, G.Y. Metabolic regulation and genetic engineering of pharmaceutical component tanshinone biosynthesis in Salvia miltiorrhiza. J. Med. Plant Res. 2010, 4, 2591–2597. [Google Scholar]
- Wang, X.; Morris-Natschke, S.L.; Lee, K.H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med. Res. Rev. 2007, 27, 133–148. [Google Scholar] [CrossRef]
- Dong, Y.; Morris-Natschke, S.L.; Lee, K.H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat. Prod. Rep. 2011, 28, 529–542. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.; Rahman, L.U. Biotransformation studies using hairy root culture—A review. Biotechnol. Adv. 2012, 30, 461–468. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, L. Transformed hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Hairy root research: Recent scenario and exciting prospects. Curr. Opin. Plant Biol. 2006, 9, 341–346. [Google Scholar] [CrossRef]
- Hu, Z.B.; Alfermann, A.W. Diterpenoid production in hairy root cultures of Salvia miltiorrhiza. Phytochemistry 1993, 32, 699–703. [Google Scholar] [CrossRef]
- Zhou, L.G.; Wu, J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006, 23, 789–810. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar]
- Choi, D.W.; Jung, J.D.; Ha, Y.I.; Park, H.W.; In, D.S.; Chung, H.J.; Liu, J.R. Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep. 2005, 23, 557–566. [Google Scholar] [CrossRef]
- Li, W.Y.; Gao, W.; Shao, A.J.; He, Y.F.; Huang, L.Q. Induction and manipulation of secondary metabolic on effective ingredients in Salvia miltiorrhiza by elicitors. China J. Chin. Mater. Med. 2011, 36, 258–262. [Google Scholar]
- Khalili, M.; Hasanloo, T.; Tabar, S.K.K. Ag+ enhanced silymarin production in hairy root cultures of Silybum marianum (L.) Gaertn. Plant Omics J. 2010, 3, 109–114. [Google Scholar]
- Ge, X.C.; Wu, J.Y. Tanshinone production and isoprenoid pathways in Salvia miltionrrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci. 2005, 168, 487–491. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell 2009, 98, 25–33. [Google Scholar]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell culture. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef]
- Gao, W.; Hillwig, M.L.; Huang, L.Q.; Cui, G.H.; Wang, X.; Kong, J.; Yang, B.; Peters, R.J. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org. Lett. 2009, 11, 5170–5173. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Gao, W.; Rong, Q.X.; Jin, G.J.; Chu, H.Y.; Liu, W.J.; Yang, W.; Zhu, Z.W.; Li, G.H.; Zhu, G.F.; Huang, L.Q.; Zhao, Z.B. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J. Am. Chem. Soc. 2012, 134, 3234–3241. [Google Scholar] [CrossRef]
- Hu, Z.B.; Du, M. Hairy root and its application in plant genetic engineering. J. Integr. Plant Biol. 2006, 48, 121–127. [Google Scholar] [CrossRef]
- Stiles, A.R.; Liu, C.Z. Hairy root culture: Bioreactor design and process intensification. Adv. Biochem. Eng. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Kai, G.Y.; Liao, P.; Xu, H.; Wang, J.; Zhou, C.C.; Zhou, W.; Qi, Y.P.; Xiao, J.B.; Wang, Y.L.; Zhang, L. Molecular mechanism of elicitor-induced tanshinone accumulation in Salvia miltiorrhiza hairy root cultures. Acta Physiol. Plant. 2012, 34, 1421–1433. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F. Effect of yeast elicitor on the secondary metabolism of Ti-transformed Salvia miltiorrhiza cell suspension cultures. Plant Cell Rep. 2000, 19, 710–717. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, Q.; Cheuk, W.K.; Wu, J.Y. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root culture by Ag+ elicitation and nutrient feeding. Planta Med. 2004, 70, 147–151. [Google Scholar] [CrossRef]
- Shi, M.; Kwok, K.W.; Wu, J.Y. Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy-root culture by hyperosmotic stress and yeast elicitor. Biotechnol. Appl. Biochem. 2007, 46, 191–196. [Google Scholar] [CrossRef]
- Zhang, S.C.; Liu, Y.; Shen, S.; Liang, Z.S.; Yang, D.F. Effects of elicitors on accumulation of phenolic acids and tanshinones in Salvia miltiorrhiza hairy root. China J. Chin. Meter. Med. 2011, 36, 1269–1274. [Google Scholar]
- Zhong, J.J.; Yue, C.J. Plant cells: Secondary metabolite heterogeneity and its manipulation. Adv. Biochem. Eng. Biotechnol. 2005, 100, 53–88. [Google Scholar]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Kang, Y.M.; Park, D.J.; Jung, H.N.; Kim, S.W.; Choi, M.S. Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. In Vitro Cell Dev. Biol. Plant. 2006, 42, 44–49. [Google Scholar] [CrossRef]
- Peters, R.J.; Croteau, R.B. Metabolic Engineering of Plant Secondary Metabolism. In Handbook of Plant Biotechnology: Applications of Plant Biotechnology in Agriculture, the Pharmaceutical Industry, and Other Industries; Kishore, G., Ed.; John Wiley & Sons Ltd.: London, UK, 2004; Volume 2, pp. 609–628. [Google Scholar]
- Xu, M.M.; Hillwig, M.L.; Prisic, S.; Coates, R.M.; Peters, R.J. Functional identification of rice syn-copalyl diphosphate synthase and its role in initiating biosynthesis of diterpenoid. Plant J. 2004, 39, 309–318. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, Q.; He, Y.; Li, G.; Liu, Y.; Gao, W.; Huang, L. Effects of Combined Elicitors on Tanshinone Metabolic Profiling and SmCPS Expression in Salvia miltiorrhiza Hairy Root Cultures. Molecules 2013, 18, 7473-7485. https://doi.org/10.3390/molecules18077473
Cheng Q, He Y, Li G, Liu Y, Gao W, Huang L. Effects of Combined Elicitors on Tanshinone Metabolic Profiling and SmCPS Expression in Salvia miltiorrhiza Hairy Root Cultures. Molecules. 2013; 18(7):7473-7485. https://doi.org/10.3390/molecules18077473
Chicago/Turabian StyleCheng, Qiqing, Yunfei He, Geng Li, Yujia Liu, Wei Gao, and Luqi Huang. 2013. "Effects of Combined Elicitors on Tanshinone Metabolic Profiling and SmCPS Expression in Salvia miltiorrhiza Hairy Root Cultures" Molecules 18, no. 7: 7473-7485. https://doi.org/10.3390/molecules18077473




