Enhancement of the Controlled-Release Properties of Chitosan Membranes by Crosslinking with Suberoyl Chloride
Abstract
:1. Introduction
2. Results and Discussion
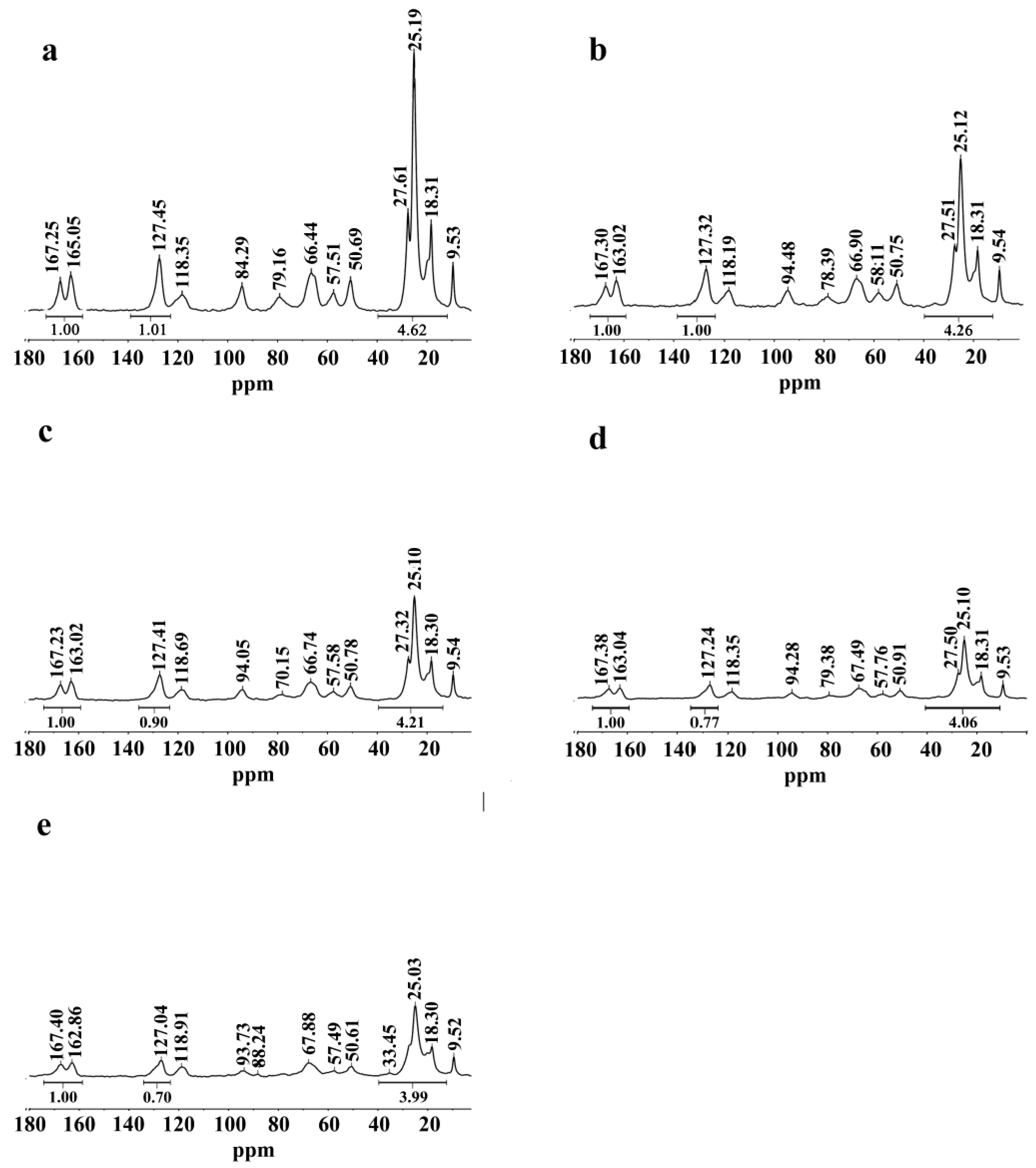
2.1. Chemical Structure
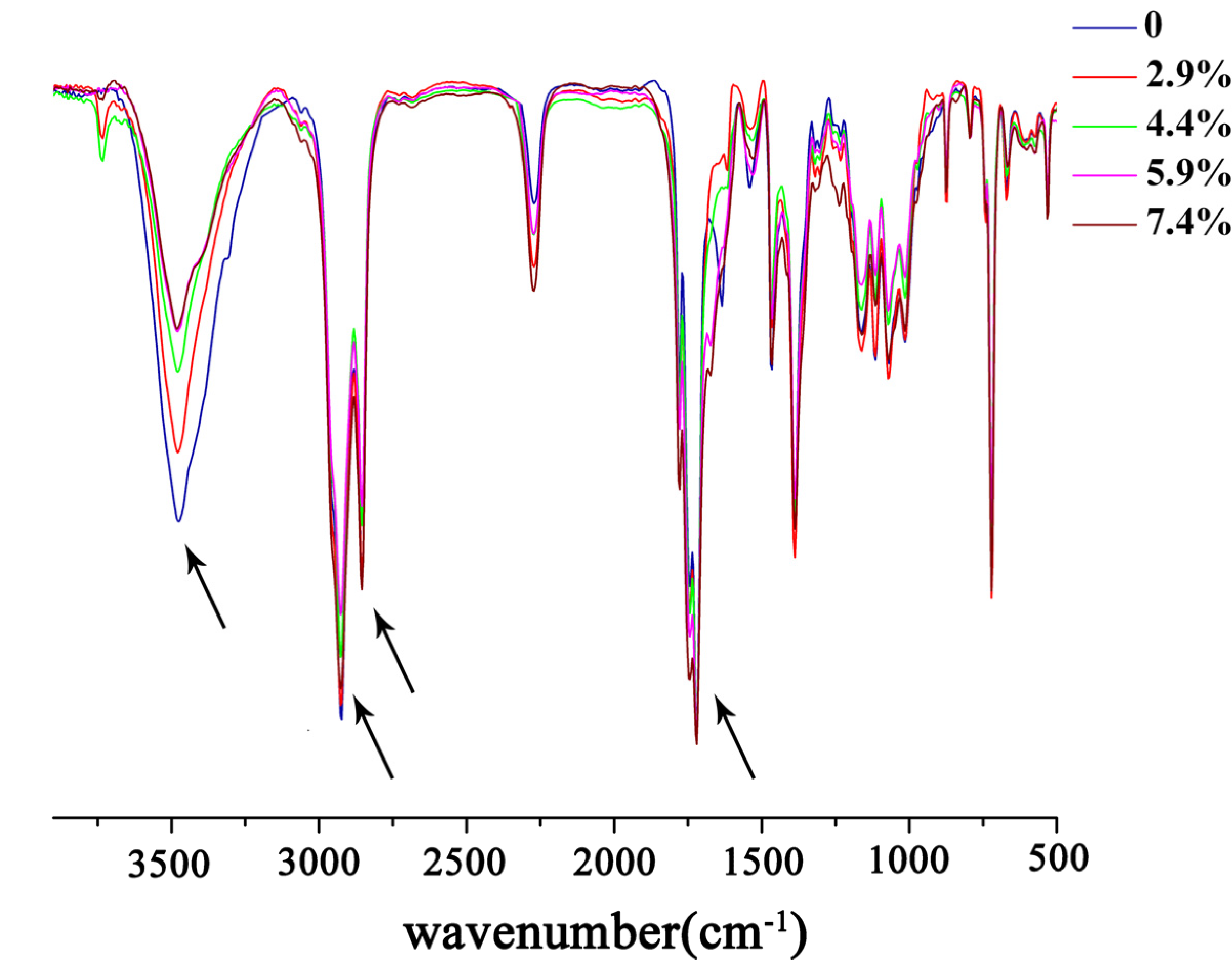
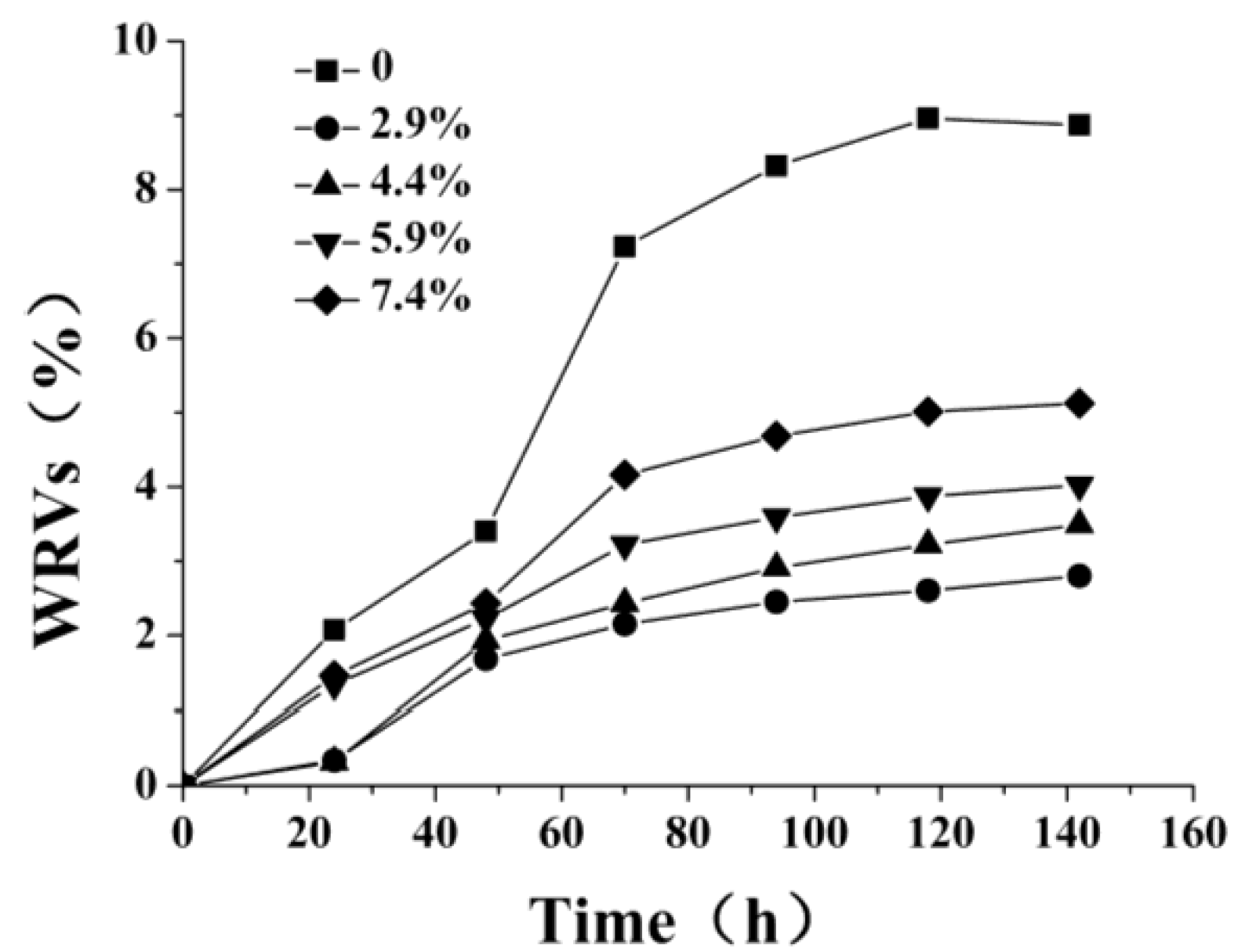
2.2. Measurement of Hydrophobicity
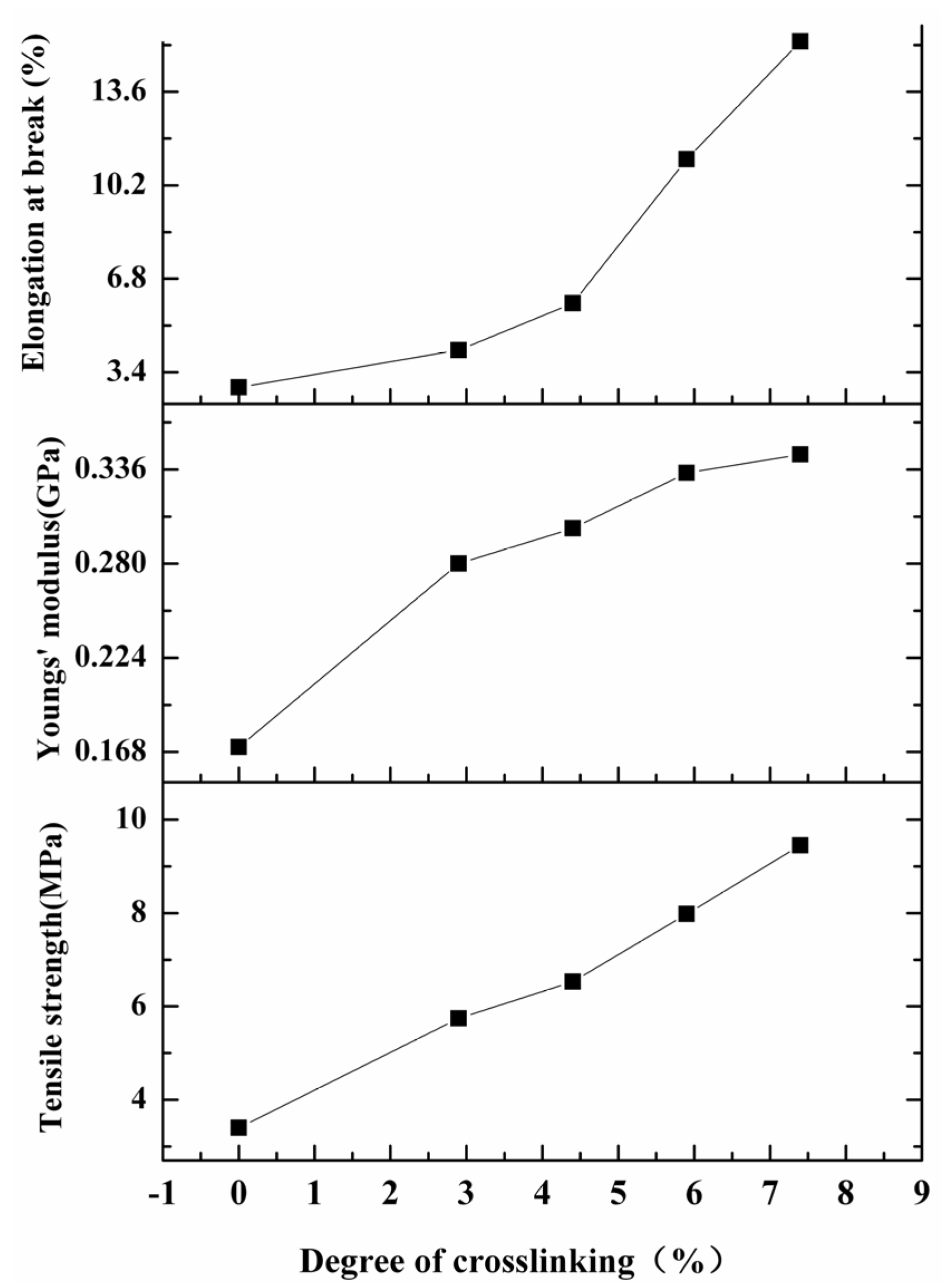
2.3. Mechanical Properties
2.4. Permeability of Macro-Nutrients and Micro-Nutrients
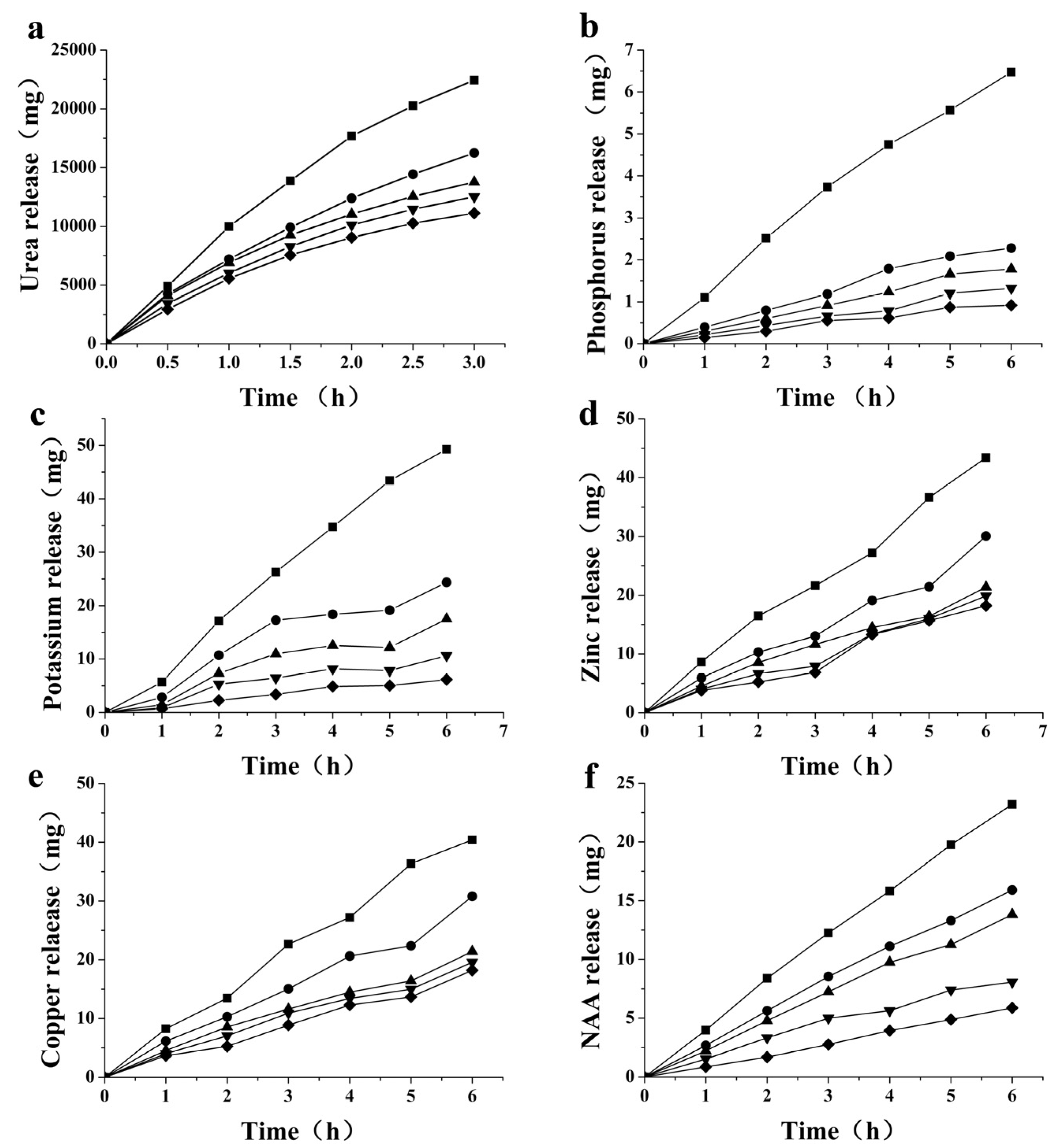
2.5. Permeability of Plant Growth Regulator NAA
3. Experimental
3.1. Materials
3.2. Synthesis and Film Forming of Crosslinked N-Phthaloyl Acylated Chitosan
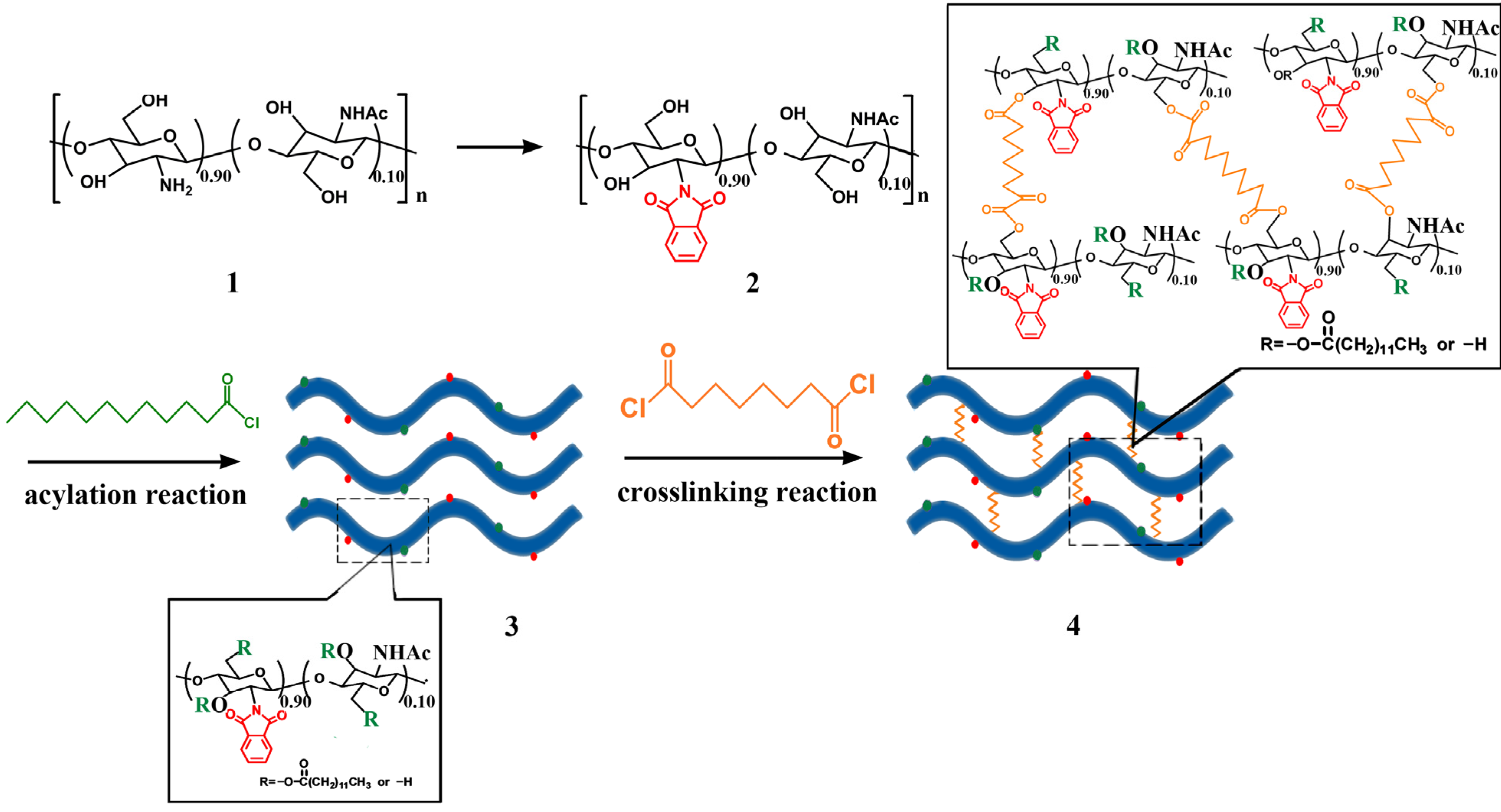
3.3. FT-IR Spectroscopy
3.4. Solid-State 13C-NMR
3.5. Mechanical Properties
3.6. Water Retention Values (WRVs)
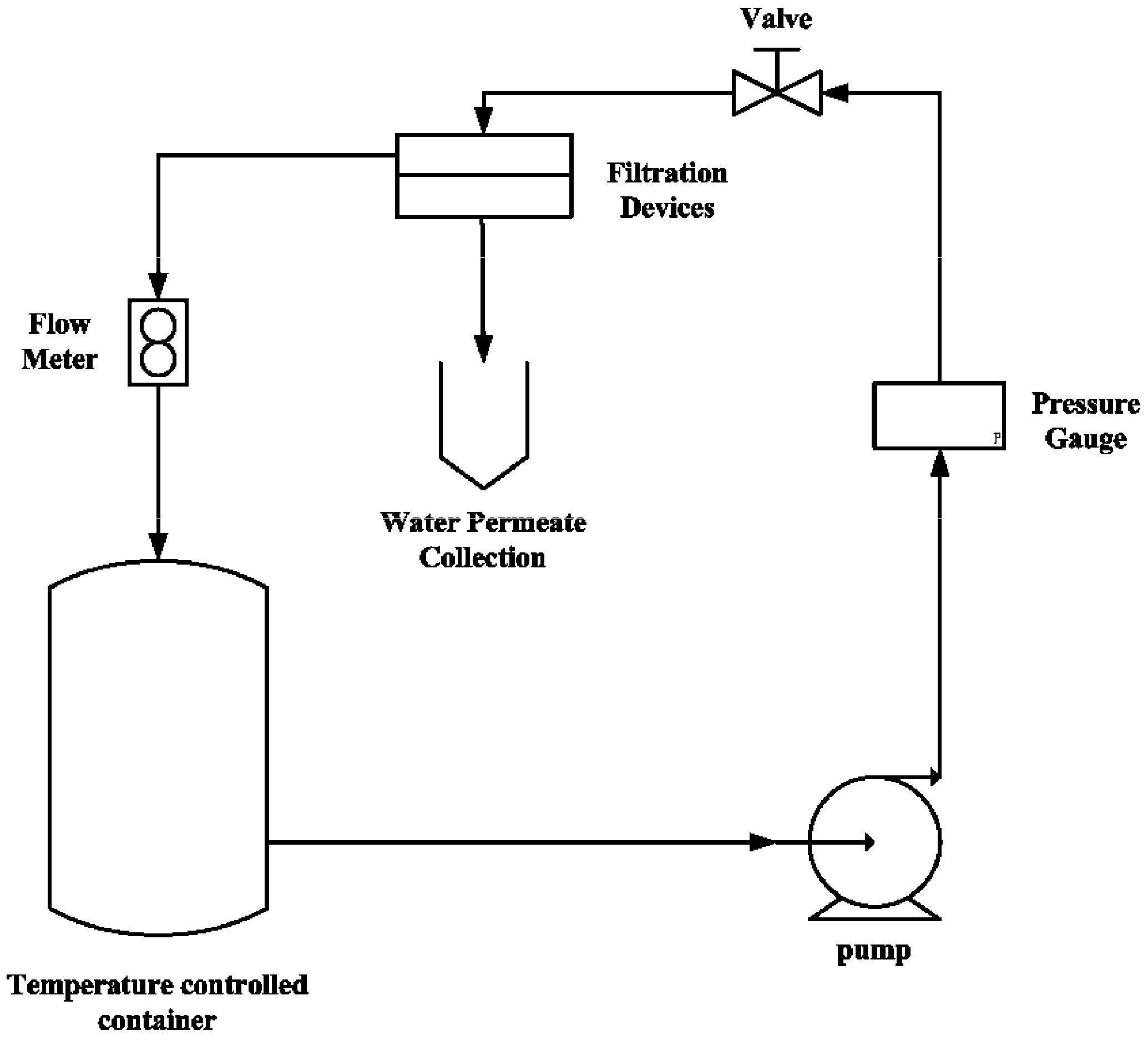
3.7. Permeability of N/P/K, NAA, Cu2+ and Zn2+
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.; Lin, V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Delive. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J.; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm. 2001, 212, 19–28. [Google Scholar] [CrossRef]
- Holland, S.J.; Tighe, B.J. Polymers for biodegradable medical devices. 1. The potential of polyesters as controlled macromolecular release systems. J. Control. Release 1986, 4, 155–180. [Google Scholar] [CrossRef]
- Song, S.; Hidajat, K.; Kawi, S. Functionalized SBA-15 materials as carriers for controlled drug delivery: Influence of surface properties on matrix-drug interactions. Langmuir 2005, 21, 9568–9575. [Google Scholar] [CrossRef]
- Polk, A.; Amsden, B.; Yao, K.D.; Peng, T.; Goosen, M.F.A. Controlled release of albumin from chitosan-alginate microcapsules. J. Pharm. Sci. 1994, 83, 178–185. [Google Scholar] [CrossRef]
- Hussain, M.R.; Devi, R.R.; Maji, T.K. Controlled release of urea from chitosan microspheres prepared by emulsification and cross-linking method. Iranian Polym. J. 2012, 21, 473–479. [Google Scholar]
- Jarosiewicz, A.; Tomaszewska, M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003, 51, 413–417. [Google Scholar] [CrossRef]
- Celis, R.; Hermosín, M.C.; Carrizosa, M.J.; Cornejo, J. Inorganic and organic clays as carriers for controlled release of the herbicide hexazinone. J. Agric. Food Chem. 2002, 50, 2324–2330. [Google Scholar]
- Taki, S.; Badens, E.; Charbit, G. Controlled release system formed by supercritical anti-solvent coprecipitation of a herbicide and a biodegradable polymer. J. Supercrit. Fluids 2001, 21, 61–70. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Aminabhavi, T.M. Preparation and characterization of interpenetrating network beads of poly (vinyl alcohol)-grafted-poly (acrylamide) with sodium alginate and their controlled release characteristics for cypermethrin pesticide. J. Appl. Polym. Sci. 2001, 84, 552–560. [Google Scholar] [CrossRef]
- Gerstl, Z.; Nasser, A.; Mingelgrin, U. Controlled release of pesticides into soils from clay-polymer formulations. J. Agric. Food Chem. 1998, 46, 3797–3802. [Google Scholar] [CrossRef]
- Hussein, M.Z.; Zainal, Z.; Yahaya, A.H.; Foo, D.W.V. Controlled release of a plant growth regulator, α-naphthaleneacetate from the lamella of Zn-Al-layered double hydroxide nanocomposite. J. Control. Release 2002, 82, 417–427. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotech. Adv. 2011, 29, 792–803. [Google Scholar]
- Chen, Y.; Ding, J.; Qin, W. Polycation-sensitive membrane electrode for determination of heparin based on controlled release of protamine. Analyst 2012, 137, 1944–1949. [Google Scholar] [CrossRef]
- Kawashima, T.; Nagai, N.; Kaji, H.; Kumasaka, N.; Onami, H.; Ishkawa, Y.; Osumi, N.; Nishizawa, M.; Abe, T. A scalable controlled-release device for transscleral drug delivery to the retina. Biomaterials 2011, 32, 1950–1956. [Google Scholar] [CrossRef]
- Loh, X.J.; Peh, P.; Liao, S.; Sng, C.; Li, J. Controlled drug release from biodegradable thermo-responsive physical hydrogel nanofibers. J. Control. Release 2010, 143, 175–182. [Google Scholar] [CrossRef]
- Sutton, S.; Campbell, N.L.; Cooper, A.I.; Kirkland, M.; Frith, W.J.; Adams, D.J. Controlled release from modified amino acid hydrogels governed by molecular size or network dynamics. Langmuir 2009, 25, 10285–10291. [Google Scholar] [CrossRef]
- Thornton, P.D.; Mart, R.J.; Webb, S.J.; Ulijin, R.V. Enzyme-responsive hydrogel particles for the controlled release of proteins: designing peptide actuators to match payload. Soft Matter 2008, 4, 821–827. [Google Scholar] [CrossRef]
- Wang, G.; Xie, R.; Ju, X.; Chu, L. Thermo-responsive polyethersulfone composite membranes blended with poly (N-isopropylacrylamide) nanogels. Chem. Eng. Technol. 2012, 35, 2015–2022. [Google Scholar] [CrossRef]
- Shoji, S.; Kanno, H. Use of polyolefin-coated fertilizers for increasing fertilizer efficiency and reducing nitrate leaching and nitrous oxide emissions. Fertilizer Res. 1994, 39, 147–152. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Belmonte, M.M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Kennedy, J.F.; Nie, J. Synthesize and characterization of organic-soluble acylated chitosan. Carbohydr. Polym. 2009, 75, 390–394. [Google Scholar] [CrossRef]
- Xiao, S.; Feng, X.; Huang, R.Y.M. Trimesoyl chloride crosslinked chitosan membranes for CO2/N2 separation and pervaporation dehydration of isopropanol. J. Memb. Sci. 2007, 306, 36–46. [Google Scholar] [CrossRef]
- Kurita, K.; Mori, S.; Nishiyama, Y.; Harata, M. N-alkylation of chitin and some characteristics of the novel derivatives. Polym. Bull. 2002, 48, 159–166. [Google Scholar] [CrossRef]
- Morimoto, M.; Nakao, M.; Ishibashi, N.; Shigemasa, Y.; Ifuku, S.; Saimoto, H. Synthesis of novel chitosan with chitosan side chains. Carbohydr. Polym. 2011, 84, 727–731. [Google Scholar] [CrossRef]
- Hirano, S.; Zhang, M.; Chung, B.G.; Kim, S.K. The N-acylation of chitosan fiber and the N-deacetylation of chitin fiber and chitin-cellulose blended fiber at a solid state. Carbohydr. Polym. 2000, 41, 175–179. [Google Scholar] [CrossRef]
- Sreedhar, B.; Aparna, Y.; Sebalkar, M.N. Preparation and characterization of HAP/carboxymethyl chitosan nanocomposites. J. Appl. Polym. Sci. 2007, 105, 928–934. [Google Scholar] [CrossRef]
- Osifo, P.O.; Masala, A. Characterization of direct methanol fuel cell (DMFC) applications with H2SO4 modified chitosan membrane. J. Power Sources 2010, 195, 4915–4922. [Google Scholar] [CrossRef]
- Chen, C.; Tao, S.; Qiu, X.; Ren, X.; Hu, S. Long-alkane-chain modified N-phthaloyl chitosan membranes with controlled permeability. Carbohydr. Polym. 2013, 91, 269–276. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Jarosiewicz, A. Use of polysulfone in controlled-release NPK fertilizer formulations. J. Agric. Food Chem. 2002, 50, 4634–4639. [Google Scholar] [CrossRef]
- Qiu, X.; Tao, S.; Ren, X.; Hu, S. Modified cellulose films with controlled permeability and biodegradability by crosslinking with toluene diisocyanate under homogeneous conditions. Carbohydr. Polym. 2012, 88, 1272–1280. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13C-NMR. Carbohydr. Polym. 2004, 58, 409–416. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, J.; Wang, M.; Zhang, H.; Han, C. High performance ultrafiltration membrane based on modified chitosan coating and electrospun nanofibrous PVDF scaffolds. J. Memb. Sci. 2012, 394–395, 209–217. [Google Scholar]
- Sangamesh, G.K.; Kumaresh, S.S.; Tejraj, M.A. Synthesis and characterization of polyacrylamide-grafted chitosan hydrogel microspheres for the controlled release of indomethacin. J. Appl. Polym. Sci. 2002, 87, 1525–1536. [Google Scholar]
- Topacli, C.; Topacli, M.; Clivan, M.; Ercan, F.; Durmus, M.; Ahsen, V. Structural characterization of Langmuir-Blodgett films of 4,5-bis (dodecyloxy) phthalic acid. Thin Solid Films 2008, 516, 8299–8306. [Google Scholar] [CrossRef]
- Willie, A. B.; Fabio, M. Hydrogen bonding of adenine with benzoic acid in the solid state: An FTIR study. Spectrochim. Acta Part A Mol. Spectrosc. 1993, 49, 249–256. [Google Scholar]
- Goodenough, K.M.; Moran, W.J.; Raubo, D.; Harrity, J.P.A. Development of a flexible approach to nuphar alkaloids via two enantiospecific piperidine-forming reactions. J. Org. Chem. 2005, 70, 207–213. [Google Scholar] [CrossRef]
- Tao, S.; Pang, R.; Chen, C.; Ren, X.; Hu, S. Synthesis, characterization and slow release properties. Carbohydr. Polym. 2012, 88, 1189–1194. [Google Scholar] [CrossRef]
- Baldock, J.A.; Oades, J.M.; Waters, A.G.; Peng, X.; Vassallo, A.M.; Wilson, M.A. Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C-NMR spectroscopy. Biogeochemistry 1992, 16, 1–42. [Google Scholar]
- Stevelmans, S.; Van Hest, J.C.M.; Jansen, J.F.G.A.; Van Boxtel, D.A.F.J.; de Brabander-van den Berg, E.M.M.; Meijer, E.M. Synthesis, characterization, and guest-host properties of inverted unimolecular dendritic micelles. J. Am. Chem. Soci. 1996, 118, 7398–7399. [Google Scholar] [CrossRef]
- Ioannis, N.; Konstantina, T.; Stavros, M. Water retention and drainage in different brands of microcrystalline cellulose: Effect of measuring conditions. Eur. J. Pharm. Biopharm. 2006, 63, 278–287. [Google Scholar] [CrossRef]
- Siroka, B.; Noisterning, M.; Griesser, U.J.; Bechtold, T. Characterization of cellulosic fibers and fabrics by sorption/desorprion. Carbohydr. Res. 2008, 343, 2194–2199. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2458–2491. [Google Scholar]
- Tien, C.L.; Lacroix, M.; Ispas-Szabo, P.; Mateescu, M.A. N-acylated chitosan: hydrophobic matrices for controlled drug release. J. Control. Release 2003, 93, 1–13. [Google Scholar] [CrossRef]
- Kobraee, S.; Shamsi, K.; Rasekhi, B. Micronutrients fertilizer and soybean nutritional. Ann. Biol. Res. 2011, 2, 468–475. [Google Scholar]
- Shavit, U.; Shaviv, A.; Shalit, G.; Zaslavsky, D. Release characteristics of a new controlled release fertilizer. J. Control. Release 1997, 43, 131–138. [Google Scholar] [CrossRef]
- Hartikainen, H.; Pitkänen, M.; Kairesalo, T.; Tuominen, L. Co-occurrence and potential chemical competition of phosphorus and silicon in lake sediment. Water Res. 1996, 30, 2472–2478. [Google Scholar] [CrossRef]
- Reed, M.G.; Scott, A.D. Flame photometric methods of determining the potassium in potassium tetraphenylborate. Anal. Chem. 1961, 33, 773–775. [Google Scholar] [CrossRef]
- Smith, D.L.; Jamieson, D.R.; Elving, P.J. Direct titration of potassium with tetraphenylborate amperometric equivalence-point detection. Anal. Chem. 1960, 32, 1253–1258. [Google Scholar] [CrossRef]
- Zou, X.J.; Wang, Z.X.; Dai, X.M.; Zhou, Y.; Ma, X.J. Rate of controlled release urea pervasion through membrane determined by ultraviolet spectrophotometry (In Chinese). Spectrosc. Spect. Anal. 2006, 26, 1151–1154. [Google Scholar]
- Sample Availability: Samples of the compounds N-phthaloyl acylated chitosan and crosslinked N-phthaloyl acylated chitosan are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, C.; Gao, Z.; Qiu, X.; Hu, S. Enhancement of the Controlled-Release Properties of Chitosan Membranes by Crosslinking with Suberoyl Chloride. Molecules 2013, 18, 7239-7252. https://doi.org/10.3390/molecules18067239
Chen C, Gao Z, Qiu X, Hu S. Enhancement of the Controlled-Release Properties of Chitosan Membranes by Crosslinking with Suberoyl Chloride. Molecules. 2013; 18(6):7239-7252. https://doi.org/10.3390/molecules18067239
Chicago/Turabian StyleChen, Chao, Zideng Gao, Xiaoyun Qiu, and Shuwen Hu. 2013. "Enhancement of the Controlled-Release Properties of Chitosan Membranes by Crosslinking with Suberoyl Chloride" Molecules 18, no. 6: 7239-7252. https://doi.org/10.3390/molecules18067239



