General Intermediates for the Synthesis of 6-C-Alkylated DMDP-Related Natural Products
Abstract
:1. Introduction
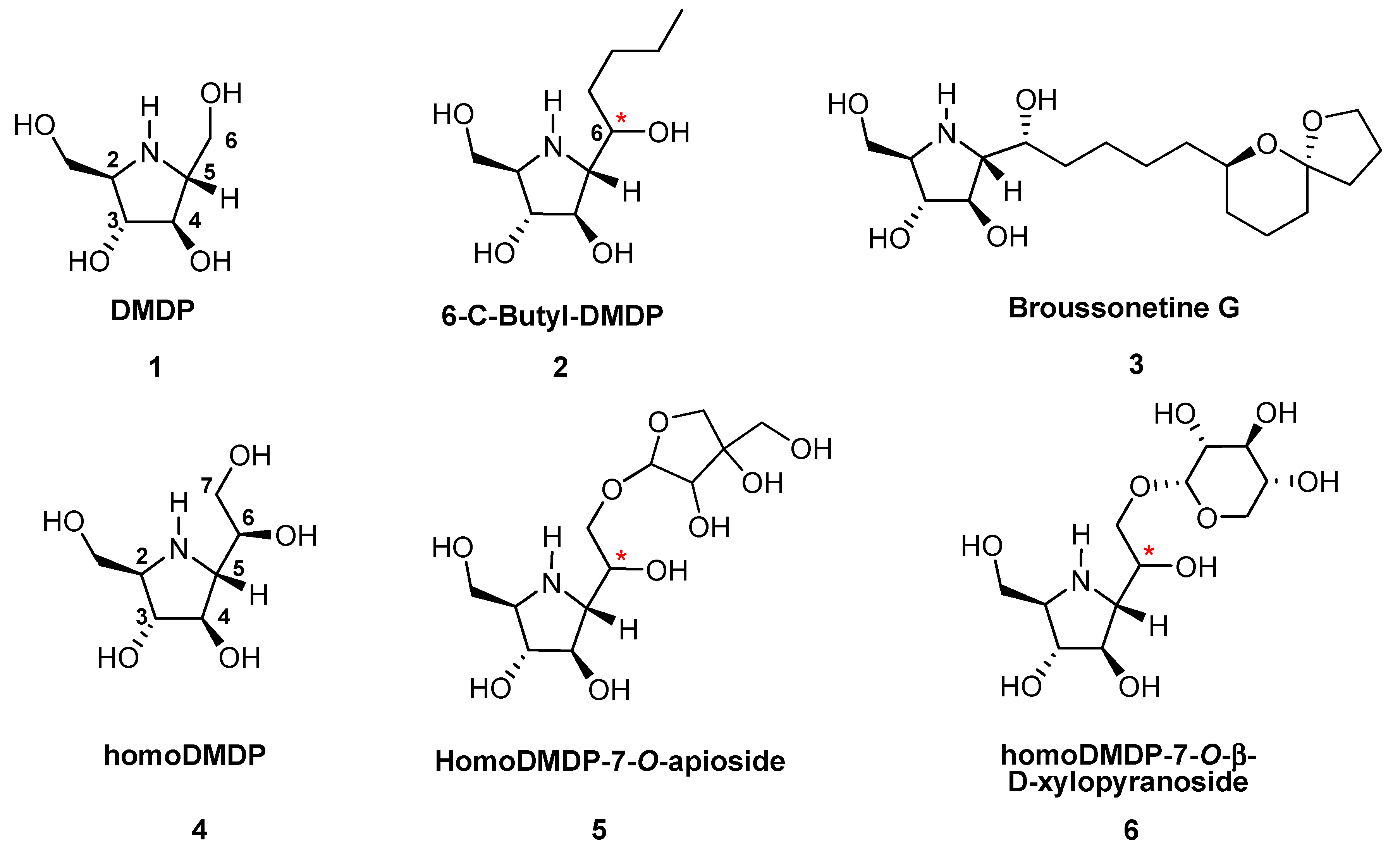
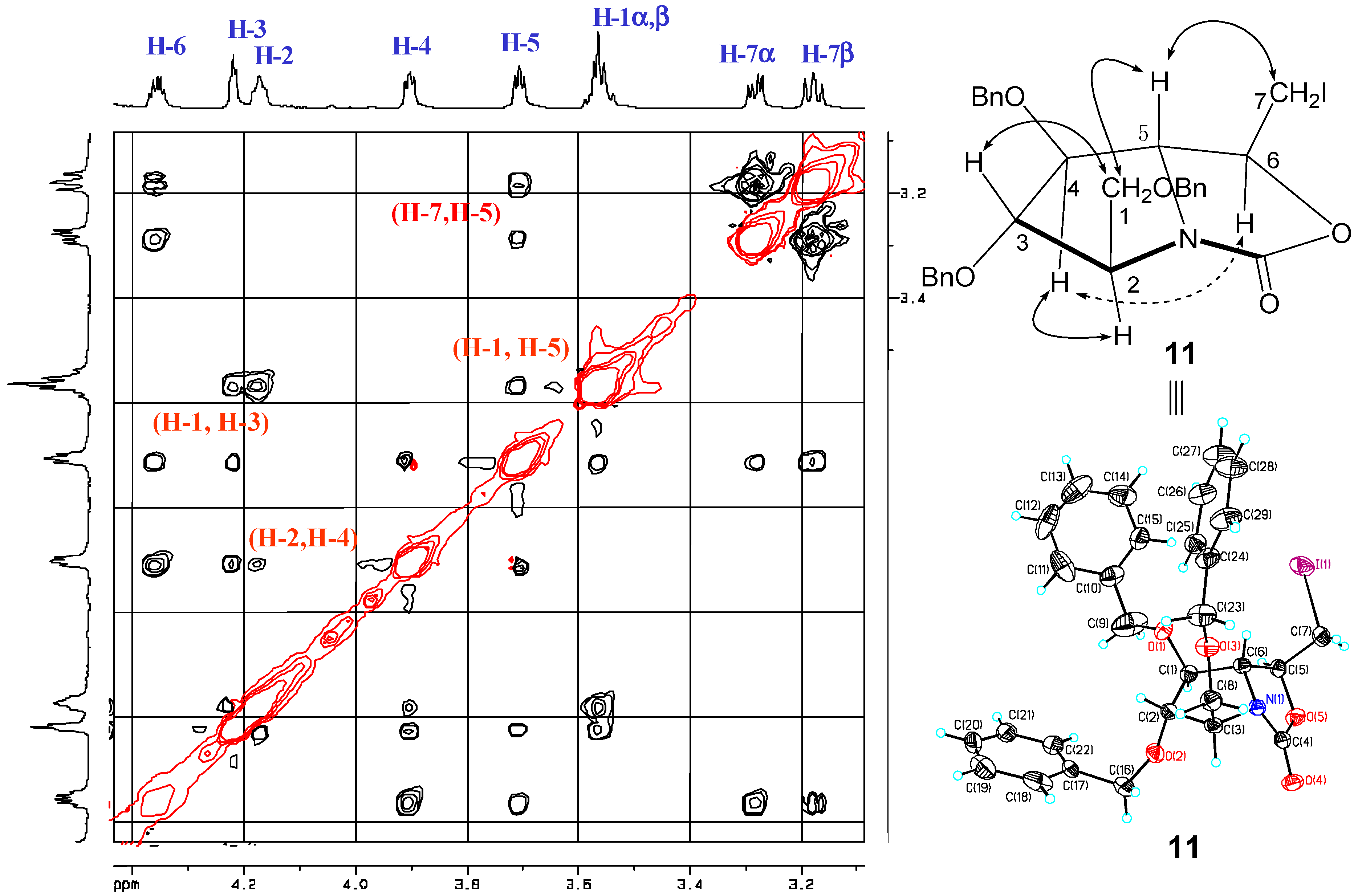
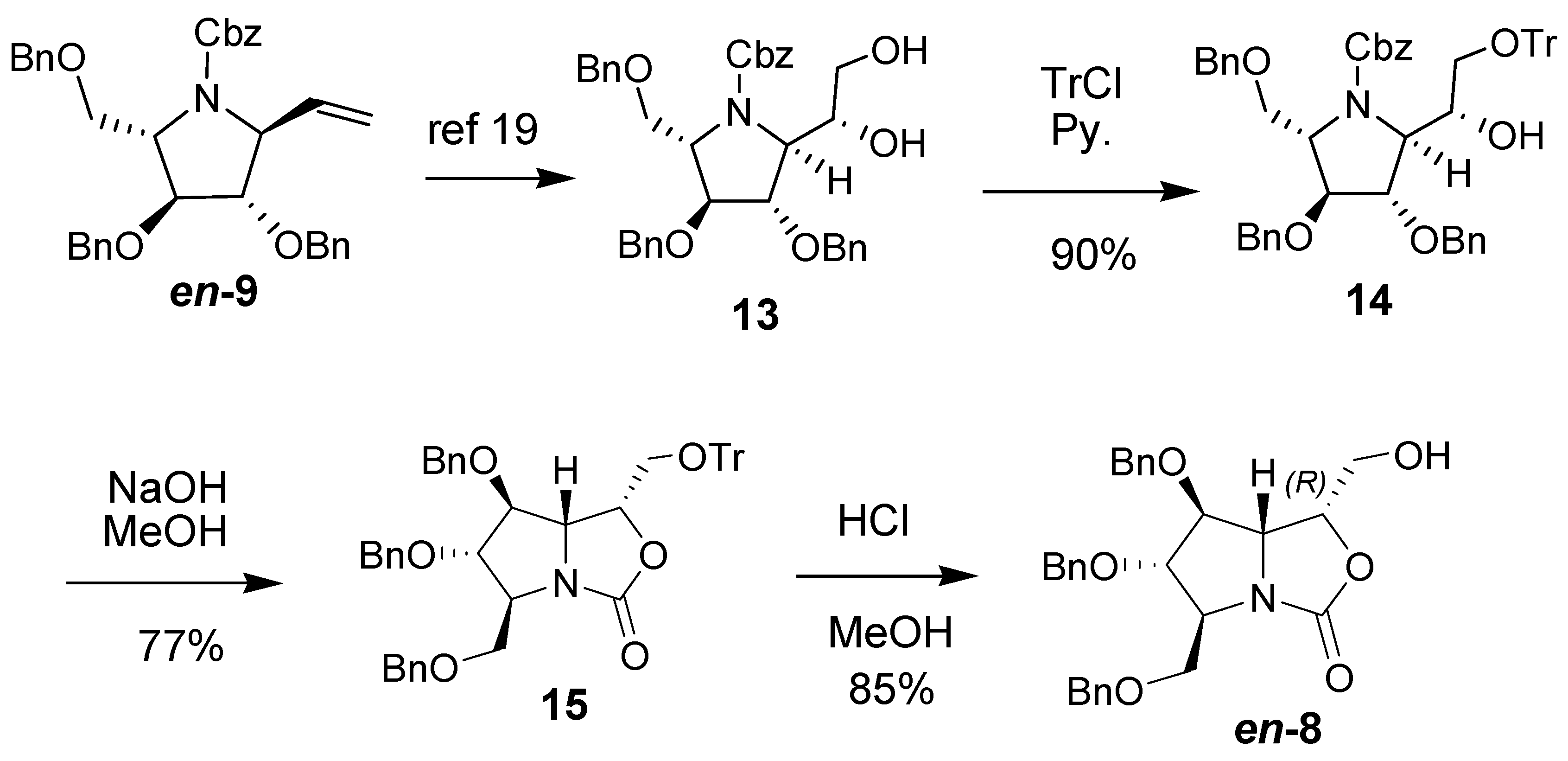
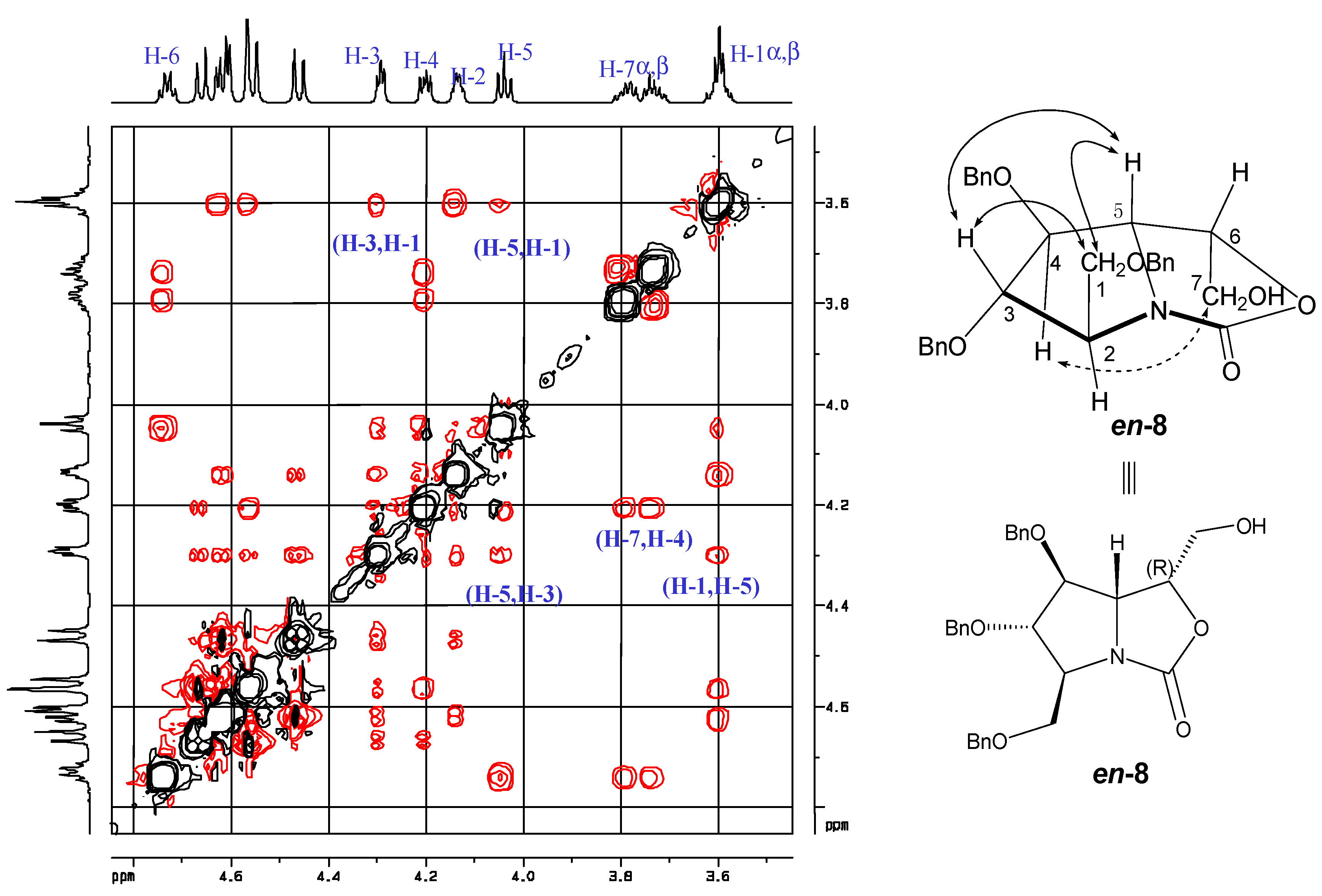
2. Results and Discussion

3. Experimental
3.1. General
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Welter, A.; Jadot, J.; Dardenne, G.; Marlier, M.; Casimir, J. 2,5-Dihydroxymethyl 3,4-dihydroxypyrrolidine dans les feuilles de Derris elliptica. Phytochemistry 1976, 15, 747–749. [Google Scholar]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W.J. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Watson, A.A.; Fleet, G.W. J.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids-natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265–295. [Google Scholar]
- Horne, G.; Wilson, F.X.; Tinsley, J.; Williams, D.H.; Storer, R. Iminosugars past, present and future: Medicines for tomorrow. Drug Discov. Today 2011, 16, 107–118. [Google Scholar] [CrossRef]
- Asano, N.; Nishida, M.; Miyauchi, M.; Ikeda, K.; Yamamoto, M.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Fleet, G.W.J. Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulaceae). Phytochemistry 2000, 53, 379–382. [Google Scholar]
- Shibano, M.; Nakamura, S.; Akazawa, N.; Kusano, G. Studies on the constituents of Broussonetia species. III. Two new pyrrolidine alkaloids, broussonetines G and H, as inhibitors of glycosidase, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull 1998, 46, 1048–1050. [Google Scholar] [CrossRef]
- Trost, B.M.; Horne, D.B.; Woltering, M.J. Palladium-catalyzed DYKAT of vinyl epoxides: Enantioselective total synthesis and assignment of the configuration of (+)-broussonetine G. Angew. Chem. Int. Ed. 2003, 42, 5987–5990. [Google Scholar] [CrossRef]
- Trost, B.M.; Horne, D.B.; Woltering, M.J. Palladium-catalyzed DYKAT of butadiene monoepoxide: Enantioselective total synthesis of (+)-DMDP, (−)-bulgecinine, and (+)-broussonetine G. Chem. Eur. J. 2006, 12, 6607–6620. [Google Scholar] [CrossRef]
- Hiranuma, S.; Shimizu, T.; Nakata, T.; Kajimoto, T.; Wong, C. Synthesis and inhibition analysis of five-membered homoazasugars from D-arabinofuranose via an SN2 reaction of the chloromethylsulfonate. Tetrahedron Lett. 1995, 36, 8247–8250. [Google Scholar]
- Watson, A.A.; Nash, R.J.; Wormald, M.R.; Harvey, D.J.; Dealler, S.; Lees, E.; Asano, N.; Kizu, H.; Kato, A.; Griffiths, R.C.; et al. Glycosidase-inhibiting pyrrolidine alkaloids from Hyacinthoides non-scripta. Phytochemistry 1997, 46, 255–259. [Google Scholar]
- Kato, A.; Adachi, I.; Miyauchi, M.; Ikeda, K.; Komae, T.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Wormald, M.R.; et al. Polyhydroxylated pyrrolidine and pyrrolizidine alkaloids from Hyacinthoides non-scripta and Scilla campanulata. Carbohydr. Res. 1999, 316, 95–103. [Google Scholar] [CrossRef]
- Jones, S.B.; Simmons, B.; Mastracchio, A.; MacMillan, D.W.C. Collective synthesis of natural products by means of organocascade catalysis. Nature 2011, 475, 183–188. [Google Scholar] [CrossRef]
- Yu, C.; Asano, N.; Ikeda, K.; Wang, M.; Butters, T.D.; Wormald, M.R.; Dwek, R.A.; Winters, A.L.; Nash, R.J.; Fleet, G.W.J. Looking glass inhibitors: L-DMDP, a more potent and specific inhibitor of [small alpha]-glucosidases than the enantiomeric natural product DMDP. Chem. Commun. 2004, 1936–1937. [Google Scholar]
- Hu, X.; Bartholomew, B.; Nash, R.J.; Wilson, F.X.; Fleet, G.W.J.; Nakagawa, S.; Kato, A.; Jia, Y.; Well, R.V.; Yu, C. Synthesis and glycosidase inhibition of the enantiomer of (−)-Steviamine, the first example of a new class of indolizidine alkaloid. Org. Lett. 2010, 12, 2562–2565. [Google Scholar] [CrossRef]
- Wang, W.; Huang, M.; Li, Y.; Rui, P.; Hu, X.; Zhang, W.; Su, J.; Zhang, Z.; Zhu, J.; Xu, W.; et al. A practical synthesis of sugar-derived cyclic nitrones: Powerful synthons for the synthesis of iminosugars. Synlett 2010, 488–492. [Google Scholar]
- Yu, C.; Huang, M. Radicamines A and B: Synthesis and revision of the absolute configuration. Org. Lett. 2006, 8, 3021–3024. [Google Scholar] [CrossRef]
- Hu, X.; Jia, Y.; Xiang, J.; Yu, C. Exploratory studies en route to 5-alkyl-hyacinthacines: Synthesis of 5-epi-(-)-hyacinthacine A(3) and (-)-hyacinthacine A(3). Synlett 2010, 982–986. [Google Scholar]
- Su, J.; Jia, Y.; He, R.; Rui, P.; Han, N.; He, X.; Xiang, J.; Chen, X.; Zhu, J.; Yu, C. A rapid synthesis of 2-aryl polyhydroxylated pyrrolidines. Synlett 2010, 1609–1616. [Google Scholar]
- Li, Y.; Huang, M.; Yamashita, Y.; Kato, A.; Jia, Y.; Wang, W.; Fleet, G.W. J.; Nash, R.J.; Yu, C. L-DMDP, L-homoDMDP and their C-3 fluorinated derivatives: Synthesis and glycosidase-inhibition. Org. Biomol. Chem. 2011, 9, 3405–3414. [Google Scholar] [CrossRef]
- Zhang, W.; Sato, K.; Kato, A.; Jia, Y.; Hu, X.; Wilson, F.X.; van Well, R.; Horne, G.; Fleet, G.W.J.; Nash, R.J.; et al. Synthesis of fully substituted polyhydroxylated pyrrolizidines via Cope-House cyclization. Org. Lett. 2011, 13, 4414–4417. [Google Scholar] [CrossRef]
- Zhang, Z.; Nakagawa, S.; Kato, A.; Jia, Y.; Hu, X.; Yu, C. A concise stereoselective synthesis of (-)-erycibelline. Org. Biomol. Chem. 2011, 9, 7713–7719. [Google Scholar] [CrossRef]
- Takebayashi, M.; Hiranuma, S.; Kanie, Y.; Kajimoto, T.; Kanie, O.; Wong, C. A versatile synthetic strategy for the preparation and discovery of new iminocyclitols as inhibitors of glycosidases. J. Org. Chem. 1999, 64, 5280–5291. [Google Scholar] [CrossRef]
- Bloch, R. Additions of organometallic reagents to CN bonds: Reactivity and selectivity. Chem. Rev. 1998, 98, 1407–1438. [Google Scholar] [CrossRef]
- Lombardo, M.; Fabbroni, S.; Trombini, C. Entropy-controlled selectivity in the vinylation of a cyclic chiral nitrone. An efficient route to enantiopure polyhydroxylated pyrrolidines. J. Org. Chem. 2001, 66, 1264–1268. [Google Scholar] [CrossRef]
- Delso, I.; Tejero, T.; Goti, A.; Merino, P. Synthesis of D-arabinose-derived polyhydroxylated pyrrolidine, indolizidine and pyrrolizidine alkaloids. Total synthesis of hyacinthacine A2. Tetrahedron 2010, 66, 1220–1227. [Google Scholar] [CrossRef]
- The crystal structure of 11 has been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number: 928245.
- Caderas, C.; Lett, R.; Overman, L.E.; Rabinowitz, M.H.; Robinson, L.A.; Sharp, M.J.; Zablocki, J. Total syntheses of allopumiliotoxins 267A, 323B' and 339A. Application of iodide-promoted iminium ion-alkyne cyclizations for forming allopumiliotoxin A alkaloids. J. Am. Chem. Soc. 1996, 118, 9073–9082. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, M.-H.; Li, Y.-X.; Jia, Y.-M.; Yu, C.-Y. General Intermediates for the Synthesis of 6-C-Alkylated DMDP-Related Natural Products. Molecules 2013, 18, 6723-6733. https://doi.org/10.3390/molecules18066723
Huang M-H, Li Y-X, Jia Y-M, Yu C-Y. General Intermediates for the Synthesis of 6-C-Alkylated DMDP-Related Natural Products. Molecules. 2013; 18(6):6723-6733. https://doi.org/10.3390/molecules18066723
Chicago/Turabian StyleHuang, Mu-Hua, Yi-Xian Li, Yue-Mei Jia, and Chu-Yi Yu. 2013. "General Intermediates for the Synthesis of 6-C-Alkylated DMDP-Related Natural Products" Molecules 18, no. 6: 6723-6733. https://doi.org/10.3390/molecules18066723



