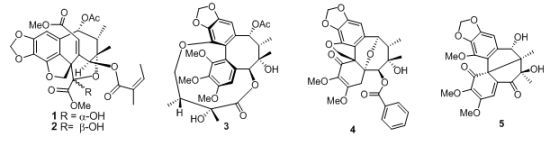
New Lignans from the Leaves and Stems of Kadsura philippinensis
Abstract
:1. Introduction
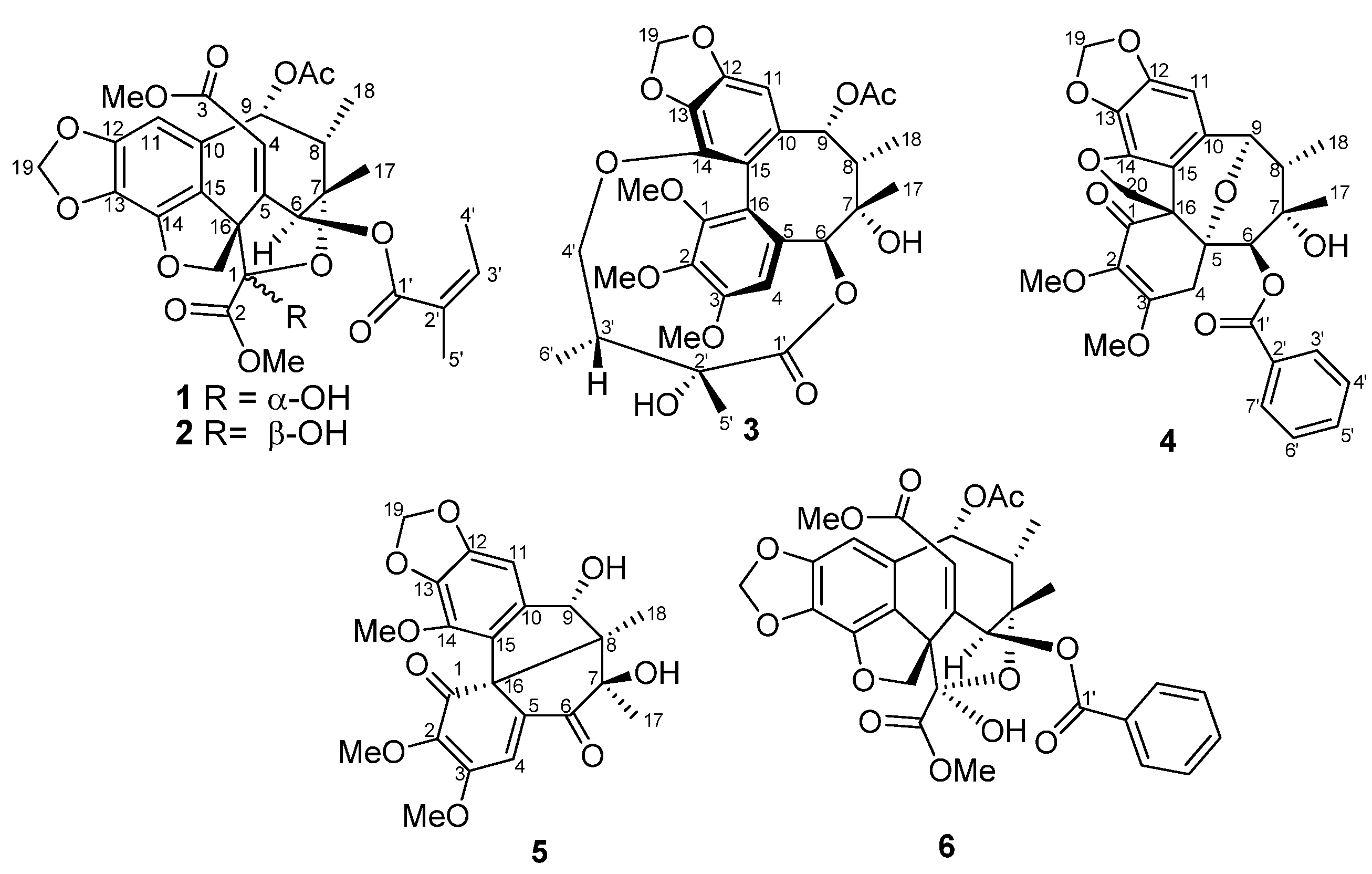
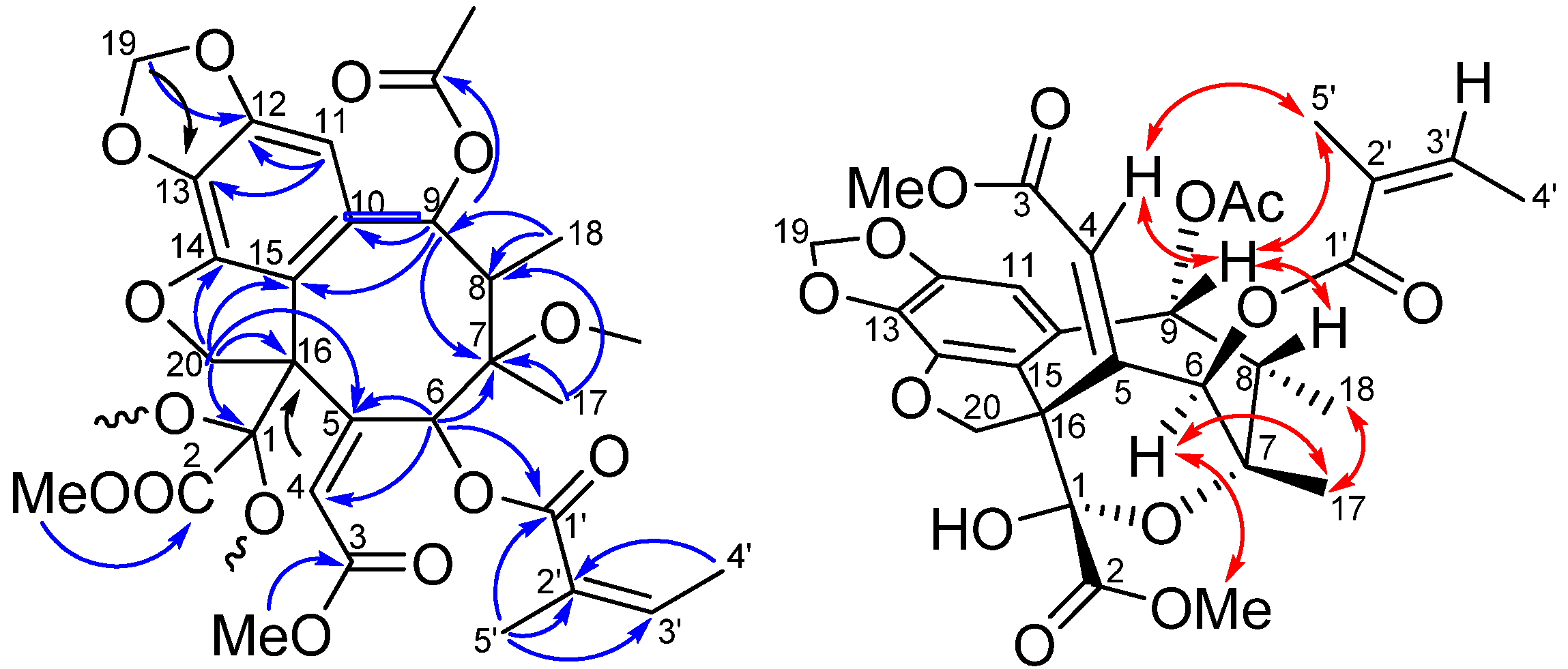
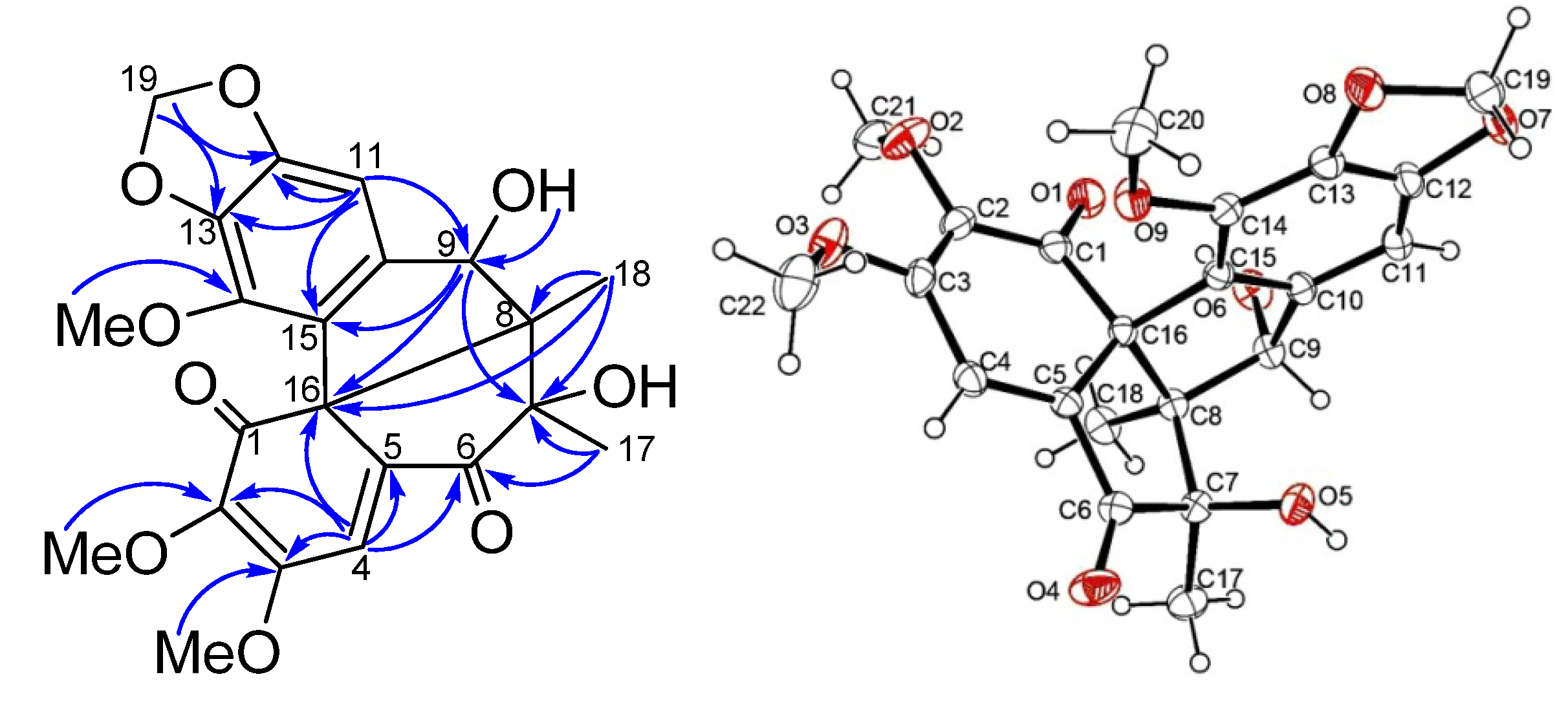
2. Results and Discussion
| Position | 1 a | 2 b | 3 a | 4 b | 5 a |
|---|---|---|---|---|---|
| 4 | 5.99, brs | 6.06, d (2.4) | 6.84, s | 3.08, d (18.4 ) | 7.34, s |
| 3.17, d (18.4 ) | |||||
| 6 | 6.28, d (2.7) | 6.69, d (2.4) | 5.76, s | 5.42, s | |
| 8 | 2.23, m | 2.23, m | 1.97, m | 2.00, m | |
| 9 | 6.55, d (2.7) | 6.63, d (2.8) | 5.48, s | 4.82, brs | 4.87, d (12.9) |
| 11 | 6.45, s | 6.44, s | 6.47, s | 6.28, s | 6.71, s |
| 17 | 1.31, s | 1.34, s | 1.37, s | 0.97, s | 1.31, s |
| 18 | 1.04, d (6.9) | 1.02, d (6.8) | 1.30, d (6.9) | 1.36, d (7.6) | 0.98, s |
| 19 | 5.97, s | 5.93, s | 5.94, s | 5.83, s | 5.90, d (1.5) |
| 5.98, s | 5.94, s | 6.03, s | 5.98, s | 5.91, d (1.5) | |
| 20 | 4.53, d (10.2) | 4.59, d (10.0) | 4.30, d (9.6) | ||
| 5.00, d (10.2) | 4.98, d (10.0) | 4.43, d (9.6) | |||
| OMe-1 | 3.46, s | ||||
| OMe-2 | 3.93, s | 3.57, s | 3.84, s | 3.66, s | 3.77, s |
| OMe-3 | 3.59, s | 3.58, s | 3.92, s | 4.07, s | 4.11, s |
| OMe-14 | 3.81, s | ||||
| OAc | 2.13, s | 2.13, s | 1.49, s | ||
| 1' | |||||
| 2' | |||||
| 3' | 6.28, overlap | 6.23, q (7.2) | 1.92, m | 7.32, m | |
| 4' | 2.05, d (7.2) | 2.06, d (7.2) | 3.63, dd (5.0, 8.0) | 7.35, m | |
| 4.16, dd (5.0, 8.0) | |||||
| 5' | 1.99, s | 2.00, s | 1.23, s | 7.55, d (7.2) | |
| 6' | 0.96,d (7.2) | 7.35, m | |||
| 7' | 7.32, m | ||||
| OH-9 | 4.28, d (12.9) |

| Position | 1 a | 2 b | 3 a | 4 b | 5 a |
|---|---|---|---|---|---|
| 1 | 97.5, C | 97.6, C | 151.5,C | 193.0, C | 196.3, C |
| 2 | 171.0, C | 170.1, C | 141.8,C | 132.5, C | 140.6, C |
| 3 | 165.4, C | 165.5, C | 152.3, C | 157.4, C | 159.0, C |
| 4 | 117.2, CH | 118.3, CH | 111.0, CH | 40.7, CH2 | 123.4, CH |
| 5 | 150.5, C | 149.8, C | 130.8, C | 77.6, C | 143.7, C |
| 6 | 72.5, CH | 72.5, CH | 86.6 , CH | 77.3, CH | 200.7, C |
| 7 | 79.2, C | 78.4, C | 73.8, C | 72.5, C | 80.7, C |
| 8 | 45.4, CH | 45.5, CH | 44.1, CH | 43.7, CH | 60.4, C |
| 9 | 70.3, CH | 70.2, CH | 83.9, CH | 77.3, CH | 75.7, CH |
| 10 | 127.9, C | 127.8, C | 132.8,C | 127.9, C | 144.5, C |
| 11 | 98.7, CH | 99.9, CH | 102.5, CH | 95.9, CH | 100.4, CH |
| 12 | 150.4, C | 149.8, C | 148.6, C | 151.3, C | 151.2, C |
| 13 | 129.1, C | 128.9, C | 137.5, C | 129.5, C | 136.4, C |
| 14 | 144.9, C | 142.6, C | 139.0, C | 140.9, C | 139.5, C |
| 15 | 118.0, C | 120.5, C | 121.2, C | 121.3, C | 125.7, C |
| 16 | 57.0, C | 58.6, C | 121.7, C | 56.9, C | 69.5, C |
| 17 | 28.2, CH3 | 28.5, CH3 | 28.4, CH3 | 23.1, CH3 | 19.9, CH3 |
| 18 | 8.9, CH3 | 8.5, CH3 | 17.8, CH3 | 15.3, CH3 | 15.1, CH3 |
| 19 | 101.8, CH2 | 101.5, CH2 | 101.5, CH2 | 101.3, CH2 | 101.7, CH2 |
| 20 | 80.4, CH2 | 78.5, CH2 | 78.2, CH2 | ||
| OMe-1 | 60.5, CH3 | ||||
| OMe-2 | 53.6, CH3 | 54.0, CH3 | 60.7, CH3 | 60.7, CH3 | 60.2, CH3 |
| OMe-3 | 51.8, CH3 | 51.7, CH3 | 56.2, CH3 | 58.9, CH3 | 58.3, CH3 |
| OMe-14 | 59.4, CH3 | ||||
| OAc | 168.9, C | 168.9, C | 168.7, C | ||
| 21.0, CH3 | 20.3, CH3 | 20.3, CH3 | |||
| 1' | 166.0, C | 166.1, C | 172.4, C | 165.4, C | |
| 2' | 126.3, C | 126.5, C | 76.6, C | 129.5, C | |
| 3' | 142.3, CH | 141.7, CH | 42.4, CH | 128.3, CH | |
| 4' | 16.0, CH3 | 15.9, CH3 | 72.4, CH2 | 129.7, CH | |
| 5' | 20.4, CH3 | 21.0, CH3 | 21.4, CH3 | 133.9, CH | |
| 6' | 12.8, CH3 | 129.7, CH | |||
| 7' | 128.3, CH |

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ookawa, N.; Ikeya, Y.; Taguchi, H.; Yosioka. The Constituents of Kadsura Japonica DUNAL. I. The Structures of Three New Lignans, Acetyl-, Angeloyl- and Caproyl-binankadsurin A. Chem. Pharm. Bull. 1981, 29, 123–127. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.K.; Zhu, Q.S. Zhong Hua Ben Cao; (in Chinese). Shanghai Scientific & Techincal Publishers: Shanghai, China, 1999; Volume 2, pp. 912–913. [Google Scholar]
- Charlton, J.L. Antiviral Activity of Lignans. J. Nat. Prod. 1998, 61, 1447–1451. [Google Scholar] [CrossRef]
- Chang, J.B.; Reiner, J.; Xie, J.G. Progress on the chemistry of dibenzocyclooctadiene lignans. Chem. Rev. 2005, 105, 4581–4609. [Google Scholar] [CrossRef]
- Wu, M.D.; Huang, R.L.; KuoYang, L.M.; Hung, C.C.; Ong, C.W.; Kuo, Y.H. The Anti-HBsAg (human type B hepatitis, surface antigen) and anti-hbeag (human type B hepatitis, e antigen) C18 dibenzocyclooctadiene lignans from Kadsura matsudai and Schizandra arisanensi. Chem. Pharm. Bull. 2003, 51, 1233–1236. [Google Scholar] [CrossRef]
- Li, L.N. Biologically active components from traditional Chinese medicines. Pure Appl. Chem. 1998, 70, 547–554. [Google Scholar] [CrossRef]
- Chen, D.F.; Zhang, S.X.; Kozuka, M.; Sun, Q.Z.; Feng, J.; Wang, Q.; Mukainaka, T.; Nobukuni, Y.; Tokuda, H.; Nishino, H.; et al. Interiotherins C and D, two new lignans from Kadsura interior and antitumor-promoting effects of related neolignans on Epstein−Barr virus activation. J. Nat. Prod. 2002, 65, 1242–1245. [Google Scholar] [CrossRef]
- Chen, D.F.; Zhang, S.X.; Wang, H.K.; Zhang, S.Y.; Sun, Q.Z.; Cosentino, L.M.; Lee, K.H. Novel Anti-HIV Lancilactone C and Related Triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999, 62, 94–97. [Google Scholar] [CrossRef]
- Li, H.L.; Chaw, S.M. Schisandraceae-Flora of Taiwan; Epoch: Taipei, Taiwan, 1996; Volume 2, p. 425. [Google Scholar]
- Shen, Y.C.; Lin, Y.C.; Kuo, Y.H.; Cheng, Y.B.; Liaw, C.C. Taiwankadsulins A, B and C, three new C-19 Homolignans from Kadsura philippinensis. Org. Lett. 2005, 7, 5297–5300. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, Y.C.; Chiang, M.Y.; Yeh, S.F.; Cheng, Y.B.; Liaw, C.C. Kadsuphilactones A and B, Two New Triterpene Dilactones from Kadsura philippinensis. Org. Lett. 2005, 7, 3307–3310. [Google Scholar] [CrossRef]
- Shen, Y.C.; Liaw, C.C.; Cheng, Y.B.; Ahmed, A.F.; Lai, M.C.; Liou, S.S.; Wu, T.S.; Kuo, Y.H.; Lin, Y.C.; Khalil, A.T. C-18 Dibenzocyclooctadiene Lignans from Kadsura philippinensis. J. Nat. Prod. 2006, 69, 963–966. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, Y.C.; Ahmed, A.F.; Cheng, Y.B.; Chen, C.T.; Liaw, C.C.; Kuo, Y.H. Four new nonaoxygenated C18 dibenzocylcooctadiene lignans from Kadsura philippinensis. Chem. Pharm. Bull. 2007, 55, 280–283. [Google Scholar] [CrossRef]
- Shen, Y.C.; Cheng, Y.B.; Lan, T.W.; Liaw, C.C.; Liou, S.S.; Kuo, Y.H.; Khalil, A.T. Kadsuphilols A-H, new oxygenated lignans from Kadsura philippinensis. J. Nat. Prod. 2007, 70, 1139–1145. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, Y.C.; Cheng, Y.B.; Chang, C.J.; Lan, T.W.; Liou, S.S.; Chien, C.T.; Liaw, C.C.; Khalil, A.T. New Oxygenated Lignans from Kadsura philippinensis. Helv. Chim. Acta. 2008, 91, 483–494. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, Y.C.; Cheng, Y.B.; Chiang, M.Y.; Liou, S.S.; Khalil, A.T. Dibenzocyclooctadiene lignans from Kadsura philippinensis. Phytochemistry 2009, 70, 114–120. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Lin, Y.C.; Khalil, A.K.; Liou, S.S.; Lee, G.C.; Kuo, Y.H.; Shen, Y.C. Seven new lignan esters from Kadsura philippinensis. Helv. Chim. Acta 2011, 94, 148–158. [Google Scholar] [CrossRef]
- Liu, J.S.; Li, L. Schisandrins L–O and acetyl schisandrin L from Kadsura coccinea. Phytochemistry 1993, 32, 1293–1296. [Google Scholar] [CrossRef]
- Ikeya, Y.; Taguchi, H.; Yosioka, I.; Iitaka, Y.; Kobayashi, H. The constituents of schizandra chinensis baill. ii. the structure of a new lignan, gomisin d. Chem. Pharm. Bull. 1979, 27, 1395–1401. [Google Scholar] [CrossRef]
- Yang, X.W.; Miyashiro, H.; Hattori, M.; Namba, T.; Tezuka, Y.; Kikuchi, T.; Chen, D.F.; Xu, G.J.; Hori, T.; Extine, M.; et al. Isolation of novel lignans, heteroclitins F and G, from the Stems of Kadsura heteroclita, and anti-lipid peroxidative actions of heteroclitins A–G and related compounds in the in vitro Rat Liver Homogenate System. Chem. Pharm. Bull. 1992, 40, 1510–1516. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, D.F. Kadsutherins A–C: Three new dibenzocyclooctane lignans from the stems of Kadsura species. Helv. Chim. Acta 2006, 89, 895–901. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-86. Program for the solution of crystal structures; University of Göttingen: Göttingen, Germany, 1985. [Google Scholar]
- Sheldrick, G.M. SHELXS-97. Program for the solution of crystal structures.; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Samples of the compounds may not be available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, Y.-C.; Cheng, Y.-B.; Liaw, C.-C.; Lo, I.-W.; Kuo, Y.-H.; Chiang, M.Y.; Chou, C.-H.; Shen, Y.-C. New Lignans from the Leaves and Stems of Kadsura philippinensis. Molecules 2013, 18, 6573-6583. https://doi.org/10.3390/molecules18066573
Lin Y-C, Cheng Y-B, Liaw C-C, Lo I-W, Kuo Y-H, Chiang MY, Chou C-H, Shen Y-C. New Lignans from the Leaves and Stems of Kadsura philippinensis. Molecules. 2013; 18(6):6573-6583. https://doi.org/10.3390/molecules18066573
Chicago/Turabian StyleLin, Yu-Chi, Yuan-Bin Cheng, Chia-Ching Liaw, I-Wen Lo, Yao-Haur Kuo, Michael Y. Chiang, Chang-Hung Chou, and Ya-Ching Shen. 2013. "New Lignans from the Leaves and Stems of Kadsura philippinensis" Molecules 18, no. 6: 6573-6583. https://doi.org/10.3390/molecules18066573