Chemical Composition of Aspidosperma ulei Markgr. and Antiplasmodial Activity of Selected Indole Alkaloids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolated Substances from A. ulei

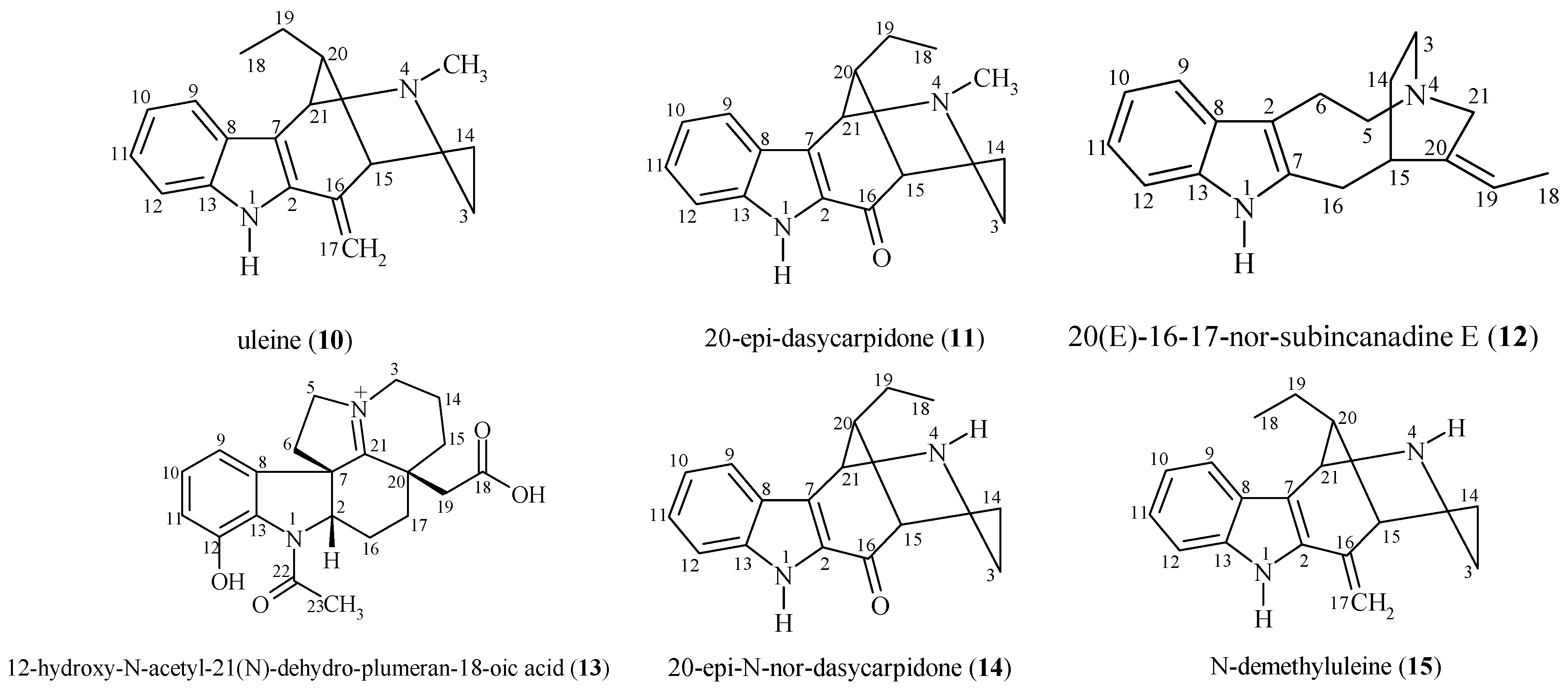
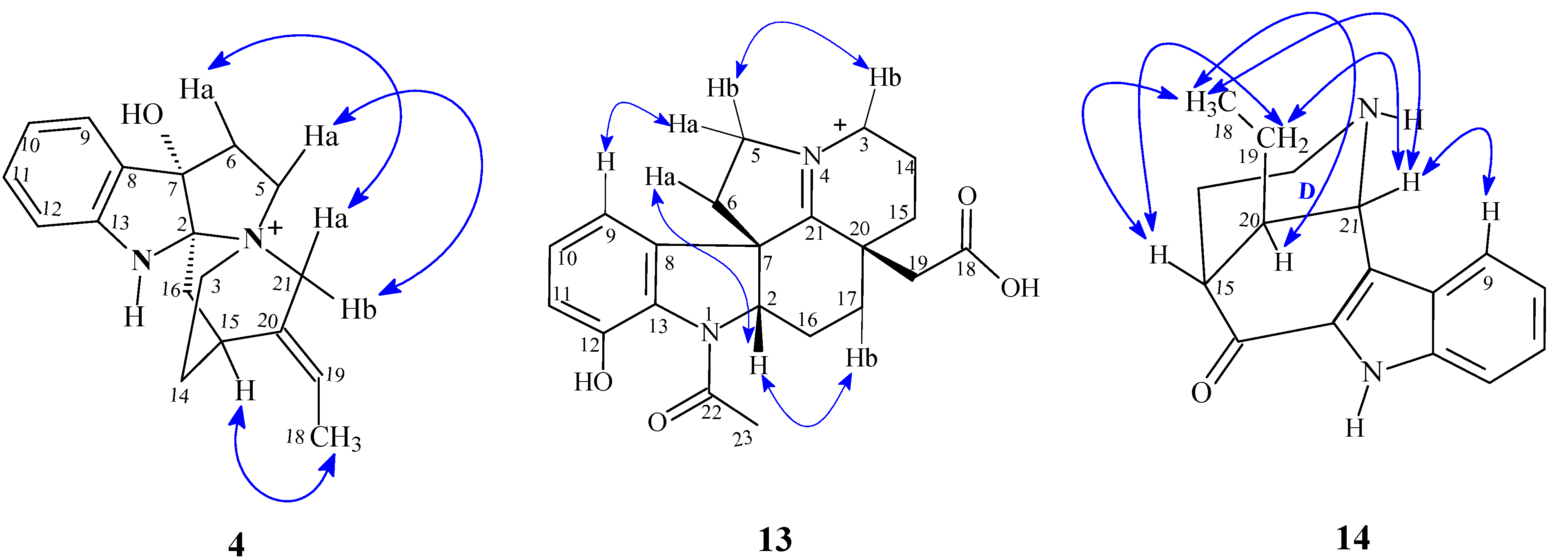
2.2. Analysis of Spectral and Physical Data for Isolated Compounds
2.3. In Vitro Inhibition of P. falciparum and Cytotoxicity
| Nº | Name | IC50 ± SD | IC50 ± SD | Result |
| µg/mL | μM | |||
| 3 | 3,4,5,6-tetradehydro-β-yohimbine | 14.0 ± 2.7 | 39.9 ± 7.7 | I |
| 4 | 19E-hunteracine | > 50.0 | > 176 | I |
| 11 | 20-epi-dasycarpidone | 4.5 ± 0.2 | 16.7 ± 0.7 | MA |
| 12 | 20(E)-nor-subincanadine E | 14.5 ± 2.8 | 54.3 ± 10.5 | I |
| 13 | 12-hydroxy-N-acetyl-21(N)-dehydroplumeran-18-oic acid | > 50.0 | > 135 | I |
| DS | chloroquine diphosphate | 0.17 ± 0.1 | 0.33 ± 0.19 | A |
| DS | quinine sulphate | 0.12 ± 0.05 | 0.30 ± 0.15 | A |
3. Experimental
3.1. General Procedures
3.2. Collection, Botanic Identification and Processing of Plant Materials
3.3. Preparation of Extracts of A. ulei and Isolation Procedures
| Dry, powdered plant material | Dry plant extract | |||||
|---|---|---|---|---|---|---|
| Part | Mass extracted (kg) | Name | Yield (g) | % Yield | Description | |
| Heartwood | 3.0 | HWEE | 52 | 1.7 | Yellow powder | |
| Leaf | 1.0 | LEE | 98 | 9.8 | Green powder | |
| Root bark | 3.0 | RBEE | 274 | 9.1 | Viscous residue | |
| Root wood | 3.0 | RWEE | 122 | 4.1 | Viscous residue | |
| Stem bark | 2.0 | SBEE | 173 | 8.7 | Viscous residue | |
3.3.1. Isolation of Chemical Components from Leaf Extracts
3.3.2. Isolation of Chemical Components from Stem Bark Extracts
3.3.3. Acid-Base Fractionation of EtOH Extracts
3.3.3.1. Isolation of Chemical Components from Acidic Fractions
3.3.3.2. Isolation of Chemical Components from Basic Fractions
3.4. Spectrometric Data for Isolated Compounds
3.5. Biological activity of isolated compounds from Aspidosperma ulei
3.5.1. In Vitro Culture of Plasmodium Falciparum and in Vitro Antiplasmodial Assay
3.5.2. Cell Culture and Cytotoxicity Test Using the Alamar BlueTM Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Malaria Report; World Health Organization: Geneva, Switzerland, 2011.
- Willcox, M.L.; Bodeker, G. Clinical Review. Traditional herbal medicines for malaria. Med. J. 2004, 329, 1156–1159. [Google Scholar] [CrossRef]
- Milliken, W. Plants for Malaria. Plants for Fever; The Royal Botanic Gardens: Kew, UK, 1997. [Google Scholar]
- Dolabela, M.F.; Oliveira, S.G.; Peres, J.M.; Nascimento, J.M.S.; Póvoa, M.M.; Oliveira, A.B. In vitro antimalarial activity of six Aspidosperma species from the state of Minas Gerais (Brazil). An. Acad. Bras. Ciên. 2012, 84, 899–910. [Google Scholar] [CrossRef]
- Pereira, M.M.; Jácome, L.R.P.; Alcântara, A.F.C.; Alves, R.B.; Raslan, D.S. Alcalóides indólicos isolados de espécies do gênero Aspidosperma (Apocynaceae). Quím. Nova 2007, 30, 970–983. [Google Scholar]
- Oliveira, V.B.; Freitas, M.S.M.; Mathias, L.; Braz-Filho, R.; Vieira, I.J.C. Atividade biológica e alcalóides indólicos do gênero Aspidosperma (Apocynaceae): uma revisão. Rev. Bras. Plantas Med. 2009, 11, 92–99. [Google Scholar] [CrossRef]
- Henrique, M.C.; Nunomura, S.M.; Pohlit, A.M. Alcalóides indólicos das cascas de Aspidosperma vargasii e A. desmanthum. Quím. Nova 2010, 33, 2284–2287. [Google Scholar]
- Büchi, G.; Warnhoff, E.W. The structure of uleine. J. Am. Chem. Soc. 1959, 81, 4433–4434. [Google Scholar]
- Büchi, G.; Gould, S.J.; Näf, F. Stereospecific syntheses of uleine and epiuleine. J. Am. Chem. Soc. 1971, 93, 2492–2501. [Google Scholar] [CrossRef]
- Mitaine-Offer, A.C.; Sauvain, M.; Valentin, A.; Callapa, J.; Mallié, M.; Zèches-Hanrot, M. Antiplasmodial activity of Aspidosperma indole alkaloids. Phytomedicine 2002, 9, 142–145. [Google Scholar] [CrossRef]
- Dominique, M.; Roland, M. Use of uleine for the prevention and/or the treatment of infectious diseases. PCT Int. Appl. WO 2011160684 A1 20111229.
- Oliveira, A.B. Standardized extracts and fractions of husks of Aspidosperma parvifolium and/or ulein for pharmaceutical use. Brazilian Patent PI BR 20095584 A2 20110823, 2011. [Google Scholar]
- Oliveira, A.B.; Dolabela, M.; Póvoa, M.; Santos, C.A.M.; Varotti, F.P. Antimalarial activity of ulein and proof of its action on the Plasmodium falciparum digestive vacuole. Malaria J. 2010, 9. Oral Presentation O9. [Google Scholar]
- Andrade-Neto, V.F.; Pohlit, A.M.; Pinto, A.C.; Silva, E.C.; Nogueira, K.L.; Melo, M.R.; Henrique, M.C.; Amorim, R.C.; Silva, L.F.; Costa, M.R.; et al. In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants. Mem. Inst. Oswaldo Cruz 2007, 102, 359–365. [Google Scholar]
- Rocha e Silva, L.F.; Montoia, A.; Amorim, R.C.N.; Melo, M.R.; Henrique, M.C.; Nunomura, S.M.; Costa, M.R.F.; Andrade Neto, V.F.; Costa, D.S.; Dantas, G.; et al. Comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. Phytomedicine 2012, 20, 71–76. [Google Scholar] [CrossRef]
- Uchoa, D.E.A. Aplicação de técnicas contemporâneas de ressonância magnética nuclear no estudo fitoquímico de Aspidosperma ulei Markgf. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2006. [Google Scholar]
- Ondetti, M.A.; Deulofeu, V. Alkaloids from Aspidosperma australe Mull Argov. Relationship of olivacine to u-alkaloid C. The structure of olivacine and u-alkaloid C (guatambuine). Tetrahedron Lett. 1959, 7, 1–4. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Spectral assignments and reference data. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Lemes, G.F.; Ferri, P.H.; Lopes, M.N. Constituintes químicos de Hyptidendron canun (Pohl ex Benth.) R. Harley (Lamiaceae). Quím. Nova 2011, 34, 39–42. [Google Scholar] [CrossRef]
- Endringer, D.C.; Pezzuto, J.M.; Soares, C.M.; Braga, F.C. L-(+)-Bornesitol. Acta Crystallogr. Sect. E 2007, 63, 1067–1068. [Google Scholar]
- Waschsmuth, R; Matusch, R. Anhydronium bases from Rauvolfia serpentina. Phytomedicine 2002, 61, 705–709. [Google Scholar]
- Wenkert, E.; Chang, C.-J.; Chawla, H.P.S.; Cochran, D.W.; Hagaman, E.W.; King, J.C.; Orito, K. General methods of synthesis on indole alkaloids. 14. Short routes of construction of yohimboid and ajmalicinoid alkaloid systems and their 13C nuclear magnetic resonance spectral analysis. J. Am. Chem. Soc. 1976, 98, 3645–3655. [Google Scholar] [CrossRef]
- Passemar, C.; Saléry, M.; Soh, P.N.; Linas, M.-D.; Ahond, A.; Poupat, C.; Benoit-Vical, F. Indole and aminoimidazole moieties appear as key structural units in antiplasmodial molecules. Phytomedicine 2011, 18, 1118–1125. [Google Scholar] [CrossRef]
- Hu, Y.; Coates, A.R.M. Novel combination for treatment of microbial infections. Patent PCT Int. Appl. WO 2012032360 CA 2809203, 2012. [Google Scholar]
- Pascale, C.; Richard, B.; Deverre, J.R.; Sevenet, T.; Zeches, M.; Le Men-Oliver, L. Alkaloids from leaves and root bark of Ervatamia hirta. Phytomedicine 1991, 30, 3785–3792. [Google Scholar]
- Bartlett, M.F.; Korzun, B.; Sklar, R.; Smith, A.F.; Taylor, W.I. The alkaloids of Hunteria eburnea Pichon. II: The quaternary bases. J. Org. Chem. 1963, 28, 1445–1449. [Google Scholar] [CrossRef]
- Burnell, R.H.; Chapelle, A.; Khalil, M.F. Hunteracine: The crystal structure of a quaternary alkaloid from Hunteria eburnea Pichon. J. Chem. Soc. Chem. Comm. 1970, 12, 722–723. [Google Scholar]
- Burnell, R.H.; Chapelle, A.; Khalil, M.F. Quaternary bases from Hunteria eburnea Pichon. Can. J. Chem. 1974, 52, 2327–2330. [Google Scholar] [CrossRef]
- Marini-Bettolo, G.B.; Nicoletti, M.; Messana, I.; Patamia, M.; Galeffi, C.; Oguakwa, J.U.; Portalone, G.; Vaciago, A. Research on Africa medicinal plants-IV: Boonein, a new C-9 terpenoid lactone from Alstonia boonei: A possible precursor in the indole alkaloid biogenesis. Tetrahedron 1983, 39, 323–329. [Google Scholar] [CrossRef]
- Borris, R.P.; Larris, D.C.; Cordell, G.A. Studies on the uleine alkaloids I. Carbon-13-NMR studies on uleine, 20-epiuleine and (4S)-uleine-Nb-oxide. J. Nat. Prod. 1983, 46, 200–205. [Google Scholar] [CrossRef]
- Jácome, R.L.R.P.; Oliveira, A.B.; Raslan, D.S.; Wagner, H. Estudo químico e perfil cromatográfico de Aspidosperma parvifolium A. DC. (“pau-pereira”). Quím. Nova 2004, 27, 897–900. [Google Scholar]
- Amat, M.; Pérez, M.; Llor, N.; Escolano, C.; Luque, F.J.; Molins, E.; Bosch, J. Conjugate additions to phenylglycinol-derived unsaturated δ-lactams enantioselective synthesis of uleine alkaloids. J. Org. Chem. 2004, 69, 8681–8693. [Google Scholar] [CrossRef]
- Forns, P.; Diez, A.; Rubiralta, M. Synthetic applications of 2-(1,3-dithian-2-yl) indoles VI. Synthesis of 20-epidasycarpidone. Tetrahedron 1996, 52, 3564–3574. [Google Scholar]
- Gràcia, J.; Casamitjana, N.; Bonjoch, J.; Bosch, J. Total synthesis of uleine-type and Strychnos alkaloids through a common intermediate. J. Org. Chem. 1994, 59, 3939–3951. [Google Scholar] [CrossRef]
- Figueiredo, E.R.; Vieira, I.J.C.; Souza, J.J.; Braz-Filho, R.; Mathias, L.; Kanashiro, M.M.; Côrtes, F.H. Isolamento, identificação e avaliação da atividade antileucêmica de alcalóides indólicos monoterpênicos de Tabernaemontana salzmannii (A. DC.), Apocynaceae. Rev. Bras. Farmacogn. 2010, 20, 675–681. [Google Scholar] [CrossRef]
- Kobayashi, J.; Sekiguchi, M.; Shimamoto, S.; Shigemori, H.; Ishiyama, H.; Ohsaki, A. Subicanadines A–C, novel quaternary índole alkaloids from Aspidosperma subincanum. J. Org. Chem. 2002, 67, 6449–6455. [Google Scholar] [CrossRef]
- Amat, M.; Coll, M.-D.; Passarella, D.; Bosch, J. An enantioselective synthesis of the Strychnos alkaloid (−)-tubifoline. Tetrahedron-Asymmetry 1996, 7, 2775–2778. [Google Scholar] [CrossRef]
- Amat, M.; Coll, M.D.; Bosch, J.; Espinosa, E.; Molins, E. Total synthesis of the Strychnos indole alkaloids (−)-tubifoline, (−)-tubifolidine and (−)-19,20-dihydroakuammicine. Tetrahedron-Asymmetr. 1997, 8, 935–948. [Google Scholar] [CrossRef]
- Milborrow, B.V.; Djerassi, C. Alkaloids studies: Part LXI: The structure of twelve new alkaloids from Aspidosperma cylindrocarpon. J. Chem. Soc. C 1969, 417–424. [Google Scholar] [CrossRef]
- Guimarães, A.H.; Braz-Filho, R.; Vieira, I.J.C. 1H and 13C-NMR data of the simplest plumeran indole alkaloids isolated from Aspidosperma species. Molecules 2012, 17, 3025–3043. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Rocha e Silva, L.F.; Lima, E.S.; Vasconcellos, M.C.; Aranha, E.S.P.; Costa, D.S.; Santos, E.V.M.; Silva, T.C.M.; Morais, S.K.R.; Quignard, E.L.J.; Alecrim, M.G.C.; et al. In vitro and in vivo antimalarial activity and cytotoxicity of extracts, fractions and a substance isolated from the Amazonian plant Tachia grandiflora (Gentianaceae). Mem. Inst. Oswaldo Cruz 2013, 108. in press. [Google Scholar]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Meth. 1994, 15, 15211–15224. [Google Scholar]
- Sample Availability: In general, samples of the compounds isolated herein are unavailable from the authors due to their isolation on a small scale. They are readily isolated using the procedures described.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dos Santos Torres, Z.E.; Silveira, E.R.; Rocha e Silva, L.F.; Lima, E.S.; De Vasconcellos, M.C.; De Andrade Uchoa, D.E.; Filho, R.B.; Pohlit, A.M. Chemical Composition of Aspidosperma ulei Markgr. and Antiplasmodial Activity of Selected Indole Alkaloids. Molecules 2013, 18, 6281-6297. https://doi.org/10.3390/molecules18066281
Dos Santos Torres ZE, Silveira ER, Rocha e Silva LF, Lima ES, De Vasconcellos MC, De Andrade Uchoa DE, Filho RB, Pohlit AM. Chemical Composition of Aspidosperma ulei Markgr. and Antiplasmodial Activity of Selected Indole Alkaloids. Molecules. 2013; 18(6):6281-6297. https://doi.org/10.3390/molecules18066281
Chicago/Turabian StyleDos Santos Torres, Zelina Estevam, Edilberto Rocha Silveira, Luiz Francisco Rocha e Silva, Emerson Silva Lima, Marne Carvalho De Vasconcellos, Daniel Esdras De Andrade Uchoa, Raimundo Braz Filho, and Adrian Martin Pohlit. 2013. "Chemical Composition of Aspidosperma ulei Markgr. and Antiplasmodial Activity of Selected Indole Alkaloids" Molecules 18, no. 6: 6281-6297. https://doi.org/10.3390/molecules18066281