An Efficient Synthesis of Aldohexose-Derived Piperidine Nitrones: Precursors of Piperidine Iminosugars
Abstract
:1. Introduction
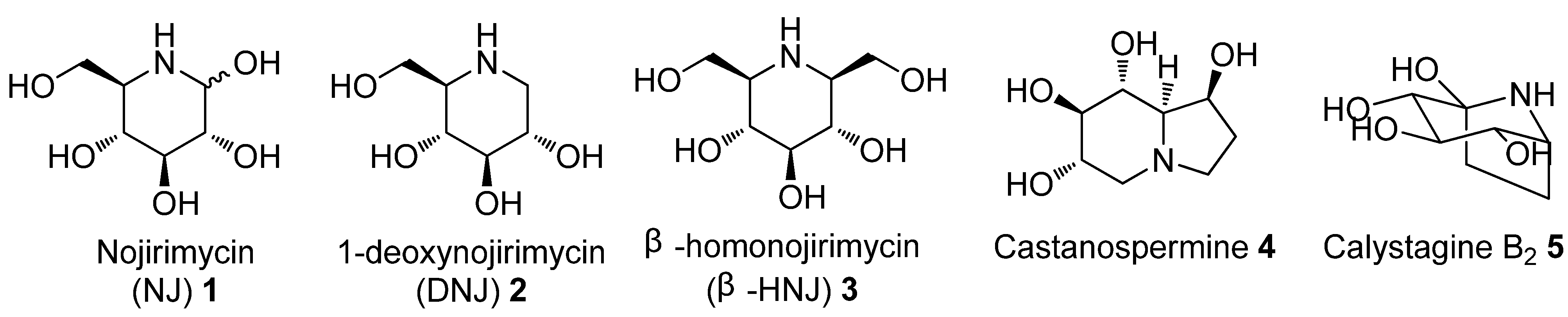
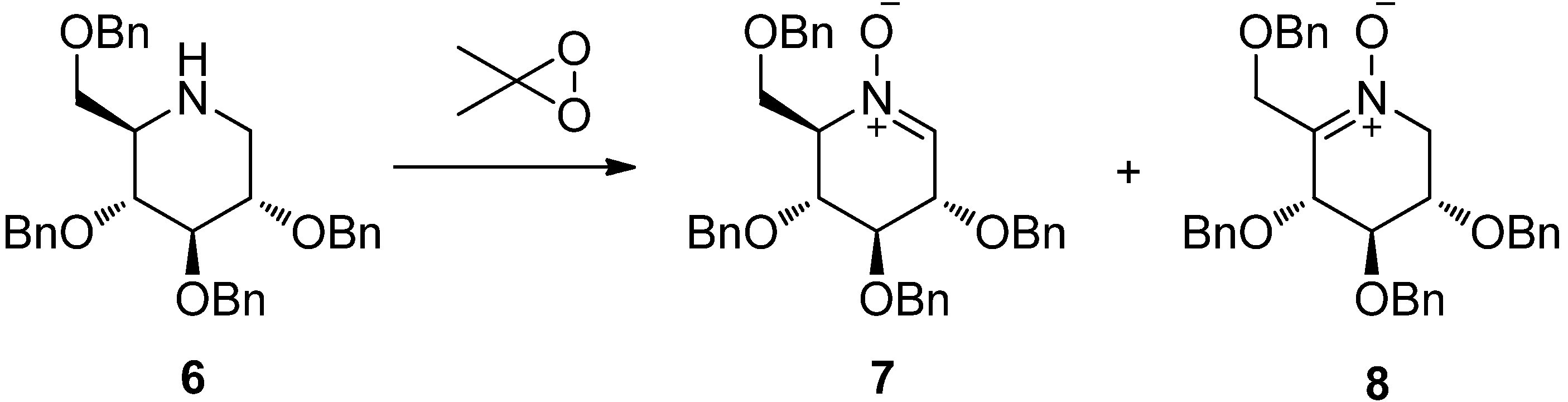
2. Results and Discussion
2.1. The Unsuccessful N-alkylation Strategy

2.2. The Preparation of Diethyl Dithioacetal
| Entry | Additives | Eq. | Solvent | Tem. (°C) | Yield a (%) |
|---|---|---|---|---|---|
| 1 | TMSCl | 0.2 | CHCl3 | RT | - |
| 2 | BF3·Et2O | 0.2 | CHCl3 | RT | - |
| 3 | AcCl | 0.2 | CH2Cl2 | RT | - |
| 5 | conc. HCl | excess | MeOH | RT | - |
| 6 | HCl (g) | excess | Dioxane | RT | 56 |
| 7 | BDMS | 0.2 | CH3CN | 0- RT | trace |
| 8 | BDMS | 0.2 | CH2Cl2 | 0- RT | trace |
| 9 | BDMS | 0.2 | EtSH | RT | 84 b |
2.3. Completion of the Synthesis

3. Experimental
3.1. General Methods
3.2. Synthesis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Winchester, B.; Fleet, G.W.J. Amino-sugar glycosidase inhibitors: Versatile tools for glycobiologists. Glycobiology 1992, 2, 199–210. [Google Scholar] [CrossRef]
- Sears, P.; Wong, C.H. Mechanism-based inhibition of carbohydrate-mediated biological recognitions. Chem. Commun. 1998, 1161–1170. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. Design, synthesis and biological evaluation of iminosugar-based glycosyltransferase inhibitors. Curr. Top. Med. Chem. 2003, 3, 541–560. [Google Scholar] [CrossRef]
- Stütz, A.E. Iminosugars as Glycosidase Inhibitors: Nojirimycin and Beyond; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutic Applications; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Afarinkia, K.; Bahar, A. Recent advances in the chemistry of azapyranose sugars. Tetrahedron: Asymmetry 2005, 16, 1239–1287. [Google Scholar] [CrossRef]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W.J. Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron: Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Asano, N.; Ikeda, K.; Yu, L.; Kato, A.; Takebayashi, K.; Adachi, I.; Kato, I.; Ouchi, H.; Takahata, H.; Fleet, G.W.J. The L-enantiomers of D-sugar-mimicking iminosugars are noncompetitive inhibitors of D-glycohydrolase? Tetrahedron: Asymmetry 2005, 16, 223–229. [Google Scholar] [CrossRef]
- Chang, M.Y.; Kung, Y.H.; Ma, C.C.; Chen, S.T. Synthesis of DMJ analogs with seven- and eight-membered iminocyclitols. Tetrahedron 2007, 63, 1339–1344. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Igarashi, Y.; Ichikawa, M.; Suhara, Y. 1-N-Iminosugars: Potent and selective inhibitors of β-glycosidases. J. Am. Chem. Soc. 1998, 120, 3007–3018. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Zwitterionic glycosidase inhibitors: salacinol and related analogues. Carbohydr. Res 2007, 342, 1551–1580. [Google Scholar] [CrossRef]
- Pearson, M.S.M.; Mathé-Allainmat, M.; Fargeas, V.; Lebreton, J. Recent advances in the total synthesis of piperidine azasugars. Eur. J. Org. Chem. 2005, 2159–2191. [Google Scholar]
- Poitout, L.; Le Merrer, Y.; Depezay, J.C. Synthesis of azasugars. Part 1 isomerization of polyhydroxylated piperidines. Tetrahedron Lett. 1996, 37, 1609–1612. [Google Scholar] [CrossRef]
- Aravind, A.; Kumar, P.S.; Sankar, M.G.; Baskaran, S. Diversity-oriented synthesis of useful chiral building blocks from d-mannitol. Eur. J. Org. Chem. 2011, 6980–6988. [Google Scholar]
- Chandrasekhar, B.; Rao, J.P.; Rao, B.V.; Naresh, P. 2,3 -Wittig rearrangement approach to iminosugar C-glycosides: 5-epi-ethylfagomine, 2-epi-5-deoxyadenophorine and formal synthesis of indolizidine 167B and 209D. Tetrahedron Lett. 2011, 52, 5921–5925. [Google Scholar] [CrossRef]
- Deschamp, J.; Mondon, M.; Nakagawa, S.; Kato, A.; Alonzi, D.S.; Butters, T.D.; Zhang, Y.; Sollogoub, M.; Bleriot, Y. Towards a stable noeuromycin analog with a d-manno configuration: Synthesis and glycosidase inhibition of d-manno-like tri- and tetrahydroxylated azepanes. Bioorg. Med. Chem. 2012, 20, 641–649. [Google Scholar] [CrossRef]
- Dragutan, I.; Dragutan, V.; Demonceau, A. Targeted drugs by olefin metathesis: piperidine-based iminosugars. RSC Adv. 2012, 2, 719–736. [Google Scholar] [CrossRef]
- Kim, I.S.; Jung, Y.H. Recent advances in the total synthesis of indolizidine iminosugars. Heterocycles 2011, 83, 2489–2507. [Google Scholar] [CrossRef]
- Pawar, N.J.; Parihar, V.S.; Chavan, S.T.; Joshi, R.; Joshi, P.V.; Sabharwal, S.G.; Puranik, V.G.; Dhavale, D.D. alpha-Geminal dihydroxymethyl piperidine and pyrrolidine iminosugars: synthesis, conformational analysis, glycosidase inhibitory activity, and molecular docking studies. J. Org. Chem. 2012, 77, 7873–7882. [Google Scholar] [CrossRef]
- Sousa, C.A.D.; Rizzo-Aguiar, F.; Vale, M.L.C.; Garcia-Mera, X.; Caamano, O.; Rodriguez-Borges, J.E. A route to selective functionalization of polyhydroxypyrrolidines. Tetrahedron Lett. 2012, 53, 1029–1032. [Google Scholar]
- Takahata, H. Chiral Synthesis of Iminosugars. Heterocycles 2012, 85, 1351–1376. [Google Scholar] [CrossRef]
- Fusaro, M.B.; Chagnault, V.; Postel, D. Synthesis of glycosylamines and glyconamides using molecular iodine. Tetrahedron 2013, 69, 542–550. [Google Scholar] [CrossRef]
- Liautard, V.; Christina, A.E.; Desvergnes, V.; Martin, O.R. Diastereoselective synthesis of novel iminosugar-containing UDP-Galf mimics: Potential inhibitors of UDP-gal mutase and UDP-Galf transferases. J. Org. Chem. 2006, 71, 7337–7345. [Google Scholar] [CrossRef]
- Pasniczek, K.; Socha, D.; Jurczak, M.; Solecka, J.; Chmielewski, M. Synthesis of 8-homocastanospermine. Can. J. Chem. 2006, 84, 534–539. [Google Scholar] [CrossRef]
- Bande, O.P.; Jadhav, V.H.; Puranik, V.G.; Dhavale, D.D.; Lombardo, M. Stereo-controlled approach to pyrrolidine iminosugar C-glycosides and 1,4-dideoxy-1,4-imino-L-allitol using a d-mannose-derived cyclic nitrone. Tetrahedron Lett. 2009, 50, 6906–6908. [Google Scholar] [CrossRef]
- Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F.M.; Goti, A. Stereocontrolled cyclic nitrone cycloaddition strategy for the synthesis of pyrrolizidine and indolizidine alkaloids. Chem. Eur. J. 2009, 15, 7808–7821. [Google Scholar] [CrossRef]
- Hu, X.G.; Bartholomew, B.; Nash, R.J.; Wilson, F.X.; Fleet, G.W.J.; Nakagawa, S.; Kato, A.; Jia, Y.M.; van Well, R.; Yu, C.Y. Synthesis and glycosidase inhibition of the enantiomer of (−)-steviamine, the first example of a new class of indolizidine alkaloid. Org. Lett. 2010, 12, 2562–2565. [Google Scholar] [CrossRef]
- Hu, X.G.; Jia, Y.M.; Xiang, J.F.; Yu, C.Y. Exploratory Studies en Route to 5-Alkyl-Hyacinthacines: Synthesis of 5-epi-(−)-Hyacinthacine A3 and (−)-Hyacinthacine A3. Synlett 2010, 982–986. [Google Scholar]
- Su, J.K.; Jia, Y.M.; He, R.R.; Rui, P.X.; Han, N.Y.; He, X.H.; Xiang, J.F.; Chen, X.; Zhu, J.H.; Yu, C.Y. A rapid synthesis of 2-Aryl polyhydroxylated pyrrolidines. Synlett 2010, 1609–1616. [Google Scholar]
- Khangarot, R.K.; Kaliappan, K.P. A stereoselective synthesis of sugar-derived chiral beta-lactams. Eur. J. Org. Chem. 2011, 6117–6127. [Google Scholar] [CrossRef]
- Li, Y.X.; Huang, M.H.; Yamashita, Y.; Kato, A.; Jia, Y.M.; Wang, W.B.; Fleet, G.W.J.; Nash, R.J.; Yu, C.Y. l-DMDP, l-homoDMDP and their C-3 fluorinated derivatives: synthesis and glycosidase-inhibition. Org. Biomol. Chem. 2011, 9, 3405–3414. [Google Scholar] [CrossRef]
- Zhang, W.; Sato, K.; Kato, A.; Jia, Y.M.; Hu, X.G.; Wilson, F.X.; van Well, R.; Horne, G.; Fleet, G.W.J.; Nash, R.J.; Yu, C.Y. Synthesis of fully substituted polyhydroxylated pyrrolizidines via cope-house cyclization. Org. Lett. 2011, 13, 4414–4417. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Nakagawa, S.; Kato, A.; Jia, Y.M.; Hu, X.G.; Yu, C.Y. A concise stereoselective synthesis of (-)-erycibelline. Org. Biomol. Chem. 2011, 9, 7713–7719. [Google Scholar] [CrossRef]
- Buchlovic, M.; Hebanova, S.; Potacek, M. 1,3-Dipolar cycloadditions of new 2,5-bifunctionalized five-membered cyclic nitrones. Tetrahedron 2012, 68, 3117–3122. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Kukushkin, V.Y. Theoretical study of reactant activation in 1,3-Dipolar cycloadditions of cyclic nitrones to free and Pt-bound nitriles. J. Org. Chem. 2005, 71, 582–592. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Nazarov, A.A.; Kozlova, L.V.; Kukushkin, V.Y. Theoretical study of Chemo-, Regio-, and Stereoselectivity in 1,3-dipolar cycloadditions of nitrones and nitrile oxides to free and Pt-bound bifunctional dipolarophiles. J. Org. Chem. 2007, 72, 4475–4485. [Google Scholar] [CrossRef]
- Marradi, M.; Corsi, M.; Cicchi, S.; Bonanni, M.; Cardona, F.; Goti, A. N-glycosylhydroxylamines as masked polyhydroxylated chiral nitrones in cycloaddition reactions: an access to pyrrolizidines. Heterocycles 2008, 79, 883–896. [Google Scholar]
- Merino, P.; Tejero, T.; Diez-Martinez, A.; Gultekin, Z. Nitrone ylides: Two possible 1,3-Dipolar cycloadditions but only one stepwise formation of all-cis-5-Aryl-2,3,5-trisubstituted N-hydroxypyrrolidines. Eur. J. Org. Chem. 2011, 6567–6573. [Google Scholar]
- Morozov, D.A.; Kirilyuk, I.A.; Komarov, D.A.; Goti, A.; Bagryanskaya, I.Y.; Kuratieva, N.V.; Grigor'ev, I.A. Synthesis of a chiral C-2-Symmetric sterically hindered pyrrolidine nitroxide radical via combined iterative nucleophilic additions and intramolecular 1,3-dipolar cycloadditions to cyclic nitrones. J. Org. Chem. 2012, 77, 10688–10698. [Google Scholar] [CrossRef]
- Bădoiu, A.; Brinkmann, Y.; Viton, F.; Kündig, E.P. Asymmetric lewis acid-catalyzed 1,3-dipolar cycloadditions. Pure. Appl. Chem. 2008, 80, 1013–1018. [Google Scholar] [CrossRef]
- Berini, C.; Minassian, F.; Pelloux-Leon, N.; Denis, J.N.; Vallée, Y.; Philouze, C. Efficient stereoselective nucleophilic addition of pyrroles to chiral nitrones. Org. Biomol. Chem. 2008, 6, 2574–2586. [Google Scholar] [CrossRef]
- Delso, I.; Tejero, T.; Goti, A.; Merino, P. Synthesis of D-arabinose-derived polyhydroxylated pyrrolidine, indolizidine and pyrrolizidine alkaloids. Total synthesis of hyacinthacine A2. Tetrahedron 2010, 66, 1220–1227. [Google Scholar] [CrossRef]
- Li, X.L.; Qin, Z.B.; Wang, R.; Chen, H.; Zhang, P.Z. Stereoselective synthesis of novel C-azanucleoside analogues by microwave-assisted nucleophilic addition of sugar-derived cyclic nitrones. Tetrahedron 2011, 67, 1792–1798. [Google Scholar]
- Merino, P.; Delso, I.; Tejero, T.; Cardona, F.; Marradi, M.; Faggi, E.; Parmeggiani, C.; Goti, A. Nucleophilic additions to cyclic nitrones en route to iminocyclitols - Total syntheses of DMDP, 6-deoxy-DMDP, DAB-1, CYB-3, nectrisine, and radicamine B. Eur. J. Org. Chem. 2008, 2929–2947. [Google Scholar]
- Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Nucleophilic additions to chiral nitrones: new approaches to nitrogenated compounds. Synlett 2000, 442–454. [Google Scholar]
- Merino, P.; Revuelta, J.; Tejero, T.; Cicchi, S.; Goti, A. Fully stereoselective nucleophilic addition to a novel chiral pyrroline N-oxide: Total syntheses of (2S,3R)-3-hydroxy-3-methylproline and its (2R)-epimer. Eur. J. Org. Chem. 2004, 776–782. [Google Scholar]
- Murga, J.; Portolés, R.; Falomir, E.; Carda, M.; Marco, J.A. Stereoselective addition of organometallic reagents to a chiral acyclic nitrone derived from l-erythrulose. Tetrahedron: Asymmetry 2005, 16, 1807–1816. [Google Scholar] [CrossRef]
- Yang, S.H.; Caprio, V. A concise and flexible synthesis of the core structure of pinnaic acid. Synlett 2007, 1219–1222. [Google Scholar]
- Masson, G.; Zeghida, W.; Cividino, P.; Py, S.; Vallée, Y. A concise formal synthesis of (S)-Vigabatrin based on nitrone umpolung. Synlett 2003, 1527–1529. [Google Scholar]
- Chavarot, M.; Rivard, M.; Rose-Munch, F.; Rose, E.; Py, S. First asymmetric SmI2-induced cross-coupling of Cr(CO)3 aromatic nitrone complexes with carbonyl compounds. Chem. Commun. 2004, 2330–2331. [Google Scholar]
- Desvergnes, S.; Py, S.; Vallée, Y. Total synthesis of (+)-Hyacinthacine A2 based on SmI2-induced nitrone umpolung. J. Org. Chem. 2005, 70, 1459–1462. [Google Scholar] [CrossRef]
- Masson, G.; Philouze, C.; Py, S. cis-Stereoselective SmI2-promoted reductive coupling of keto-nitrones: First synthesis of 1-epitrehazolamine. Org. Biomol. Chem. 2005, 3, 2067–2069. [Google Scholar] [CrossRef]
- Argyropoulos, N.G.; Panagiotidis, T.; Coutouli-Argyropoulou, E.; Raptopoulou, C. Asymmetric nitrone cycloadditions and their application to the synthesis of enantiopure pyrrolidine and pyrrolizidine derivatives. Tetrahedron 2007, 63, 321–330. [Google Scholar] [CrossRef]
- Desvergnes, S.; Desvergnes, V.; Martin, O.R.; Itoh, K.; Liu, H.-W.; Py, S. Stereoselective synthesis of β-1-C-substituted 1,4-dideoxy-1,4-imino-d-galactitols and evaluation as UDP-galactopyranose mutase inhibitors. Bioorg. Med. Chem. 2007, 15, 6443–6449. [Google Scholar] [CrossRef]
- Wu, S.F.; Zheng, X.; Ruan, Y.P.; Huang, P.Q. A new approach to 3-hydroxyprolinol derivatives by samarium diiodide-mediated reductive coupling of chiral nitrone with carbonyl compounds. Org. Biomol. Chem. 2009, 7, 2967–2975. [Google Scholar] [CrossRef]
- Wu, S.F.; Ruan, Y.P.; Zheng, X.; Huang, P.Q. Samarium diiodide-mediated reductive couplings of chiral nitrones with aldehydes/ketones and acyl chlorides. Tetrahedron 2010, 66, 1653–1660. [Google Scholar] [CrossRef]
- Merino, P. Nitrones and its Cyclic Analogues. In Science of Synthesis; Bellus, D., Padwa, A., Eds.; George Thieme: Stuttgart, Germany, 2004; Volume 27, pp. 511–580. [Google Scholar]
- Merino, P. Nitrones and cyclic analogues. An update. In Science of Synthesis; Schaumann, E., Ed.; George Thieme: Stuttgart, Germany, 2011; Volume 2010/4, pp. 325–403. [Google Scholar]
- Tsou, E.L.; Chen, S.Y.; Yang, M.H.; Wang, S.C.; Cheng, T.R.R.; Cheng, W.C. Synthesis and biological evaluation of a 2-aryl polyhydroxylated pyrrolidine alkaloid-based library. Bioorg. Med. Chem. 2008, 16, 10198–10204. [Google Scholar]
- Stecko, S.; Mames, A.; Furman, B.; Chmielewski, M. Asymmetric kinugasa reaction of cyclic nitrones and nonracemic acetylenes. J. Org. Chem. 2009, 74, 3094–3100. [Google Scholar] [CrossRef]
- Ueda, T.; Inada, M.; Okamoto, I.; Morita, N.; Tamura, O. Synthesis of Maremycins A and D1 via Cycloaddition of a Nitrone with (E)-3-Ethylidene-1-methylindolin-2-one. Org. Lett. 2008, 10, 2043–2046. [Google Scholar] [CrossRef]
- Cardona, F.; Moreno, G.; Guarna, F.; Vogel, P.; Schuetz, C.; Merino, P.; Goti, A. New concise total synthesis of (+)-lentiginosine and some structural analogues. J. Org. Chem. 2005, 70, 6552–6555. [Google Scholar]
- Goti, A.; Cacciarini, M.; Cardona, F.; Cordero, F.M.; Brandi, A. total synthesis of (−)-rosmarinecine by intramolecular cycloaddition of (S)-malic acid derived pyrroline N-oxide. Org. Lett. 2001, 3, 1367–1369. [Google Scholar] [CrossRef]
- Toyao, A.; Tamura, O.; Takagi, H.; Ishibashi, H. A concise synthesis of (-)-codonopsinine and an approach to synthesis of (+)-hyacinthacines A1 and A2 from a polyhydroxylated cyclic nitrone. Synlett 2003, 35–38. [Google Scholar]
- Gurjar, M.K.; Borhade, R.G.; Puranik, V.G.; Ramana, C.V. Total synthesis of (−)-radicamine B. Tetrahedron Lett. 2006, 47, 6979–6981. [Google Scholar] [CrossRef]
- Revuelta, J.; Cicchi, S.; Goti, A.; Brandi, A. Enantiopure Cyclic Nitrones: A Useful Class of Building Blocks for Asymmetric Syntheses. Synthesis 2007, 485–504. [Google Scholar]
- Cicchi, S.; Marradi, M.; Corsi, M.; Faggi, C.; Goti, A. Preparation of N-Glycosylhydroxylamines and their oxidation to nitrones for the enantioselective synthesis of isoxazolidines. Eur. J. Org. Chem. 2003, 4152–4161. [Google Scholar]
- Cardona, F.; Gorini, L.; Goti, A. Tetra-n-Propylammonium Perruthenate (TPAP) catalyzed aerobic oxidation of hydroxylamines to nitrones. Lett. Org. Chem. 2006, 3, 118–120. [Google Scholar] [CrossRef]
- Bonanni, M.; Marradi, M.; Cicchi, S.; Goti, A. Formal mixed double addition to N-glycosylnitrones through Addition-Oxidation-Addition to N-glycosylhydroxylamines. Synlett 2008, 197–202. [Google Scholar]
- Cao, Q.Y.; Zhang, J.F.; Ren, W.X.; Choi, K.; Kim, J.S. Ferrocene-based novel electrochemical In3+ sensor. Tetrahedron Lett. 2011, 52, 4464–4467. [Google Scholar] [CrossRef]
- Robinson, A.J.; DeLucca, I.; Drummond, S.; Boswell, G.A. Steroidal nitrone inhibitors of 5α-reductase. Tetrahedron Lett. 2003, 44, 4801–4804. [Google Scholar] [CrossRef]
- Goti, A.; Nannelli, L. Synthesis of nitrones by methyltrioxorhenium catalyzed direct oxidation of secondary amines. Tetrahedron Lett. 1996, 37, 6025–6028. [Google Scholar] [CrossRef]
- Murray, R.W.; Iyanar, K.; Chen, J.; Wearing, J.T. Synthesis of Nitrones Using the Methyltrioxorhenium/Hydrogen Peroxide System. J. Org. Chem. 1996, 61, 8099–8102. [Google Scholar] [CrossRef]
- Cardona, F.; Bonanni, M.; Soldaini, G.; Goti, A. One-Pot Synthesis of Nitrones from Primary Amines and Aldehydes Catalyzed by Methyltrioxorhenium. ChemSusChem 2008, 1, 327–332. [Google Scholar] [CrossRef]
- Soldaini, G.; Cardona, F.; Goti, A. Catalytic Oxidation of Imines Based on Methyltrioxorhenium/Urea Hydrogen Peroxide: A Mild and Easy Chemo- and Regioselective Entry to Nitrones. Org. Lett. 2007, 9, 473–476. [Google Scholar] [CrossRef]
- Chackalamannil, S.; Wang, Y. An enantioselective route to trans-2,6-disubstituted piperidines. Tetrahedron 1997, 53, 11203–11210. [Google Scholar] [CrossRef]
- Oppolzer, W.; Deerberg, J.; Tamura, O. Diastereo- and Enantiocontrolled Synthesis of (–)-Allosedamine via Cycloaddition of a Chiral Nitrone. Helv. Chim. Acta 1994, 77, 554–560. [Google Scholar] [CrossRef]
- Cicchi, S.; Marradi, M.; Vogel, P.; Goti, A. One-Pot synthesis of cyclic nitrones and their conversion to pyrrolizidines: 7a-epi-Crotanecine inhibits α-Mannosidases. J. Org. Chem. 2006, 71, 1614–1619. [Google Scholar] [CrossRef]
- Tsou, E.L.; Yeh, Y.T.; Liang, P.H.; Cheng, W.C. A convenient approach toward the synthesis of enantiopure isomers of DMDP and ADMDP. Tetrahedron 2009, 65, 93–100. [Google Scholar] [CrossRef]
- Wang, W.B.; Huang, M.H.; Li, Y.X.; Rui, P.X.; Hu, X.G.; Zhang, W.; Su, J.K.; Zhang, Z.L.; Zhu, J.S.; Xu, W.H.; Xie, X.Q.; Jia, Y.M.; Yu, C.Y. A Practical Synthesis of Sugar-Derived Cyclic Nitrones: Powerful Synthons for the Synthesis of Iminosugars. Synlett 2010, 488–492. [Google Scholar]
- Tamura, O.; Toyao, A.; Ishibashi, H. TBAT-Mediated Nitrone Formation of ω-Mesyloxy-O-tert-butyldiphenylsilyl-oximes: Facile Synthesis of Cyclic Nitrones from Hemiacetals. Synlett 2002, 1344–1346. [Google Scholar] [CrossRef]
- Chan, T.H.; Chang, Y.F.; Hsu, J.J.; Cheng, W.C. Straightforward Synthesis of Diverse 1-Deoxyazapyranosides via Stereocontrolled Nucleophilic Additions to Six-Membered Cyclic Nitrones. Eur. J. Org. Chem. 2010, 5555–5559. [Google Scholar]
- Peer, A.; Vasella, A. Synthesis of an L-Fucose-Derived Cyclic Nitrone and its Conversion to α-L-Fucosidase Inhibitors. Helv. Chim. Acta. 1999, 82, 1044–1065. [Google Scholar] [CrossRef]
- van den Broek, L.A.G.M. 1,3-Dipolar cycloaddition reactions of homochiral cyclic nitrones derived from 1-deoxynojirimycin. Tetrahedron 1996, 52, 4467–4478. [Google Scholar] [CrossRef]
- Gracias, V.; Zeng, Y.; Desai, P.; Aubé, J. Ring Expansive Routes to Quinolizidine Alkaloids: Formal Synthesis of (−)-Lasubine II. Org. Lett. 2003, 5, 4999–5001. [Google Scholar] [CrossRef]
- Zhao, W.B.; Nakagawa, S.; Kato, A.; Adachi, I.; Jia, Y.M.; Hu, X.G.; Fleet, G.W.J.; Wilson, F.X.; Horne, G.; Yoshihara, A.; Izumori, K.; Yu, C.Y. General Synthesis of Sugar-derived Azepane Nitrones: Precursors of Azepane Iminosugars. J. Org. Chem. 2013, 78, 3208–3221. [Google Scholar] [CrossRef]
- Khan, A.T.; Khan, M.M. Bromodimethylsulfonium bromide (BDMS) mediated dithioacetalization of carbohydrates under solvent-free conditions. Carbohydr. Res. 2010, 345, 2139–2145. [Google Scholar] [CrossRef]
- Ley, S.V.; Norman, J.; Griffith, W.P.; Marsden, S.P. Tetrapropylammonium Perruthenate, Pr4N+RuO4−, TPAP: A Catalytic Oxidant for Organic Synthesis. Synthesis 1994, 639–666. [Google Scholar]
- Moutel, S.; Shipman, M. Synthesis of (+)-nojirimycin from 2,3,4,6-tetra-O-benzyl-d-glucopyranose. J. Chem. Soc. Perkin. Trans. 1 1999, 1403–1406. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7 and 13–16 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, H.; Zhao, W.-B.; Zhu, J.-S.; Jia, Y.-M.; Yu, C.-Y. An Efficient Synthesis of Aldohexose-Derived Piperidine Nitrones: Precursors of Piperidine Iminosugars. Molecules 2013, 18, 6021-6034. https://doi.org/10.3390/molecules18056021
Zhao H, Zhao W-B, Zhu J-S, Jia Y-M, Yu C-Y. An Efficient Synthesis of Aldohexose-Derived Piperidine Nitrones: Precursors of Piperidine Iminosugars. Molecules. 2013; 18(5):6021-6034. https://doi.org/10.3390/molecules18056021
Chicago/Turabian StyleZhao, Hui, Wen-Bo Zhao, Jian-She Zhu, Yue-Mei Jia, and Chu-Yi Yu. 2013. "An Efficient Synthesis of Aldohexose-Derived Piperidine Nitrones: Precursors of Piperidine Iminosugars" Molecules 18, no. 5: 6021-6034. https://doi.org/10.3390/molecules18056021





