Triterpene Esters: Natural Products from Dorstenia arifolia (Moraceae)
Abstract
:1. Introduction
2. Results and Discussion

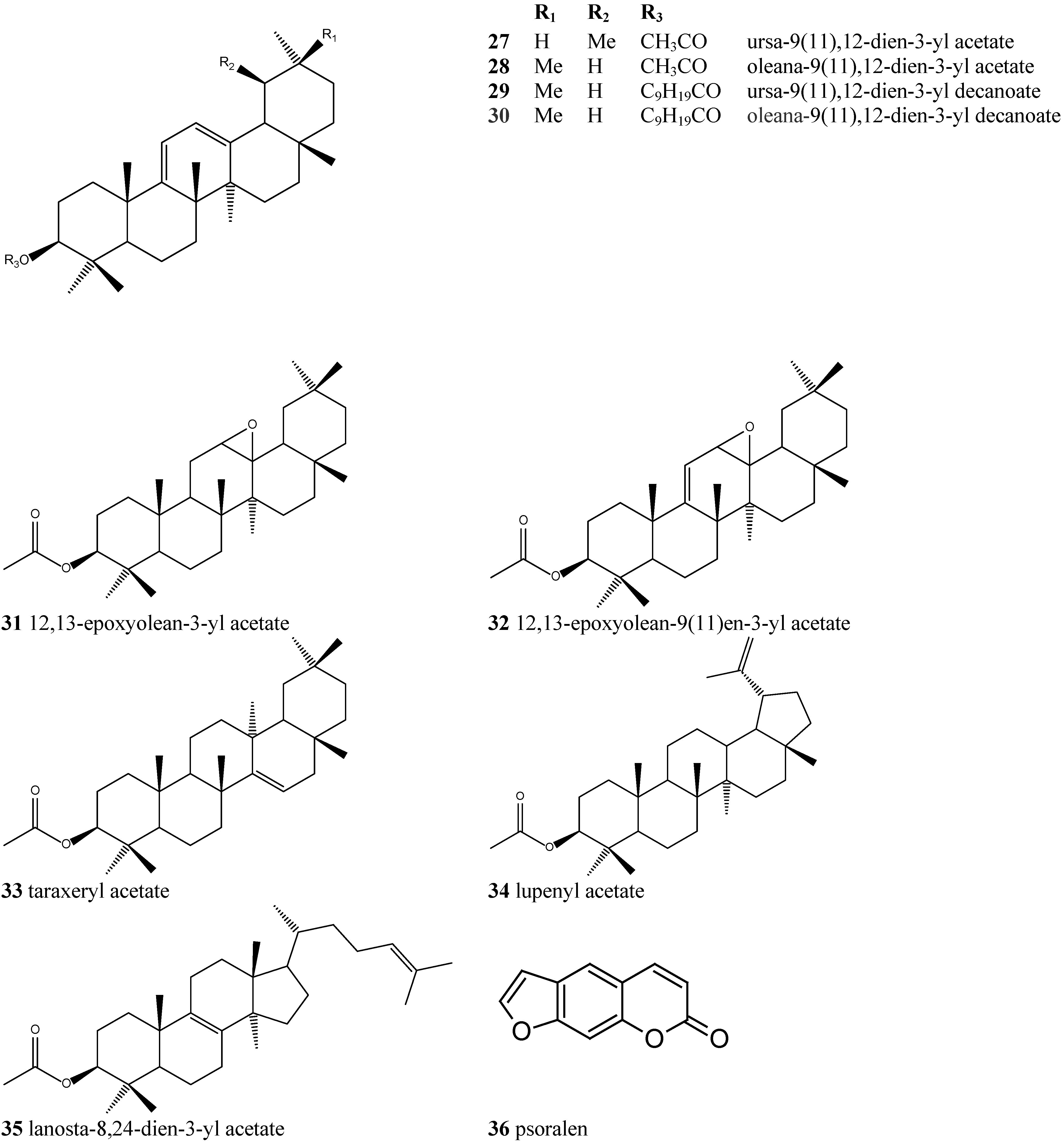
| Compound | Fragments, m/z (relative abundance) |
|---|---|
| 1 | C30H50O: 426 (M+, 11), 218 (100), 203 (22), 189 (22) |
| 2 | C30H50O: 426 (M+, 5), 218 (100), 203 (44), 189 (17) |
| 3 | C32H52O2: 468 (M+, 11), 408 ([M-HAc], 5), 218 (100), 203 (22), 189 (28) |
| 4 | C32H52O2: 468 (M+, 5), 218 (100), 203 (44), 189 (17) |
| 5 * | C38H24O2: 552 (M+, 5), 218 (100), 203 (22), 189 (33) |
| 6 * | C38H24O2: 218 (100), 203 (56), 189 (28) |
| 7 | C40H68O2: 580 (M+, 4), 409 ([M-HDec + H], 3), 218 (100), 203 (19), 189 (14) |
| 8 | C40H68O2: 580 (M+, 2), 409 ([M-HDec + H], 1), 218 (100), 203 (30), 189 (14) |
| 9 | C42H72O2: 608 (M+, 4), 409 ([M-HDod + H], 3), 218 (100), 203 (13), 189 (17) |
| 10 | C42H72O2: 608 (M+, 2), 409 ([M-HDod + H], 2), 218 (100), 203 (28), 189 (13) |
| 11 | C44H76O2: 636 (M+, 3), 409 ([M-HTet + H], 3), 218 (100), 203 (12), 189 (16) |
| 12 | C44H76O2: 636 (M+, 1), 409 ([M-HTet + H], 2), 218 (100), 203 (25), 189 (12) |
| 13 * | C46H80O2: 664 (M+, 5), 409 ([M-HHex + H], 5), 218 (100), 203 (11), 189 (17) |
| 14 * | C46H80O2: 409 ([M-Hex + H], 5), 218 (100), 203 (33), 189 (17) |
| 15 | C30H50O: 426 (M+, 5), 408 (5), 259 (100), 274 (83) |
| 16 | C32H52O2: 468 (M+, 11), 259 (100), 274 (94), 408 ([M-HAc], 5) |
| 17 | C30H48O2: 440 (M+, 22), 408 (5), 273 (89), 232 (78), 135 (100) |
| 18 | C30H48O2: 440 (M+, 11), 408 (5), 273 (100), 232 (44), 135 (67) |
| 19 | C32H50O3: 482 (M+, 5), 407 ([M-HAc + H], 5), 232 (61), 273 (61), 135 (100) |
| 20 | C32H50O3: 482 (M+, 5), 407 ([M-HAc + H], 5), 232 (55), 273 (94), 135 (100) |
| 21 | C38H62O3: 566 (M+, 5), 423 (5), 407 ([M-HOct + H], 11), 232 (83), 273 (83), 135 (100) |
| 22 | C38H62O3: 566 (M+, 5), 423 (5), 407 ([M-HOct + H], 5), 232 (55), 273 (100), 135 (72) |
| 23 | C40H66O3: 594 (M+, 5), 423 (11), 407 ([M-HDec + H], 11), 232 (89), 273 (89), 135 (100) |
| 24 | C40H66O3: 594 (M+, 5), 423 (5), 407 ([M-HDec + H], 5), 232 (50), 273 (100), 135 (67) |
| 25 | C42H70O3: 622 (M+, 5), 407 ([M-HDod + H], 11), 232 (100), 273 (94) |
| 26 | C42H70O3: 622 (M+, 5), 407 ([M-HDod + H], 15), 232 (55), 273 (100) |
| 27 * | C32H50O2: 466 (M+, 100), 451 (5), 407 ([M-HAc + H], 5), 255 (50) |
| 28 * | C32H50O2: 466 (M+, 100), 451 (11), 407 ([M-HAc + H], 5), 255 (50) |
| 29 * | C40H66O2: 578 (M+, 100), 563 (5), 407 ([M-HDec + H], 5), 391 (22), 255 (50) |
| 30 * | C40H66O2: 578 (M+, 100), 563 (22), 407 ([M-HDec + H], 11), 391 (28), 255 (28) |
| 31 | C32H52O3: 484 (M+, 17), 466 (11), 234 (100) |
| 32 * | C32H50O3: 482 (M+, 17), 466 (11), 234 (100) |
| 33 * | C32H52O2: 468 (M+, 5), 453 (11), 393 (11), 344(39), 269 (33), 204 (100) |
| 34 * | C32H52O2: 468 (M+, 11), 408 (11), 204 (11), 189 (100) |
| 35 * | C32H52O2: 468 (M+, 11), 453 (39), 393 (56), 353 (11) |
| 36 | C11H6O3: 186 (M+, 100), 158 (94), 130 (33), 102 (56) |
| Carbon | α-Am | β-Am | 11-oxo-α | 11-oxo-β |
|---|---|---|---|---|
| 3 | 79.0 | 79.0 | 78.8 | 78.8 |
| 11 | 23.6 | 23.6 | 199.8 | 200.3 |
| 12 | 124.4 | 121.8 | 130.4 | 128.1 |
| 13 | 139.5 | 145.2 | 164.9 | 170.6 |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Chromatographic Analysis
3.5. Basic Hydrolysis of Triterpene Ester Derivatives
4. Conclusions
Acknowledgments
References
- Abegaz, B.M.; Ngadjui, B.T.; Dongo, E.; Ngameni, B.; Nindi, M.N.; Bezabih, M. Chalcones and other constituents of Dorstenia prorepens and Dorstenia zenkeri. Phytochemistry 2002, 59, 877–883. [Google Scholar]
- Ngadjui, B.T.; Dongo, B.M.; Fotso, S.; Tamboue, H. Dinklagins A, B and C: Three prenylated flavonoids and other constituents from the twigs of Dorstenia dinklagei. Phytochemistry 2002, 61, 99–104. [Google Scholar]
- Omisore, N.O.A.; Adewunmi, C.O.; Iwalewa, E.O.; Ngadjui, B.T.; Watchueng, J.; Abegaz, B.M.; Ojewole, J.A.O. Antinociceptive and anti-inflammatory effects of Dorstenia barteri (Moraceae) leaf and twig extracts in mice. J. Ethnopharmacol. 2004, 95, 7–12. [Google Scholar]
- Ngameni, B.; Touaibia, M.; Patnam, R.; Belkaid, A.; Sonna, P.; Ngadjui, T.; Annabi, B.; Roy, R. Inhibition of MMP-2 secretion from brain tumor cells suggests chemopreventive properties of a furanocoumarin glycoside and of chalcones isolated from the twigs of Dorstenia turbinate. Phytochemistry 2006, 67, 2573–2579. [Google Scholar]
- Vouffo, B.; Krohn, K.; Kouam, S.F.; Hussain, H.; Dongo, E.; Meier, K.; Schulz, B. Dinklagenonoate: A new isobauerane-type triterpenoid and other minor constituents from the twigs of Dorstenia dinklagei. Biochem. Syst. Ecol. 2008, 36, 655–658. [Google Scholar]
- Tabopda, T.K.; Ngoupayo, J.; Awoussong, P.K.; Mitaine-Offer, A.C.; Ali, M.S.; Ngadjui, B.T.; Lacaille-Dubois, M.A. Triprenylated flavonoids from Dorstenia psilurus and their R-glucosidase inhibition properties. J. Nat. Prod. 2008, 71, 2068–2072. [Google Scholar] [CrossRef]
- Heinke, R.; Franke, K.; Michels, K.; Wessjohann, L.; Ali, N.A.A.; Schmidt, J. Analysis of furanocoumarins from Yemenite Dorstenia species by liquid chromatography/electrospray tandem mass spectrometry. J. Mass. Spectrom. 2012, 47, 7–22. [Google Scholar]
- Ngadjui, B.T.; Abegaz, B.M. The chemistry and pharmacology of the genus Dorstenia (Moraceae). J. Nat. Prod. 2003, 29, 761–805. [Google Scholar]
- Silva, M.L.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Pedro, S.R.; Fontana, R. Bioactive oleanane, oupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Hernández-Vázquez, L.; Mangas, S.; Palazón, J.; Navarro-Ocaña, A. Valuable medicinal plants and resins: Commercial phytochemicals with bioactive properties. Ind. Crop. Prod. 2010, 31, 476–480. [Google Scholar] [CrossRef]
- Vilegas, J.H.Y.; Lanças, F.M.; Vilegas, W.; Pozetti, G.L. Further triterpenes, steroids and furocoumarins from brazilian medicinal plants of Dorstenia genus (Moraceae). J. Braz. Chem. Soc. 1997, 8, 529–535. [Google Scholar] [CrossRef]
- Barreiros, M.L.; David, J.M.; Queiroz, L.P.; David, J.P. Flavonoids and triterpenes from leaves of Erythroxylum nummularia. Biochem. Syst. Ecol. 2005, 33, 537–540. [Google Scholar]
- Mendes, C.C.; Cruz, F.G.; David, J.M.; Nascimento, I.P.; David, J.P. Triterpenos esterificados com ácidos graxos e ácidos triterpênicos isolados de Byrosonima microphylla. Quim. Nova 1999, 22, 185–188. [Google Scholar] [CrossRef]
- Zapata-Sudo, G.; Mendes, C.F.; Kartnaller, M.A.; Fortes, TO.; Freitas, N.F.B.; Kaplan, M.A.C.; Sudo, R.T. Sedative and anticonvulsant activities of methanol extract of Dorstenia arifolia in mice. J. Ethnopharmacol. 2010, 130, 9–12. [Google Scholar] [CrossRef]
- Poumale, H.M.P.; Awoussonga, K.P.; Randrianasoloc, R.; Christophe, C.F.S.; Ngadjuia, B.T.; Shiono, Y. Long-chain alkanoic acid esters of lupeol from Dorstenia harmsiana Engl. (Moraceae). Nat. Prod. Res. 2012, 26, 749–755. [Google Scholar] [CrossRef]
- Fingolo, C.E. Phytochemical Strategies for the Biological Diversity Sustainable Use. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Sample Availability: Samples of the compounds 1–36 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fingolo, C.E.; Santos, T.D.S.; Filho, M.D.M.V.; Kaplan, M.A.C. Triterpene Esters: Natural Products from Dorstenia arifolia (Moraceae). Molecules 2013, 18, 4247-4256. https://doi.org/10.3390/molecules18044247
Fingolo CE, Santos TDS, Filho MDMV, Kaplan MAC. Triterpene Esters: Natural Products from Dorstenia arifolia (Moraceae). Molecules. 2013; 18(4):4247-4256. https://doi.org/10.3390/molecules18044247
Chicago/Turabian StyleFingolo, Catharina E., Thabata De S. Santos, Marcelo D. M. Vianna Filho, and Maria Auxiliadora C. Kaplan. 2013. "Triterpene Esters: Natural Products from Dorstenia arifolia (Moraceae)" Molecules 18, no. 4: 4247-4256. https://doi.org/10.3390/molecules18044247




