Dynamic Motion and Rearranged Molecular Shape of Heme in Myoglobin: Structural and Functional Consequences
Abstract
:1. Introduction
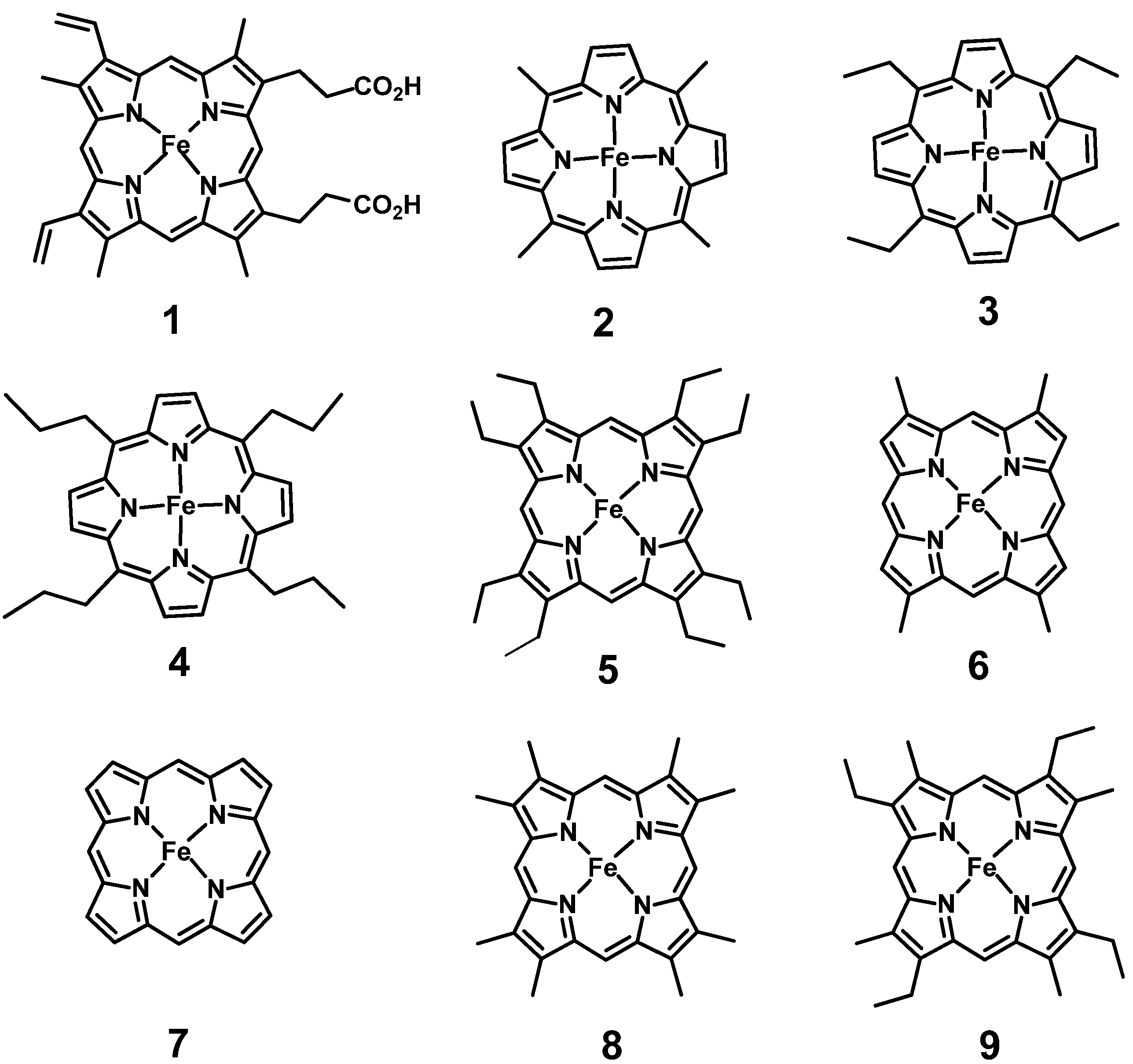
2. Heme Rotation in Myoglobin
2.1. Myoglobin Reconstitution with Alkyl Hemin
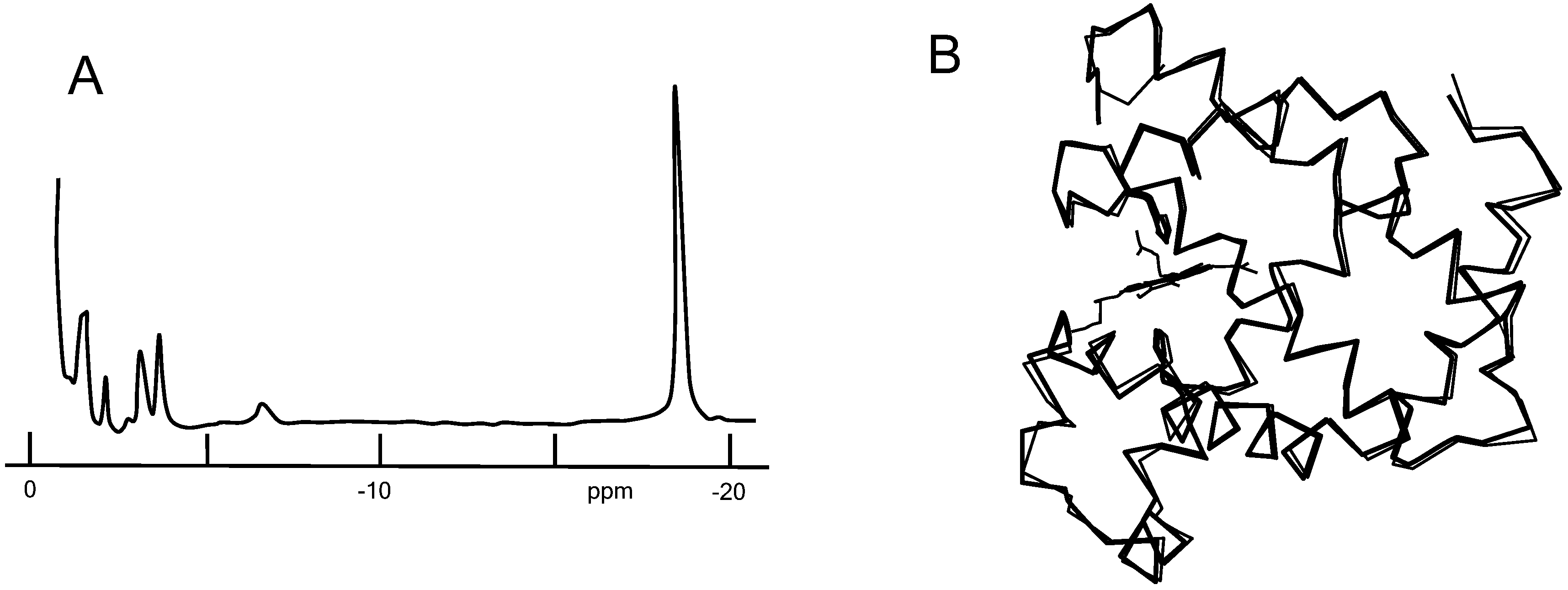
2.2. Detection of the Heme Rotation about the Iron-Histidine Bond
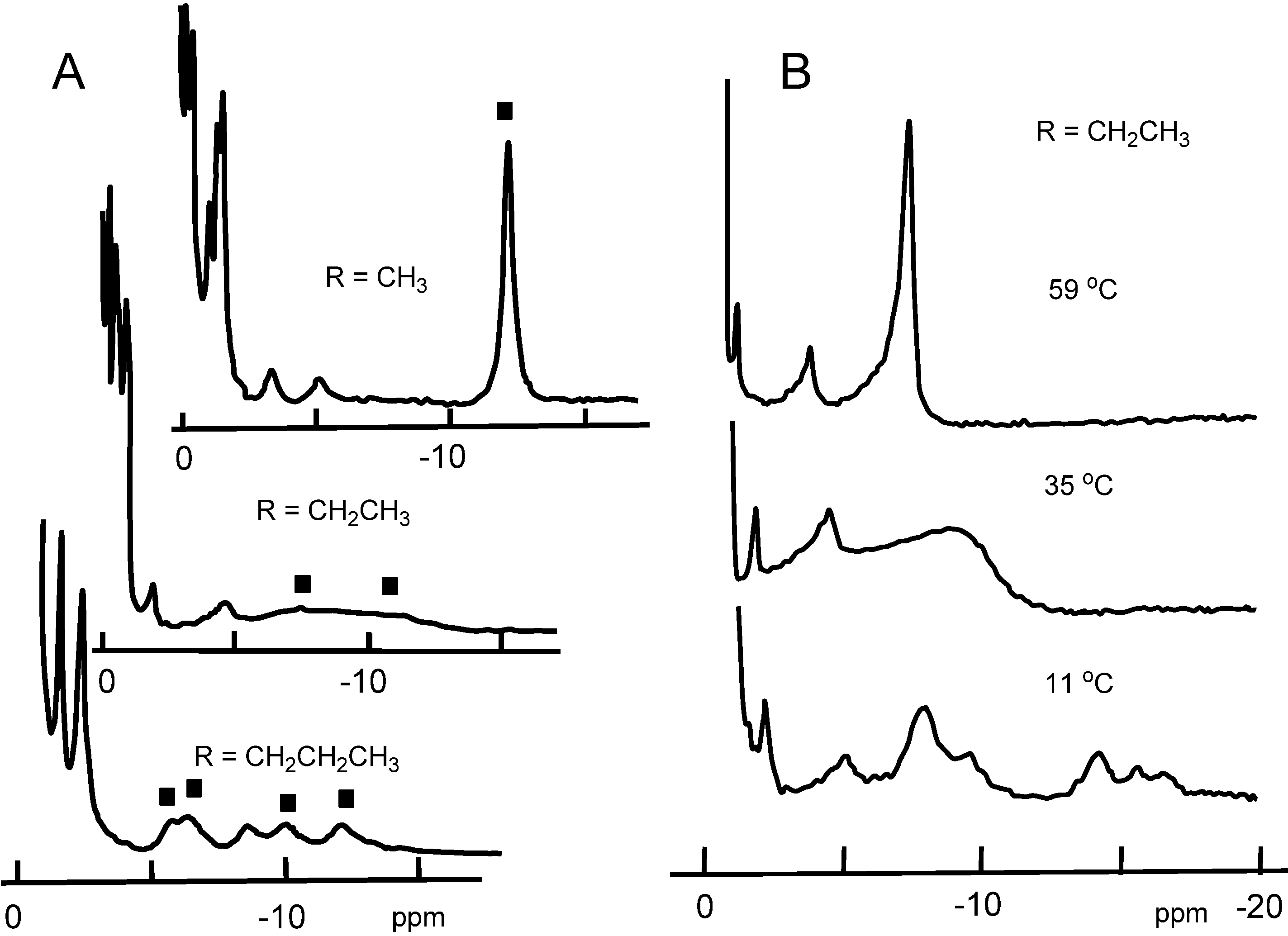
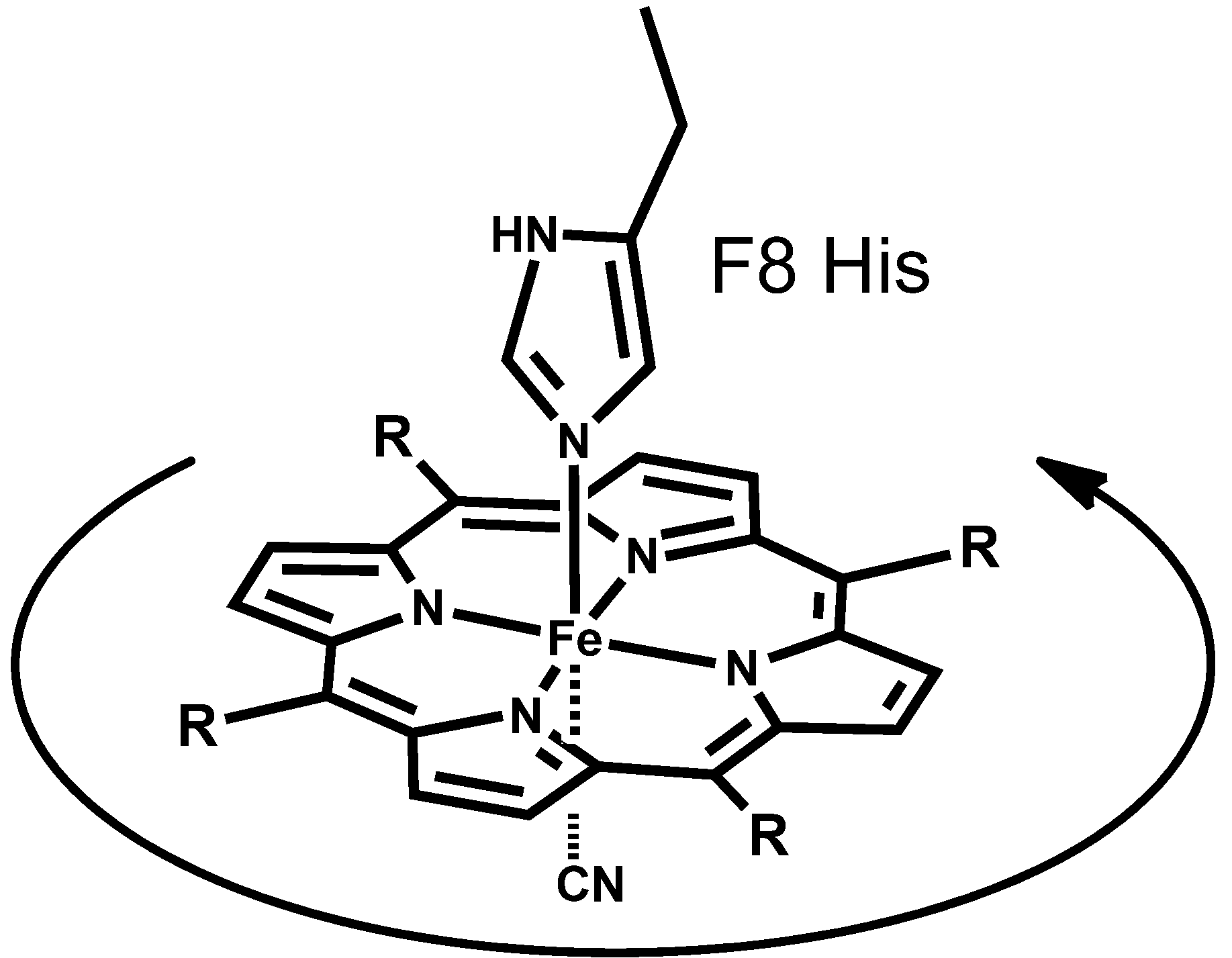
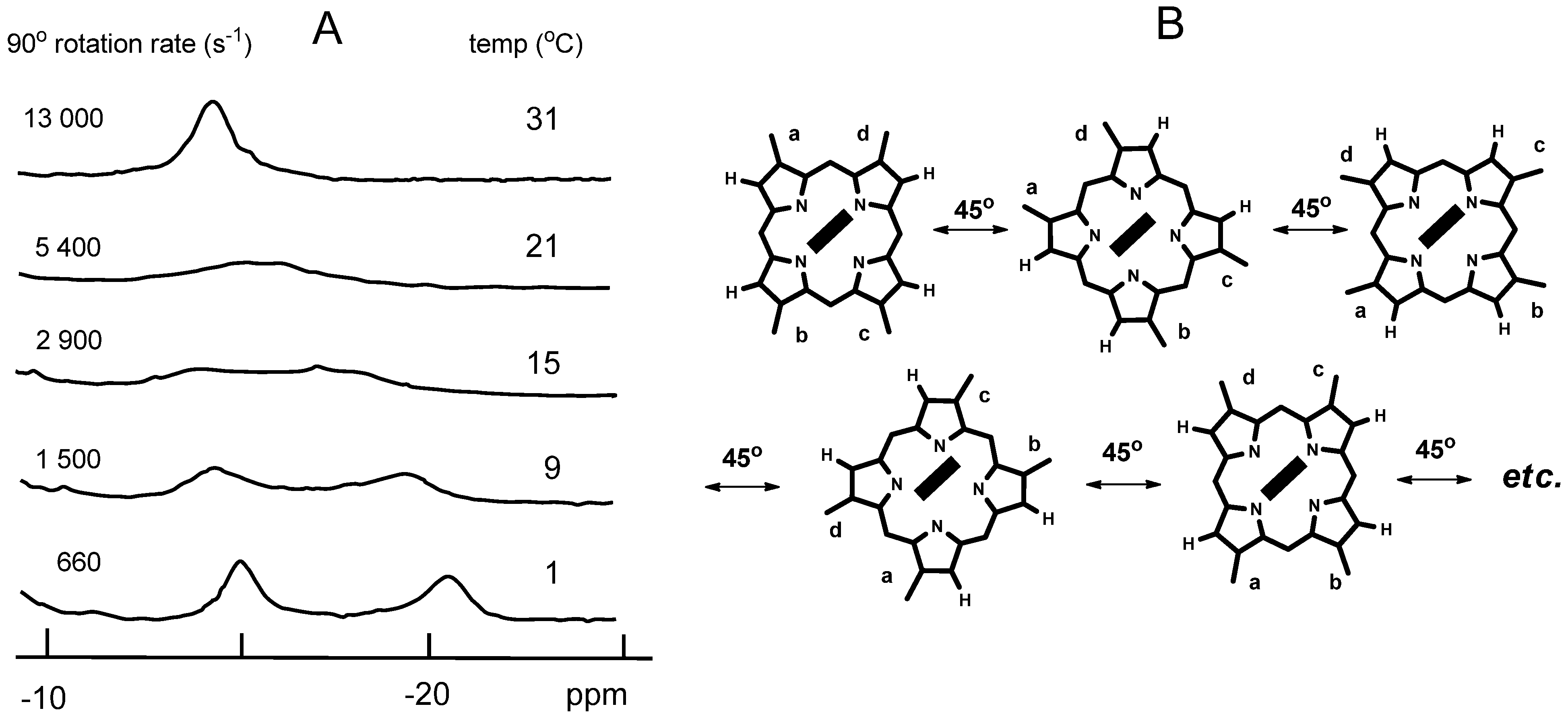
2.3. Analysis of Heme Rotation Rate
2.4. Crystallographic Structure of Mb with Rotating Heme
2.5. CO Binding to the Mb Reconstituted with Small Hemes
| Mb | kon (μM−1s−1) | koff (s−1) | K (μM−1) | Vacancy space (Å3) a |
|---|---|---|---|---|
| Mb7 | 3.6 | 0.583 | 6.2 | 399 |
| Mb2 | 12.6 | 0.323 | 39.0 | 426 |
| Mb3 | 15.1 | 0.356 | 42.0 | 438 |
| Mb4 | 7.4 | 0.180 | 41.0 | 411 |
| Mb1 | 0.7 | 0.016 | 44.0 | 385 |
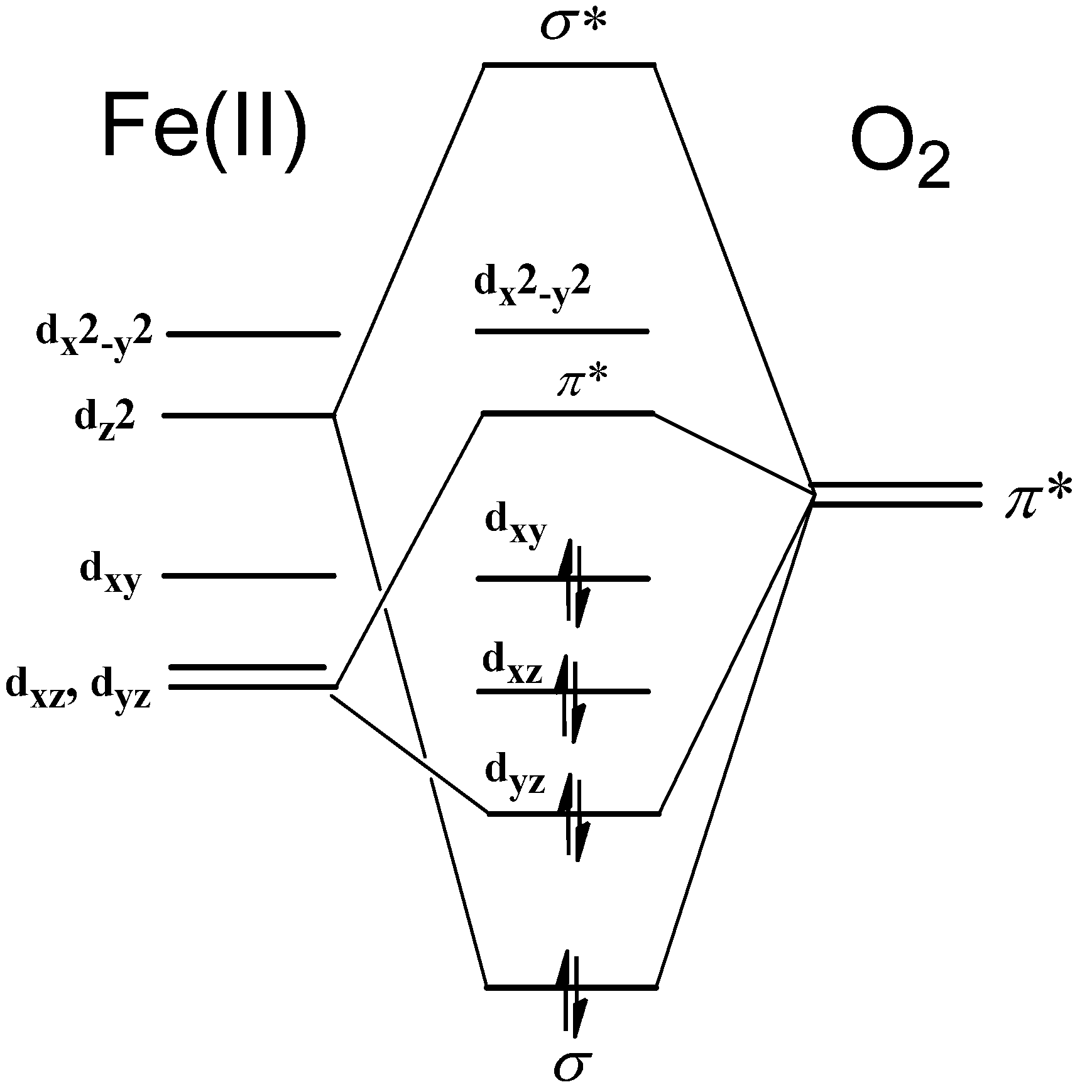
2.6. Function of the Mb Bearing Rotating Heme

| Mb | kon (μM−1s−1) | koff (s−1) | K (μM−1) |
|---|---|---|---|
| Mb8 | 9.7 | 7.5 | 1.2 |
| Mb9 | 8.4 | 8.5 | 1.0 |
| Mb1 | 14 | 12 | 1.2 |
3. Core Modified Heme Analogues
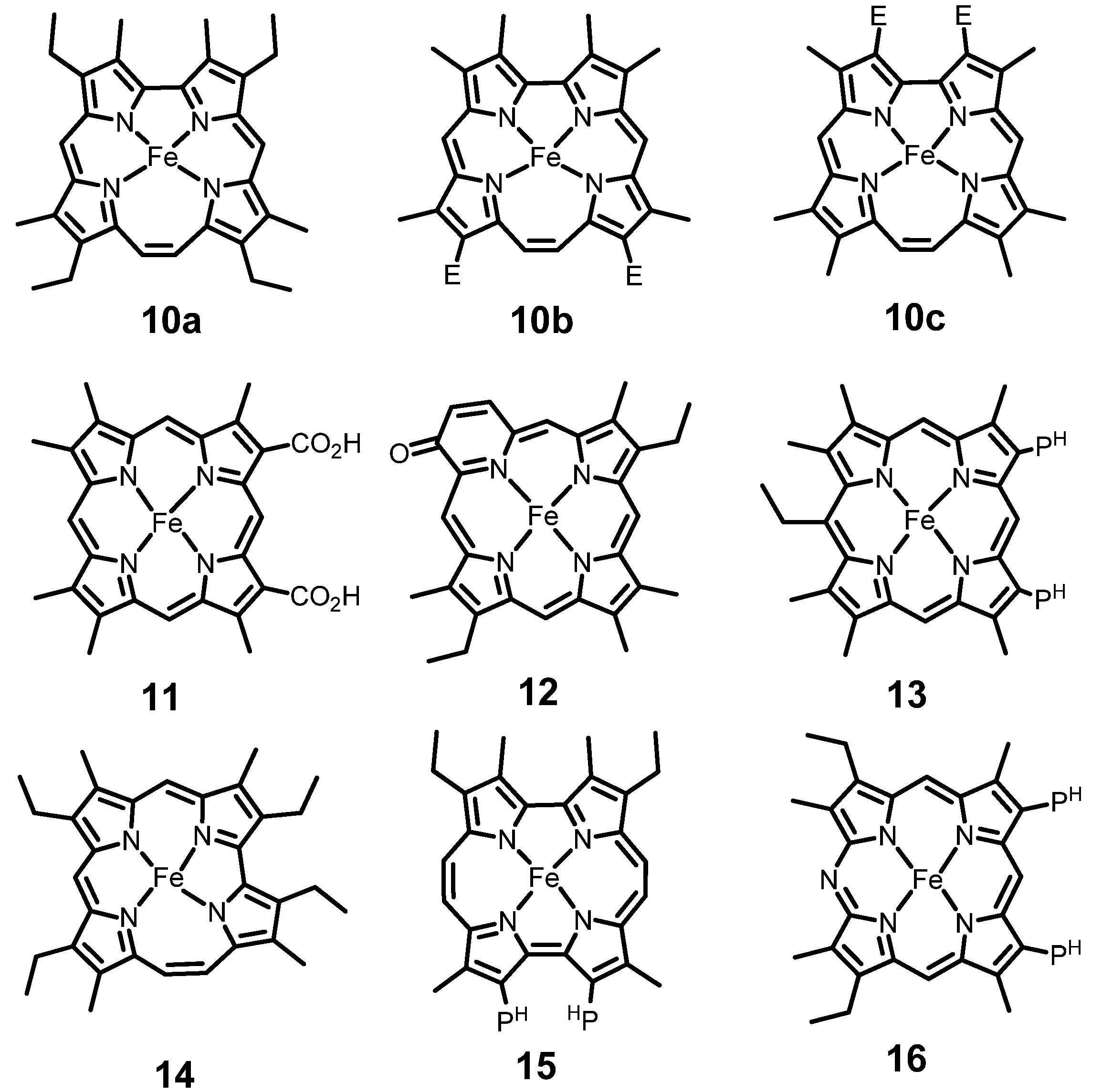
| Mb | K (M−1) | Characteristic of coordination structure |
|---|---|---|
| Mb10a | 8.2 × 104 | Corrphycene: Trapezoidal heme |
| Mb10b | 4.5 × 104 | Corrphycene: Trapezoidal heme with two ester side chains |
| Mb10c | 1.4 × 102 | Corrphycene: Trapezoidal heme with two ester side chains |
| Mb11 | 3.7 × 105 | Regular heme with two carboxyl groups |
| Mb12 | 0 | Oxypyriporphyrin: Pyridine-substituted heme |
| Mb13 | 4.4 × 104 | Nonplanar heme |
| Mb14 | 1.3 × 107 | Hemiporphycene: Asymmetric heme |
| Mb15 | 1.1 × 109 | Porphycene: Rectangular heme with two propionate groups |
| Mb16 | 5.5 × 108 | Azaporphyrin: Regular heme with one meso-nitrogen |
4. Conclusions
Acknowledgments
References
- Kendrew, J.C.; Bodo, G.; Dintzis, H.M.; Parrish, R.G.; Wycoff, H.; Phillips, D.C. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature 1958, 181, 662–666. [Google Scholar]
- Tamura, M.; Asakura, T.; Yonetani, T. Heme modification studies of myoglobin I. Biochem. Biophys. Acta 1973, 295, 467–479. [Google Scholar] [CrossRef]
- Tamura, M.; George, V.W., III; Yonetani, T. Heme modification studies of myoglobin II. Biochem. Biophys. Acta 1973, 317, 34–79. [Google Scholar] [CrossRef]
- Hayashi, T.; Hisaeda, Y. New functionalization of myoglobin by chemical modification of heme-propionates. Acc. Chem. Res. 2002, 35, 35–43. [Google Scholar] [CrossRef]
- Monzani, E.; Alzuet, G.; Casella, L.; Redaelli, C.; Bassani, C.; Sanangelantoni, A.M.; Gulotti, M.; De Gioia, M.; Santagostini, L.; Chillemi, F. Properties of myoglobin reconstituted with chemically modified protohemin complexes. Biochemistry 2000, 39, 9571–9582. [Google Scholar] [CrossRef]
- Chang, C.K.; Ward, B.; Ebina, S. Kinetic study of CO and O2 binding to horse heart myoglobin reconstituted with synthetic hemes lacking methyl and vinyl side chains. Archiv. Biochem. Biophys. 1984, 231, 366–371. [Google Scholar] [CrossRef]
- Springer, B.A.; Sliger, S.G.; Olson, J.S.; Phillips, G.N., III. Mechanism of ligand recognition in myoglobin. Chem. Rev. 1994, 94, 699–714. [Google Scholar] [CrossRef]
- La Mar, G.; Budd, D.L.; Viscio, D.B.; Smith, K.M.; Langry, K.C. Proton nuclear magnetic resonance characterization of heme disorder in hemoproteins. Proc. Natl. Acad. Sci. USA 1978, 75, 5755–5759. [Google Scholar] [CrossRef]
- La Mar, G.; Davis, L.N.; Parish, D.W.; Smith, K.M. Heme orientational disorder in reconstituted and native sperm whale myoglobin. J. Mol. Biol. 1983, 168, 887–896. [Google Scholar] [CrossRef]
- Neya, S.; Funasaki, N. Proton NMR study of the cyanide metmyoglobin reconstituted with meso-tetraalkylhemins. J. Biol. Chem. 1987, 262, 6725–6728. [Google Scholar]
- Asakura, T. Hemoglobin porphyrin modification. Methods Enzymol. 1978, 52, 447–455. [Google Scholar] [CrossRef]
- Neya, S.; Funasaki, N.; Imai, K. Etiohemin as a prosthetic group of myoglobin. Biochim. Biophys. Acta 1989, 996, 226–232. [Google Scholar] [CrossRef]
- Neya, S.; Funasaki, N.; Imai, K. Structure and function of the myoglobin containing octaethylhemin as a prosthetic group. J. Biol. Chem. 1988, 263, 8810–8815. [Google Scholar]
- La Mar, G.N. Nuclear magnetic resonance of hemoproteins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 5, pp. 185–298. [Google Scholar]
- La Mar, G.N.; Hauksson, J.B.; Dugad, L.B.; Liddell, P.A.; Venkataramana, N.; Smith, K.M. Proton NMR study of the heme rotational mobility in myoglobin. J. Am. Chem. Soc. 1991, 113, 1554–1550. [Google Scholar]
- Neya, S.; Funasaki, N.; Nakamura, M. Dynamic analysis of efficient heme rotation in myoglobin by NMR spectroscopy. Biochim. Biophys. Acta 1991, 1117, 243–250. [Google Scholar]
- Juillard, S.; Bondon, A.; Simonneaux, G. Proton NMR study of myoglobin reconstituted with 3,7-diethyl-2,8-dimethyl iron porphyrin. J. Inorg. Biochem. 2006, 100, 1441–1448. [Google Scholar] [CrossRef]
- Juillard, S.; Chevance, S.; Bondon, A.; Simonneaux, G. Dynamics of heme in hemoproteins: Proton NMR study of myoglobin reconstituted with iron 3-ethyl-2-methylporphyrin. Biochim. Biophys. Acta 2011, 1814, 1188–1194. [Google Scholar]
- Takano, T. Structure of myoglobin refined at 2.0 Å resolution. J. Mol. Biol. 1977, 110, 537–568. [Google Scholar] [CrossRef]
- Neya, S.; Funasaki, N.; Sato, T.; Igarashi, N.; Tanaka, N. Structural analysis of the myoglobin reconstituted with iron porphine. J. Biol. Chem. 1995, 268, 8935–8942. [Google Scholar]
- Sato, T.; Tanaka, N.; Moriyama, H.; Matsumoto, O.; Takenaka, A.; Neya, S.; Funasaki, N. The crystal structure of cyanide metmyoglobins reconstituted with iron(III) complexes of porphyrin, 5,10,15,20-tetramethylporphyrin and 5,10,15,20-tetraethylporphyrin. Bull. Chem. Soc. Jpn. 1992, 65, 739–745. [Google Scholar] [CrossRef]
- Hata, T.; Hata, Y.; Sato, T.; Tanaka, N.; Neya, S.; Funasaki, N.; Katsube, Y. The 2.0 Å crystal structure of cyanide metmyoglobin reconstituted with 5,10,15,20-tetrapropylhemin. Bull. Chem. Soc. Jpn. 1991, 64, 821–828. [Google Scholar] [CrossRef]
- Breslow, E.; Koehler, R.; Girotti, A.W. Properties of protoporphyrin-apomyoglobin complexes and related compounds. J. Biol. Chem. 1967, 242, 4149–4156. [Google Scholar]
- Neya, S.; Funasaki; Shiro, Y.; Iizuka, T.; Imai, K. Consequence of rapid heme rotation to the oxygen binding to myoglobin. Biochim. Biophys. Acta 1994, 1208, 31–37. [Google Scholar]
- Tran, A.-T.T.; Kalish, H.; Balch, A.L.; La Mar, G.N. Solution 1H-NMR investigation of the seating and rotational “hopping” of centrosymmetric etioheme-I in myoglobin. J. Biol. Inorg. Chem. 2000, 5, 624–633. [Google Scholar] [CrossRef]
- Jongeward, K.A.; Madge, D.S.; Taube, D.L.; Masters, J.C.; Traylor, T.G.; Sharma, V.S. Picosecond and nanosecond geminate recombination of myoglobin with carbon monoxide, oxygen, nitric oxide and isocyanides. J. Am. Chem. Soc. 1988, 110, 380–387. [Google Scholar] [CrossRef]
- Kottalam, J.; Case, D.A. Dynamics of ligand escape from the heme pocket of myoglobin. J. Am. Chem. Soc. 1988, 110, 7690–7697. [Google Scholar] [CrossRef]
- Livingstone, D.J.; Davis, N.L.; Parish, D.W.; La Mar, G.N.; Brown, W.D. Influence of heme orientation on oxygen affinity in native sperm whale myoglobin. J. Am. Chem. Soc. 1984, 106, 3025–3028. [Google Scholar] [CrossRef]
- Light, W.R.; Rohlfs, R.J.; Palmer, G.; Olson, J.S. Functional effects of heme orientational disorder in sperm whale myoglobin. J. Biol. Chem. 1987, 262, 46–52. [Google Scholar]
- Traylor, T.G. Hemoprotein oxygen transport: Models and mechanisms. In Bioinorganic Chemistry; van Tamelen, E.E., Ed.; Academic Press: New York, NY, USA, 1978; Volume 4, pp. 437–468. [Google Scholar]
- Smerdon, S.J.; Krzywda, S.; Wilkinson, A.J.; Brantley, R.E.; Carver, T.E.; Hargrove, M.S.; Olson, J.S. Serine92 (F7) contributes to the control of heme reactivity and stability in myoglobin. Biochemistry 1993, 32, 5132–5139. [Google Scholar] [CrossRef]
- Vogel, E.; Köchler, M.; Schmickler, H.; Lex, J. Porphycene, a novel porphyrin isomer. Angew. Chem. Int. Ed. Engl. 1986, 25, 257–259. [Google Scholar] [CrossRef]
- Sessler, J.L.; Gebauer, A.; Vogel, E. Porphyrin isomers. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 2, pp. 1–54. [Google Scholar]
- Neya, S.; Nakamura, M.; Imai, K.; Funasaki, N. Functional analysis of the iron(II) etiocorrphycene incorporated in the myoglobin heme pocket. Chem. Pharm. Bull. 2001, 49, 435–436. [Google Scholar]
- Neya, S.; Funasaki, N.; Hori, H.; Imai, K. Functional regulation of myoglobin by iron corrphycene. Chem. Lett. 1999, 28, 989–990. [Google Scholar]
- Neya, S.; Funasaki, N.; Igarashi, N.; Ikezaki, A.; Sato, T.; Imai, K.; Tanaka, N. Structure and function of 6,7-dicarboxyheme-substituted myoglobin. Biochemistry 1998, 37, 5489–5493. [Google Scholar]
- Neya, S.; Imai, K.; Hiramatsu, Y.; Kitagawa, T.; Hohino, T.; Hata, M.; Funasaki, N. Significance of the molecular shape of irrophycene in a protein pocket. Inorg. Chem. 2006, 45, 4238–4242. [Google Scholar]
- Neya, S.; Suzuki, M.; Ode, H.; Hoshino, T.; Furutani, Y.; Kandori, H.; Hori, H.; Imai, K.; Komatsu, T. Functional evaluation of iron oxypyriporphyrin in protein heme pocket. Inorg. Chem. 2008, 47, 10771–10778. [Google Scholar] [CrossRef]
- Neya, S.; Suzuki, M.; Hoshino, T.; Ode, H.; Imak, K.; Komatsu, T.; Ikezaki, A.; Nakamura, M.; Furutani, Y.; Kandori, H. Molecular insight into intrinsic heme distortion in ligand binding in hemoprotein. Biochemistry 2010, 49, 5642–5650. [Google Scholar] [CrossRef]
- Neya, S.; Imai, K.; Hori, H.; Ishikawa, H.; Ishimori, K.; Okuno, D.; Nagatomo, S.; Hoshino, T.; Hata, M.; Funasaki, N. Iron hemiporphycene as a functional prosthetic group for myoglobin. Inorg. Chem. 2003, 42, 1456–1461. [Google Scholar] [CrossRef]
- Hayashi, T.; Dejima, H.; Sato, H.; Murata, D.; Hisaeda, Y. Blue myoglobin with an iron porphycene shows extremely high oxygen affinity. J. Am. Chem. Soc. 2002, 124, 11226–11227. [Google Scholar] [CrossRef]
- Neya, S.; Kaku, T.; Funasaki, N.; Shiro, Y.; Iizuka, T.; Imai, K.; Hori, H. Novel ligand binding properties of the myoglobin substituted with monoazahemin. J. Biol. Chem. 1995, 270, 13118–13123. [Google Scholar]
- Ohgo, Y.; Neya, S.; Takahashi, M.; Takeda, M.; Funasaki, N.; Nakamura, M. Iodo(etiohemiporphycenato)iron(IIII). Unexpected difference in magnetic behavior in solution and solid. Chem. Lett. 2003, 32, 526–527. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Neya, S. Dynamic Motion and Rearranged Molecular Shape of Heme in Myoglobin: Structural and Functional Consequences. Molecules 2013, 18, 3168-3182. https://doi.org/10.3390/molecules18033168
Neya S. Dynamic Motion and Rearranged Molecular Shape of Heme in Myoglobin: Structural and Functional Consequences. Molecules. 2013; 18(3):3168-3182. https://doi.org/10.3390/molecules18033168
Chicago/Turabian StyleNeya, Saburo. 2013. "Dynamic Motion and Rearranged Molecular Shape of Heme in Myoglobin: Structural and Functional Consequences" Molecules 18, no. 3: 3168-3182. https://doi.org/10.3390/molecules18033168




