A New 5α,8α-Epidioxysterol from the Soft Coral Sinularia gaweli
Abstract
:1. Introduction
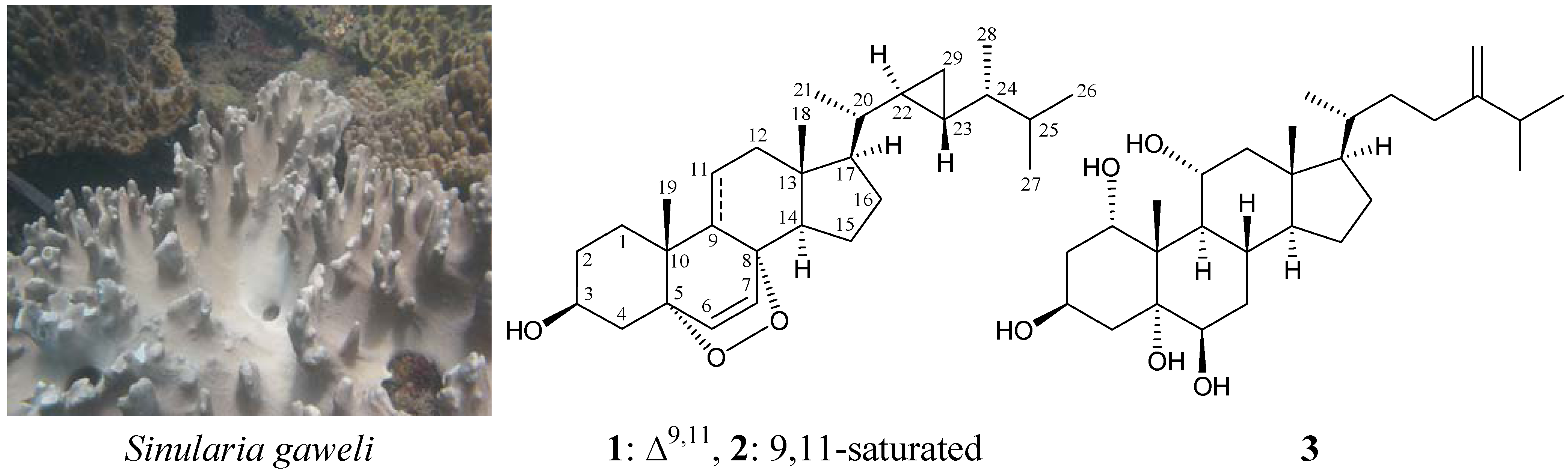
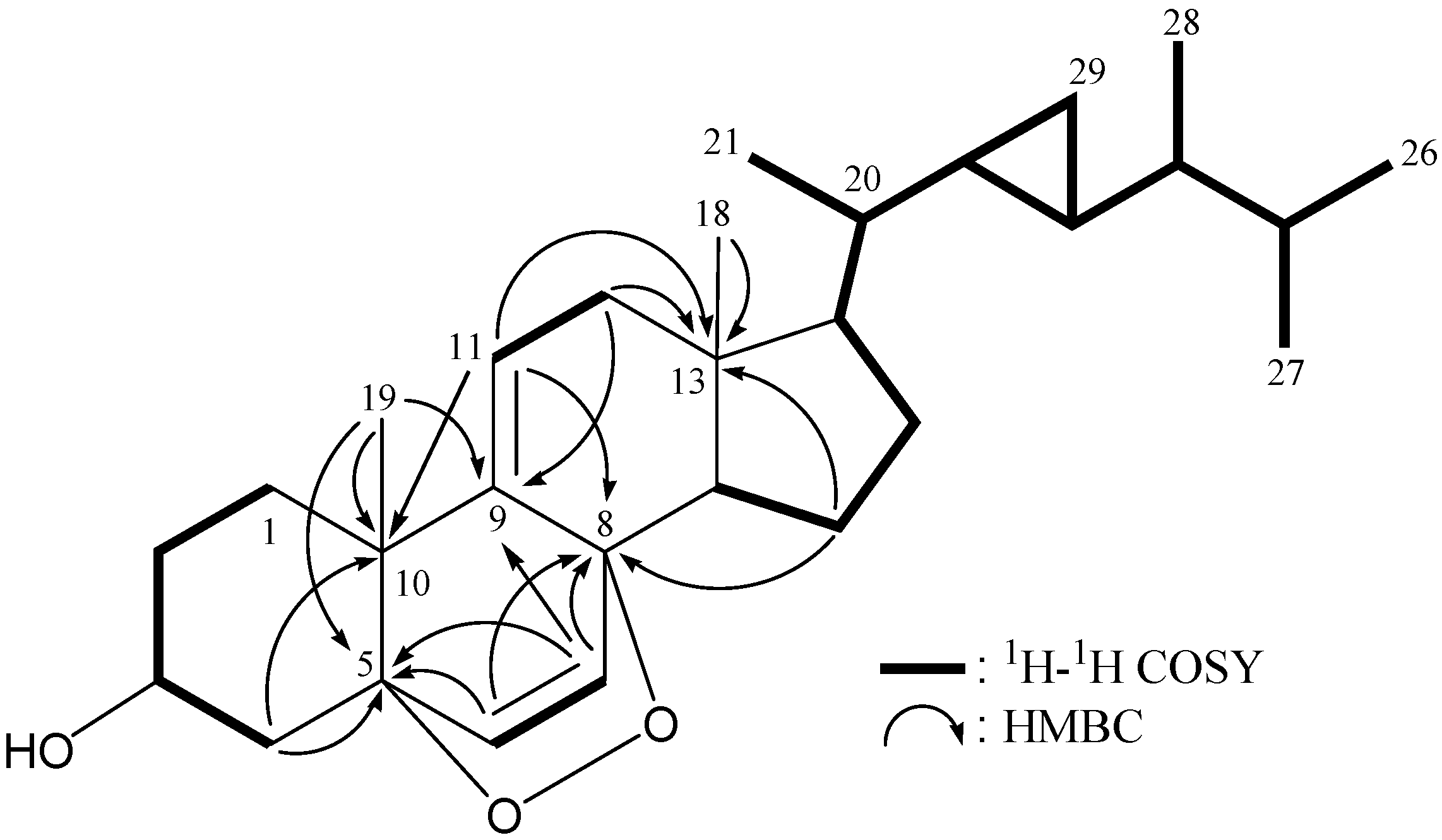
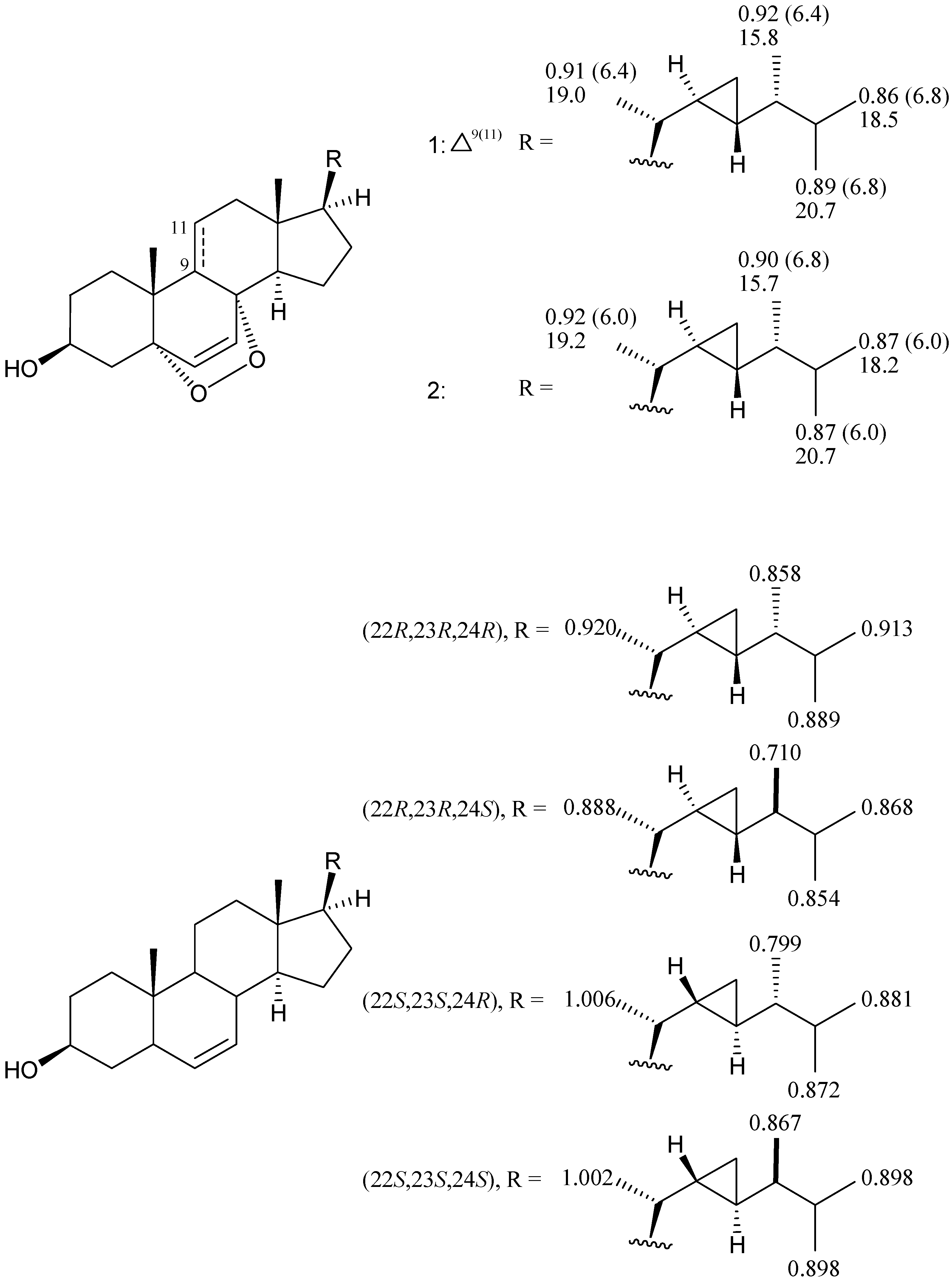
2. Results and Discussion
| Position | δH (J in Hz) | δC, Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 2.11 m; 1.70 m | 32.6, CH2 | H2-2 | n.o. |
| 2 | 1.91 m; 1.55 m | 30.6, CH2 | H2-1, H-3 | C-3 |
| 3 | 4.02 m | 66.3, CH | H2-2, H2-4 | n.o. |
| 4 | 2.14 dd (13.6, 2.0); 1.92 dd (13.6, 11.6) | 36.1, CH2 | H-3 | C-2, -3, -5, -10 |
| 5 | 82.7, C | |||
| 6 | 6.60 d (8.0) | 130.8, CH | H-7 | C-4, -5, -8 |
| 7 | 6.28 d (8.0) | 135.4, CH | H-6 | C-5, -8, -9, -14 |
| 8 | 78.4, C | |||
| 9 | 142.5, C | |||
| 10 | 37.9, C | |||
| 11 | 5.42 dd (6.0, 2.0) | 119.8, CH | H2-12 | C-8, -10, -12, -13 |
| 12 | 2.28 dd (16.8, 6.0); 2.09 dd (16.8, 2.0) | 41.2, CH2 | H-11 | C-9, -11, -13, -14, -17 |
| 13 | 44.1, C | |||
| 14 | 1.83 dd (12.0, 8.0) | 47.8, CH | H2-15 | C-12, -15 |
| 15 | 1.75 m; 1.61 m | 21.2, CH2 | H-14, H2-16 | C-8, -13, -16 |
| 16 | 2.20 m | 28.4, CH2 | H2-15, H-17 | n.o. |
| 17 | 1.49 m | 57.4, CH | H2-16, H-20 | n.o. |
| 18 | 0.68 s | 12.6, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.09 s | 25.5, CH3 | C-1, -5, -9, -10 | |
| 20 | 0.88 m | 39.7, CH | H-17, H3-21, H-22 | C-17 |
| 21 | 0.91 d (6.4) | 19.0, CH3 | H-20 | C-20, -22 |
| 22 | 0.56 m | 24.2, CH | H-20, H-23, H2-29 | n.o. |
| 23 | 0.33 m | 25.1, CH | H-22, H-24, H2-29 | n.o. |
| 24 | 0.55 m | 44.9, CH | H-23, H-25, H3-28 | n.o. |
| 25 | 1.64 m | 32.8, CH | H-24, H3-26, H3-27 | C-24 |
| 26 | 0.86 d (6.8) | 18.5, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.89 d (6.8) | 20.7, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.92 d (6.4) | 15.8, CH3 | H-24 | C-24, -25 |
| 29 | 0.14 m | 10.5, CH2 | H-22, H-23 | C-20, -22, -24 |
| Compounds | Cell lines IC50 (μg/mL) | ||
|---|---|---|---|
| K562 | MOLT-4 | HL-60 | |
| 1 | NA | 15.70 | NA |
| 2 | NA | NA | 12.14 |
| 3 | 9.71 | 6.91 | 3.39 |
| Doxorubicin a | 0.20 | 0.01 | 0.03 |
3. Experimental
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
 +158 (c 0.03, CHCl3); m.p. 218−220 °C; IR (neat) υmax 3445, 1644 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS m/z 463 [M+Na]+; HRESIMS: m/z 463.3192 (calcd for C29H44O3Na, 463.3188).
+158 (c 0.03, CHCl3); m.p. 218−220 °C; IR (neat) υmax 3445, 1644 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS m/z 463 [M+Na]+; HRESIMS: m/z 463.3192 (calcd for C29H44O3Na, 463.3188). +20 (c 0.02, CHCl3) (Ref. [4],
+20 (c 0.02, CHCl3) (Ref. [4],  +35 (c 0.1, CHCl3)); IR (neat) υmax 3438, 1638 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data were found to be in full agreement with those reported previously [4,18]; ESIMS m/z 465 [M+Na]+; HRESIMS: m/z 465.3347 (calcd for C29H46O3Na, 465.3344).
+35 (c 0.1, CHCl3)); IR (neat) υmax 3438, 1638 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data were found to be in full agreement with those reported previously [4,18]; ESIMS m/z 465 [M+Na]+; HRESIMS: m/z 465.3347 (calcd for C29H46O3Na, 465.3344). −3 (c 0.06, CHCl3) (Ref. [15],
−3 (c 0.06, CHCl3) (Ref. [15],  −4 (c 1.60, CHCl3)); IR (neat) υmax 3380, 1216 cm−1; 1H (400 MHz, C5D5N) and 13C (100 MHz, C5D5N) NMR data were found to be in full agreement with those reported previously [15]; ESIMS: m/z 487 [M+Na]+; HRESIMS: m/z 487.3402 (calcd for C28H48O5Na, 487.3399).
−4 (c 1.60, CHCl3)); IR (neat) υmax 3380, 1216 cm−1; 1H (400 MHz, C5D5N) and 13C (100 MHz, C5D5N) NMR data were found to be in full agreement with those reported previously [15]; ESIMS: m/z 487 [M+Na]+; HRESIMS: m/z 487.3402 (calcd for C28H48O5Na, 487.3399).3.4. Cytotoxicity Testing
4. Conclusions
Acknowledgments
References and Notes
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar]
- Sheu, J.-H.; Chang, K.-C.; Duh, C.-Y. A cytotoxic 5α,8α-epidioxysterol from a soft coral Sinularia species. J. Nat. Prod. 2000, 63, 149–151. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. 1α,3β,5β-Trihydroxy-24-methylenecholestan-6-one: a novel steroid from a soft coral Sinularia gibberosa. Steroids 2003, 68, 377–381. [Google Scholar] [CrossRef]
- Su, J.-H.; Tseng, Y.-J.; Huang, H.-H.; Ahmed, A.F.; Lu, C.-K.; Wu, Y.-C.; Sheu, J.-H. 9,11-Secosterols from the soft corals Sinularia lochmodes and Sinularia leptoclados. J. Nat. Prod. 2006, 69, 850–852. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Hsieh, Y.-T.; Wen, Z.-H.; Wu, Y.-C.; Sheu, J.-H. Polyoxygenated sterols from the Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2006, 69, 1275–1279. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Tai, S.-H.; Wu, Y.-C.; Sheu, J.-H. Sinugrandisterols A–D, trihydroxysteroids from the soft coral Sinularia grandilobata. Steroids 2007, 72, 368–374. [Google Scholar] [CrossRef]
- Chen, B.-W.; Su, J.-H.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Polyoxygenated steroids from a Formosan soft coral Sinularia facile. Bull. Chem. Soc. Jpn. 2008, 81, 1304–1307. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chen, H.-P.; Wang, S.-K.; Duh, C.-Y. Three new 9,11-secosterols from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2011, 84, 648–652. [Google Scholar] [CrossRef]
- Chao, C.-H.; Chou, K.-J.; Huang, C.-Y.; Wen, Z.-H.; Hsu, C.-H.; Wu, Y.-C.; Dai, C.-F.; Sheu, J.-H. Steroids from the soft coral Sinularia crassa. Mar. Drugs 2012, 10, 439–450. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Su, J.-H.; Duh, C.-Y.; Chen, B.-W.; Wen, Z.-H.; Kuo, Y.-H.; Sheu, J.-H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012, 22, 4373–4376. [Google Scholar]
- Su, J.-H.; Lo, C.-L.; Wen, Z.-H.; Huang, C.-Y.; Dai, C.-F.; Sheu, J.-H. Anti-inflammatory polyoxygenated steroids from the soft coral Sinularia sp. Bull. Chem. Soc. Jpn. 2008, 81, 1616–1620. [Google Scholar] [CrossRef]
- Yen, W.-H.; Hu, L.-C.; Su, J.-H.; Lu, M.-C.; Twan, W.-H.; Yang, S.-Y.; Kuo, Y.-C.; Weng, C.-F.; Lee, C.-H.; Kuo, Y.-H.; et al. Norcembranoidal diterpenes from a Formosan soft coral Sinularia sp. Molecules 2012, 17, 14058–14066. [Google Scholar] [CrossRef]
- Kobayashi, M.; Appa Rao, K.M.C.; Krishna, M.M.; Anjaneyulu, V. Marine sterols. Part 30. Isolation of 24-methylenecholestane-1α,3β,5α,6β,11α-pentol and its 11-monoacetate from the Andaman Sea soft coral Sinularia dissecta. J. Chem. Res. (S) 1994, 180–181. [Google Scholar]
- Anjaneyulu, A.S.R.; Venugopal, M.J.R.V.; Sarada, P. On the novel cembranoids of the soft coral Sinularia granosa of the Indian Ocean and their biogenesis. Indian J. Chem. 2000, 39B, 530–535. [Google Scholar]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 26. Isolation and structure elucidation of nine new 5α,8α-epidioxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- The 1H and 13C-NMR data of (22R,23R,24R)-5α,8α-epidioxy-22,23-methylene-24-methylcholest-6-en-3β-ol (2) were reassigned in a later study. Please see Lin, Y.-C. Isolation and biological activities of secondary metabolites from the soft coral Lobophytum sarcophytoides. Master Thesis, Department of Marine Biotechnology and Resources, National Sun Yat-sen Univeristy, Kaohsiung, Taiwan, July 2009.
- Blanc, P.-A.; Djerassi, C. Isolation and structure elucidation of 22(S),23(S)-methylenecholesterol. Evidence for direct bioalkylation of 22-dehydrocholesterol. J. Am. Chem. Soc. 1980, 102, 7113–7114. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.-C.; Wen, Z.-H.; Su, J.-H.; Sung, P.-J.; Hsu, C.-H.; Kuo, Y.-H.; Chiang, M.Y.; Dai, C.-F.; Sheu, J.-H. Steroid and cembranoids from the Dongsha atoll soft coral Lobophytum sarcophytoides. Tetrahedron 2010, 66, 7129–7135. [Google Scholar] [CrossRef]
- Aknin, M.; Gros, E.; Vacelet, J.; Kashman, Y.; Gauvin-Bialecki, A. Sterols from the Madagascar sponge Fascaplysinopsis sp. Mar. Drugs 2010, 8, 2961–2975. [Google Scholar] [CrossRef]
- Arreguín-Espinosa, R.; Arreguín, B.; Hernández-Santoyo, A.; Rodriguez-Romero, A. Sterol composition and biosynthesis in the sponge Spheciospongia vesparia. J. Chem. Technol. Biotechnol. 1998, 72, 245–248. [Google Scholar] [CrossRef]
- Albro, P.W.; Bilski, P.; Corbett, J.T.; Schroeder, J.L.; Chignell, C.F. Photochemical reactions and phototoxicity of sterols: Novel self-perpetuating mechanism for lipid photooxidation. Photochem. Photobiol. 1997, 66, 316–325. [Google Scholar] [CrossRef]
- Verseveldt, J. Alcyonaceans (Coelenterata: Octocorallia) from some Micronesian Islands. Zool. Meded. Leiden 1978, 53, 49–55. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Sample Availability: Samples of the sterols 1–3 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yen, W.-H.; Chen, W.-F.; Cheng, C.-H.; Dai, C.-F.; Lu, M.-C.; Su, J.-H.; Su, Y.-D.; Chen, Y.-H.; Chang, Y.-C.; Chen, Y.-H.; et al. A New 5α,8α-Epidioxysterol from the Soft Coral Sinularia gaweli. Molecules 2013, 18, 2895-2903. https://doi.org/10.3390/molecules18032895
Yen W-H, Chen W-F, Cheng C-H, Dai C-F, Lu M-C, Su J-H, Su Y-D, Chen Y-H, Chang Y-C, Chen Y-H, et al. A New 5α,8α-Epidioxysterol from the Soft Coral Sinularia gaweli. Molecules. 2013; 18(3):2895-2903. https://doi.org/10.3390/molecules18032895
Chicago/Turabian StyleYen, Wei-Hsuan, Wu-Fu Chen, Ching-Hsiao Cheng, Chang-Feng Dai, Mei-Chin Lu, Jui-Hsin Su, Yin-Di Su, Yu-Hsin Chen, Yu-Chia Chang, Yung-Husan Chen, and et al. 2013. "A New 5α,8α-Epidioxysterol from the Soft Coral Sinularia gaweli" Molecules 18, no. 3: 2895-2903. https://doi.org/10.3390/molecules18032895





