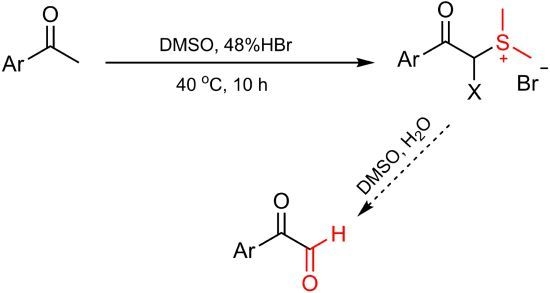
Synthesis of Dimethyl Aryl Acylsulfonium Bromides from Aryl Methyl Ketones in a DMSO-HBr System
Abstract
:1. Introduction
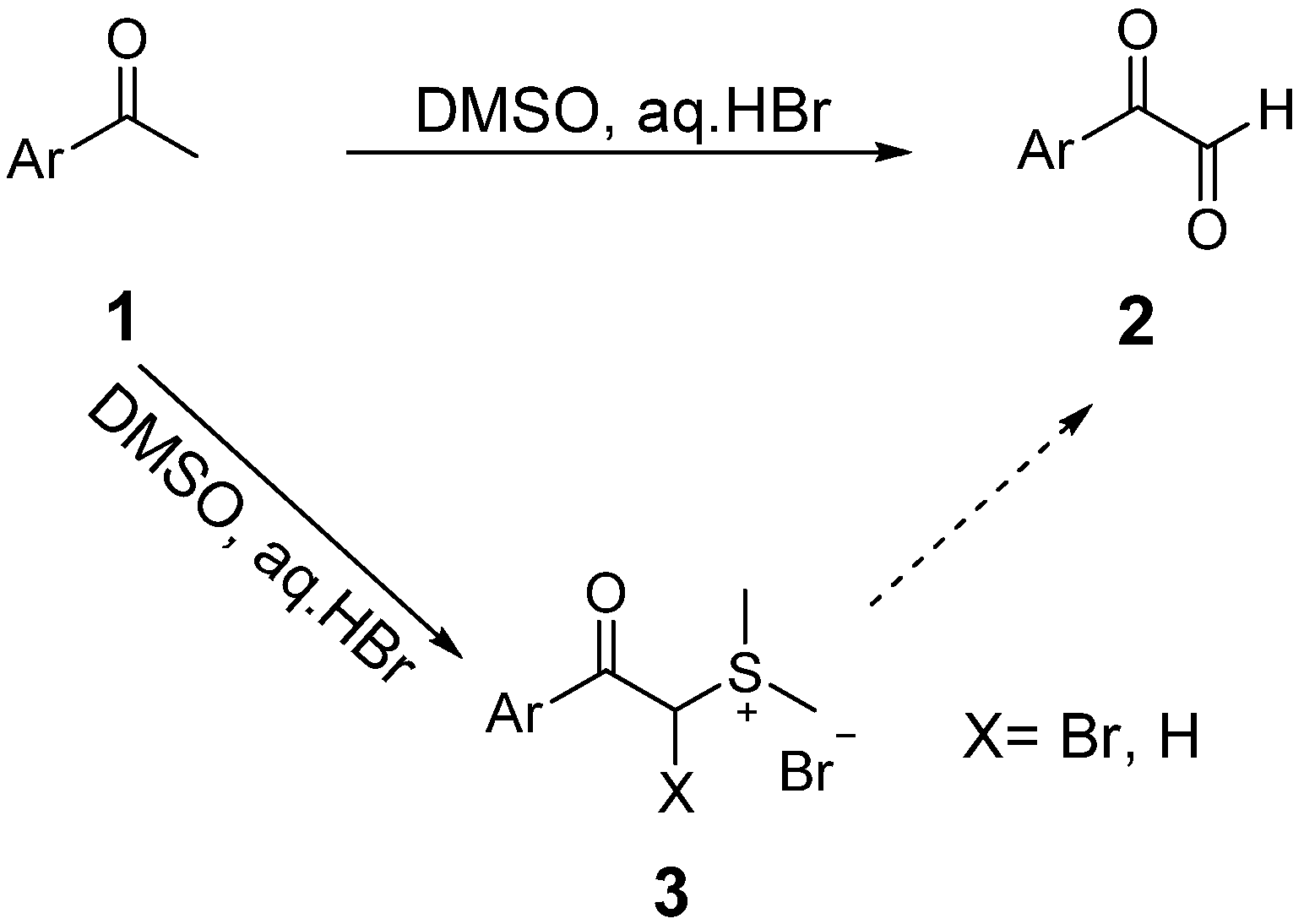
2. Results and Discussion
| Entry | Aryl Acetone | Product | Yield (%) |
|---|---|---|---|
| a |  |  | 69 |
| b |  |  | 57 |
| c |  |  | 71 |
| d |  |  | 62 |
| e |  |  | 75 |
| f |  |  | 55 |
| g |  |  | 57 |
| h |  |  | 48 |
| Entry | Molar Ratio Methyl Ketone/HBr | Time (h) | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|
| 1 | 1:3 | 6 | 40 | 41 |
| 2 | 1:3 | 10 | 40 | 69 |
| 3 | 1:3 | 12 | 40 | 56 |
| 4 | 1:3 | 10 | 55 | 45 |
| 5 | 1:1 | 12 | 40 | 12 |
| 6 | 1:5 | 6 | 40 | 61 |
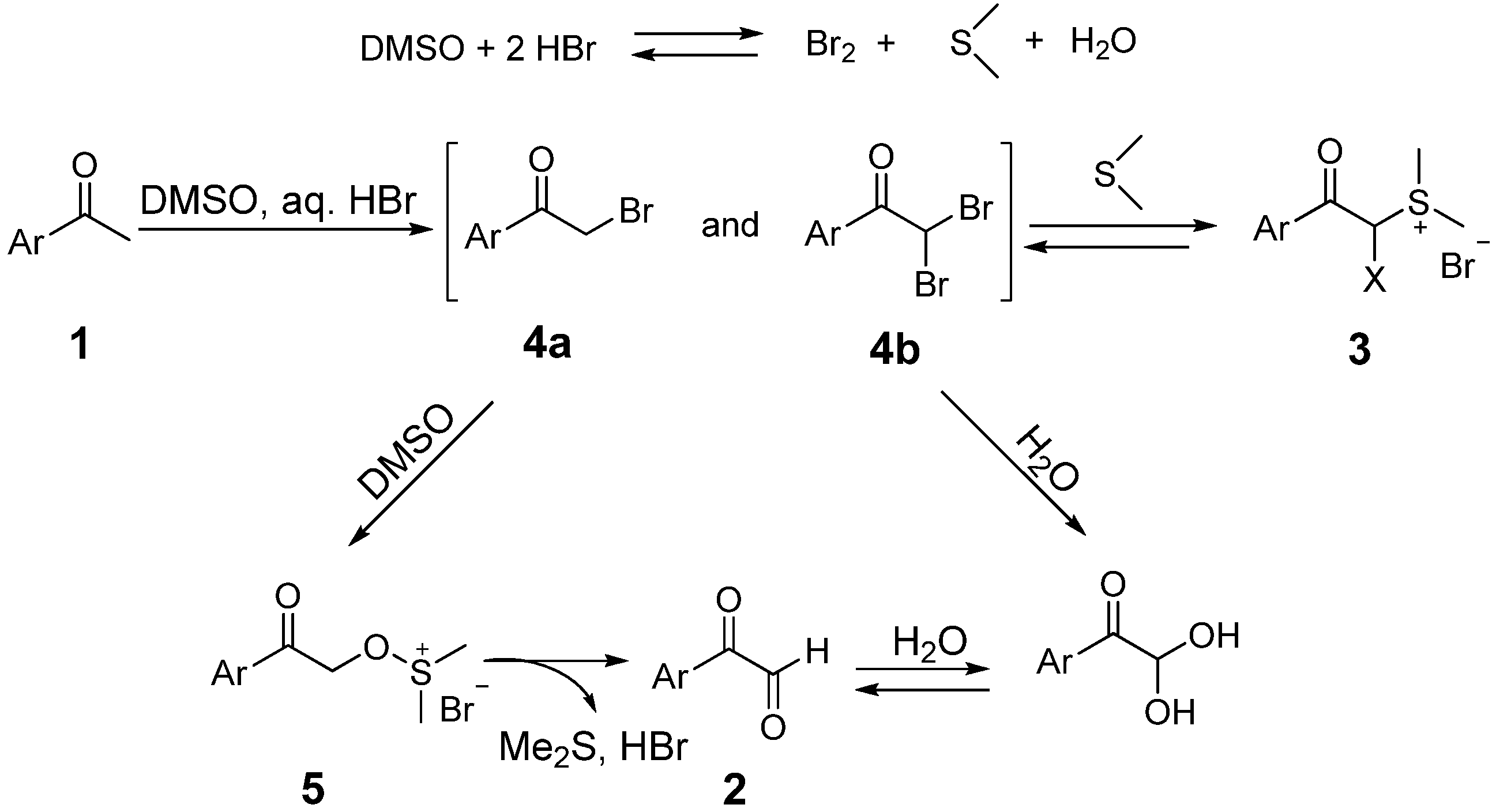
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Sulfonium Salts 3a–h
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kornblum, N.; Powers, J.W.; Anderson, G.J.; Jones, W.J.; Larson, H.O.; Levand, O.; Weaver, W.M. A new and selective method of oxidation. J. Am. Chem. Soc. 1957, 79, 6562. [Google Scholar]
- Schipper, E.; Cinnamon, M.; Rascher, L.; Chiang, Y.H.; Oroshnik, W. Oxidation of active methylenes by dimethyl sulfoxide: A new ninhydrin synthesis. Tetrahedron Lett. 1968, 9, 62016–62204. [Google Scholar]
- Floyd, M.B.; Du, M.T.; Fabio, P.F.; Jacob, L.A.; Johnson, B.D. The oxidation of acetophenones to arylglyoxals with aqueous hydrobromic acid in dimethyl sulfoxide. J. Org. Chem. 1985, 50, 5022–5027. [Google Scholar] [CrossRef]
- Zuliani, V.; Cocconcelli, G.; Fantini, M.; Ghiron, C.; Rivara, M. A practical synthesis of 2,4(5)-diarylimidazoles from simple building blocks. J. Org. Chem. 2007, 72, 4551–4553. [Google Scholar] [CrossRef]
- Juspin, T.; Terme, T.; Vanelle, P. TDAE strategy using α-diketones: Rapid access to 2,3-diphenylquinoline and acenaphtho [1,2-b] quinoline derivatives. Synlett 2009, 2009, 1485–1489. [Google Scholar] [CrossRef]
- Fischer, B.; Kabha, E.; Gendron, F.-P.; Beaudoin, A.R. Synthesis, mechanism and fluorescence Properties of 8-(aryl)-3-β-d-ribofuranosylimidazo [2,1-i] purine 5′-phosphate derivatives. Nucleos. Nucleoti. Nucleic Acids 2000, 19, 1033–1054. [Google Scholar] [CrossRef]
- Prashantha Kumar, B.; Sharma, G.K.; Srinath, S.; Noor, M.; Suresh, B.; Srinivasa, B. Microwave-assisted, solvent-free, parallel syntheses and elucidation of reaction mechanism for the formation of some novel tetraaryl imidazoles of biological interest. J. Heterocycl. Chem. 2009, 46, 278–284. [Google Scholar] [CrossRef]
- Bauer, D.P.; Macomber, R.S. Iodide catalysis of oxidations with dimethyl sulfoxide. Convenient two-step synthesis of. alpha. diketones from. alpha.-methylene ketones. J. Org. Chem. 1975, 40, 1990–1992. [Google Scholar] [CrossRef]
- Schaefer, J.P. Selenium Dioxide Oxidations. II. Base catalyzed oxidation of ketones. J. Am. Chem. Soc. 1962, 84, 717–718. [Google Scholar] [CrossRef]
- Wan, Z.; Jones, C.D.; Mitchell, D.; Pu, J.Y.; Zhang, T.Y. Practical method for transforming alkynes into α-diketones. J. Org. Chem. 2006, 71, 826–828. [Google Scholar] [CrossRef]
- Yusubov, M.S.; Filimonov, V.D.; Vasilyeva, V.P.; Chi, K.W. Chemoselective oxidation of carbon-carbon double or triple bonds to 1, 2-diketones with DMSO-based reagents. Synthesis 1995, 1995, 1234–1236. [Google Scholar] [CrossRef]
- Chuvashov, D.; Vakulin, I.; Talipov, R.; Galin, F. Study of mechanisms of sulfonium keto ylides intramolecular cyclization. J. Mol. Struct. THEOCHEM 2007, 807, 55–60. [Google Scholar] [CrossRef]
- Crivello, J.V.; Kong, S. Synthesis and characterization of second-generation dialkylphenacylsulfonium salt photoinitiators. Macromolecules 2000, 33, 825–832. [Google Scholar] [CrossRef]
- Choudhury, L.H.; Parvin, T.; Khan, A.T. Recent advances in the application of bromodimethylsulfonium bromide (BDMS) in organic synthesis. Tetrahedron 2009, 65, 9513–9526. [Google Scholar] [CrossRef]
- Chittimalla, S.K.; Bandi, C. Rapid entry to functionally rich bicyclo [4.1. 0] heptenone systems. RSC Adv. 2013, 3, 13663–13667. [Google Scholar] [CrossRef]
- Kunz, R.K.; David, W.C. Enantioselective organocatalytic cyclopropanations. The identification of a new class of iminium catalyst based upon directed electrostatic activation. J. Am. Chem. Soc. 2005, 127, 3240–3241. [Google Scholar] [CrossRef]
- Shao, Q.; Li, C. Efficient condensation between glyoxal hydrates and sulfonium salts leading to highly functionalized 1,4-diketones. Synlett 2008, 2008, 2317–2320. [Google Scholar] [CrossRef]
- Baranac-Stojanović, M.; Marković, R.; Stojanović, M. Catalytic oxidations of enolizable ketones using 2-alkylidene-4-oxothiazolidine vinyl bromide. Tetrahedron 2011, 67, 8000–8008. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3e–h are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cao, Z.; Shi, D.; Qu, Y.; Tao, C.; Liu, W.; Yao, G. Synthesis of Dimethyl Aryl Acylsulfonium Bromides from Aryl Methyl Ketones in a DMSO-HBr System. Molecules 2013, 18, 15717-15723. https://doi.org/10.3390/molecules181215717
Cao Z, Shi D, Qu Y, Tao C, Liu W, Yao G. Synthesis of Dimethyl Aryl Acylsulfonium Bromides from Aryl Methyl Ketones in a DMSO-HBr System. Molecules. 2013; 18(12):15717-15723. https://doi.org/10.3390/molecules181215717
Chicago/Turabian StyleCao, Zhiling, Dahua Shi, Yingying Qu, Chuanzhou Tao, Weiwei Liu, and Guowei Yao. 2013. "Synthesis of Dimethyl Aryl Acylsulfonium Bromides from Aryl Methyl Ketones in a DMSO-HBr System" Molecules 18, no. 12: 15717-15723. https://doi.org/10.3390/molecules181215717