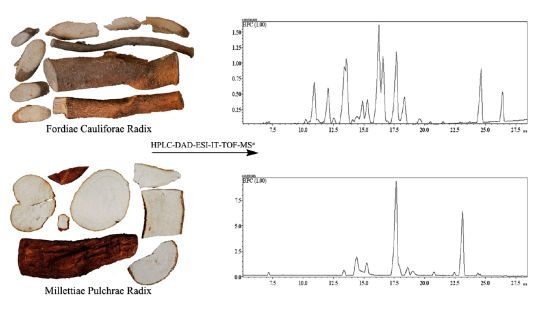
Characterization of Flavonoids in the Ethomedicine Fordiae Cauliflorae Radix and Its Adulterant Millettiae Pulchrae Radix by HPLC-DAD-ESI-IT-TOF-MSn
Abstract
:1. Introduction

2. Results and Discussion
2.1. Optimization of HPLC Conditions
2.2. Optimization of Mass Spectrometry Conditions

2.3. Rationale for the Characterization of Flavonoids
2.3.1. Identification of Furonoflavonoids

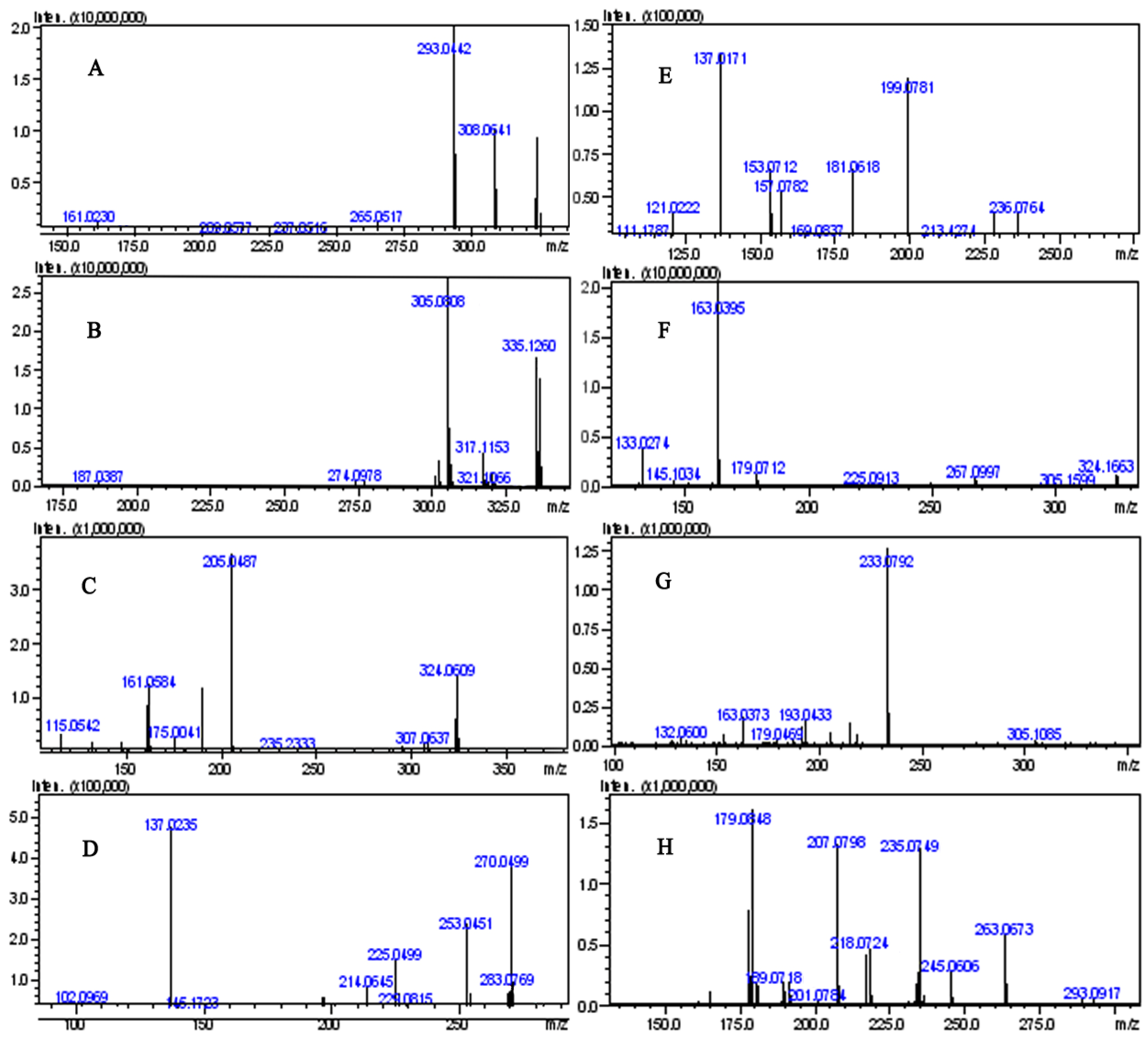
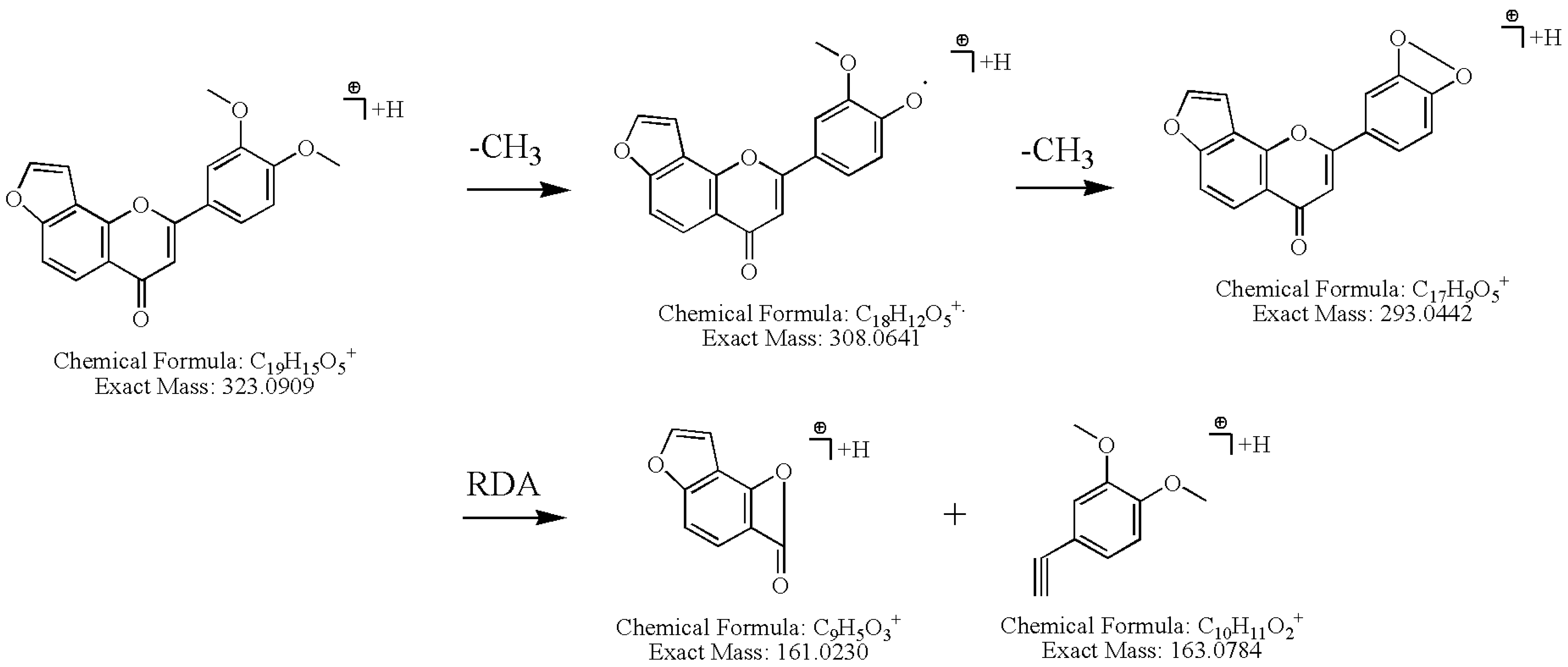
2.3.2. Identification of Pyranoflavonoids
2.3.3. Identification of Chalcones
2.3.4. Identification of Pterocarpin, Isoflavone, Flavones, Flavonones and Rotenoids
2.3.5. Chemical Characteristics of FC and MP
2.3.6. Identification of FC and MP
| No. | t R (min) | Formula | PI meas. (Da) | PI pred. (Da) | Error (ppm) | Major Fragments Ions (PI) | Identification | Peak Area in FC | Peak Area in MP |
|---|---|---|---|---|---|---|---|---|---|
| 1 b | 6.63 | C16H12O5 | 285.0755 | 285.0685 | 0.9 | 270.0499,253.0451, 225.0499, 214.0645, 137.0235 | (−)-Maackiain [24] | 303223 | 186752 |
| 2 b | 6.88 | C18H16O6 | 329.1038 | 329.1020 | 5.5 | 314.0747, 299.0560, 191.0764, 167.0669, 147.0428 | (6S,6aS,11aR)-6α-Methoxy-pterocarpin [24] | - | 691251 |
| 3 a | 7.46 | C15H10O4 | 255.0642 | 255.0652 | −1 | 236.0764, 199.0781, 181.0618, 153.0712, 137.0171, 121.0222 | 7,4'-Dihydroxyisoflavone [21] | 1124746 | 148674 |
| 4 | 8.64 | C18H12O5 | 309.0756 | 309.0758 | −0.2 | 294.0495, 238.0635, 192.0049,176.0118, 164.0106 | Pongapinnol D [34] | 760606 | - |
| 5 | 8.72 | C19H14O6 | 339.0844 | 339.0863 | −1.9 | 324.0552, 323.0544, 321.0394, 295.0583, 293.0442, 278.0568,181.0639 | Milletenin B [25] | 1482047 | - |
| 6 | 9.27 | C17H14O4 | 283.0948 | 283.0956 | −1.7 | 267.0627, 239.0704, 137.0277 | 5-Hydroxy-7-methoxy-6-methylflavone [26] | 630180 | - |
| 7 b | 9.312 | C17H14O5 | 299.0998 | 299.0914 | −3.0 | 284.0681, 257.8259, 174.0634 | Pterocarpin [24] | 1885294 | - |
| 8 a | 10.26 | C18H12O5 | 309.0749 | 309.0758 | −0.9 | 294.0496, 266.0575, 210.0791, 161.0244 | Pongapinnol C [18] | 7681020 | - |
| 9 | 10.59 | C19H14O6 | 339.0848 | 339.0863 | −1.5 | 324.0619, 309.0375, 281.0413, 279.0628, 179.0845, 161.0245 | 3'-Hydroxy,4',5'-dimethoxy furano[2'',3'':7,6]flavone [35] | 5349406 | - |
| 10 a | 10.98 | C18H12O4 | 293.0801 | 293.0808 | −0.7 | 278.0560, 250.0590, 222.0635, 194.0715, 176.0103, 148.0139 | O-Methylpongaglabol [17] | 60114615 | - |
| 11 a | 11.32 | C20H18O5 | 339.1128 | 339.1074 | 5.4 | 324.0609, 205.0487, 190.0239, 175.0041, 161.0584 | β,2',5'-Trimethoxyfurano-[4'',5'':3',4']chalcone [22] | 8167359 | - |
| 12 a | 11.57 | C16H12O4 | 269.0800 | 269.0736 | 3.1 | 254.0576, 253.0494, 237.0542, 209.0716, 181.0674, 137.0234 | 7-Hydroxy-4'-methoxyisoflavone [21] | 4302864 | 828786 |
| 13 | 12.11 | C19H14O5 | 323.0899 | 323.0914 | −1.5 | 307.0601, 305.0447, 279.0641, 277.0481, 261.0529, 161.0215, 145.0280 | Millettocalyxin C [36] | 57681679 | - |
| 14 b | 12.22 | C18H14O4 | 295.0929 | 295.0965 | −3.6 | 279.0843, 267.4780, 191.0352, 176.0113 | Pongamol [23] | 61222600 | - |
| 15 | 12.28 | C17H10O4 | 279.0670 | 279.0652 | 1.8 | 205.0783, 176.0095, 149.0231 | 7-Hydroxyfurano[2'',3'':5,6]flavone [20] | 4101706 | - |
| 16 | 13.14 | C18H10O4 | 291.0649 | 291.0652 | −0.3 | 263.0673, 235.0749, 217.0628, 207.0798, 179.0848 | Pongarotene [28] | - | 5862765 |
| 17 a | 13.37 | C18H12O4 | 293.0794 | 293.0808 | −1.4 | 278.0559, 250.0599, 222.0630, 194.0718, 176.0100, 148.0157 | Pinnatin [18] | 76746797 | - |
| 18 a | 13.65 | C20H16O6 | 353.1071 | 353.1020 | 5.1 | 338.0765, 323.0557, 295.0576, 277.0497, 161.0209, 145.0294 | Pachycarin A [15] | 97471101 | - |
| 19 a | 13.75 | C21H18O5 | 351.1158 | 351.1227 | −6.9 | 321.0751, 305.0864, 293.0823, 279.0685 | 6-Hydroxy-3-methoxy-6'',6''-dimethylpyrano[2'',3'':7,8]flavone [21] | 64574503 | - |
| 20 | 14.14 | C19H16O5 | 325.1068 | 325.1071 | −0.3 | 191.0332, 176.0125, 135.0795 | 2'-Hydroxy-3,4-dimethoxyfurano[4',3':2'',3'']chalcone [37] | 6747619 | - |
| 21 | 14.20 | C19H16O4 | 309.1126 | 309.1121 | 0.5 | 175.0380, 160.013 | O-Methylpongamol [38] | 12924865 | 17877802 |
| 22 | 15.02 | C21H18O7 | 383.1173 | 383.1125 | −0.8 | 368.0868, 353.0663, 321.0431, 307.0594, 293.0445, 279.0647 | Pachycarin C [19] | 31970912 | - |
| 23 a | 15.34 | C17H10O3 | 263.0682 | 263.0703 | −2.1 | 207.0795, 178.0780, 161.0230, 133.0248, 129.0342, 105.0352 | Lanceolatin B [27] | 36470090 | 12225716 |
| 24 | 15.57 | C19H16O4 | 309.1111 | 309.1121 | −1.0 | 175.0376, 160.0171 | Isomer of O-Methylpongamol | 757311 | - |
| 25 a | 15.95 | C19H14O5 | 323.0909 | 323.0914 | −0.4 | 308.0641, 293.0442, 265.0492, 237.0542, 181.0649, 163.0784, 161.0230 | 3',4'-Dimethoxy[2",3":7,8]-furanoflavone [15] | 140956269 | - |
| 26 a | 16.57 | C18H12O4 | 293.0796 | 293.0808 | −1.2 | 250.0607, 182.0709, 161.0231, 153.0682 | Cauliflorin A [18] | 85623000 | - |
| 27 | 16.73 | C19H16O5 | 325.1068 | 325.1071 | −0.3 | 191.0331, 176.0125, 135.0795 | Dihydroovalitenin C [39] | 7231422 | - |
| 28 | 17.07 | C19H16O4 | 309.1112 | 309.1121 | −0.9 | 175.0390, 160.0111 | Isomer of O-Methylpongamol | 4454801 | - |
| 29 a,b | 17.67 | C18H12O4 | 293.0796 | 293.0808 | −1.2 | 278.0653, 277.0495, 249.0532, 221.0587, 205.0668, 193.0633, 161.0215 | Karanjin [16] | 97751879 | 83932212 |
| 30 | 17.98 | C21H18O4 | 335.1268 | 335.1278 | −1.0 | 305.0791, 203.0344, 175.0358, 159.0459, 135.0426 | Isopongaflavone [40] | 897095 | 11229663 |
| 31 | 18.38 | C19H14O5 | 323.0901 | 323.0914 | −1.3 | 307.0588, 292.0462, 279.0613, 264.0484,173.0247, 161.0195, 145.0266 | 3,6-Dimethoxyfurano-[7,8:2'',3'']falvone [41] | 40394167 | - |
| 32 | 19.25 | C23H24O5 | 381.1695 | 381.1697 | −0.2 | 247.0951, 217.1648, 215.0691, 161.0588, 159.0810 | 3'- O-Methylpraecansone B [42] | - | 4875446 |
| 33 a | 19.54 | C21H20O4 | 337.1428 | 337.1434 | −0.6 | 305.1085, 233.0792, 193.0433, 191.0368, 163.0373 | 7-Methoxy-8-(3''-hydroxy-3''-methyl-1''-butenyl)-flavone [27] | 9062891 | 863827 |
| 34 a | 20.47 | C22H20O5 | 365.1374 | 365.1384 | −1.0 | 335.0896, 292.0686, 277.0488, 235.0713, 217.0450, 135.0529 | 3,6-Dimethoxy-6'',6''-dimethyl-pyrano [2'',3'':7,8]flavone [21] | 4240276 | - |
| 35 | 20.63 | C18H14O3 | 279.0996 | 279.1016 | −2.0 | 205.034, 175.0371, 149.0230 | Ovalitenin A [43] | - | 3619252 |
| 36 | 21.29 | C22H20O5 | 365.1382 | 365.1384 | 0.6 | 335.0899, 320.0646, 292.0768, 263.0679, 247.0674, 236.0828 | 5-Methoxykaranjachromene [44] | 1605015 | - |
| 37 | 22.50 | C22H22O4 | 351.1562 | 351.1591 | −2.9 | 217.0853,175.0415, 161.0595, 147.0450, 115.0464 | 4',7-Dimethoxy-8-prenyl-isoflavone [45] | 534978 | 3526648 |
| 38 a | 23.11 | C20H16O3 | 305.1163 | 305.1172 | −0.9 | 287.1093, 187.0367 | 6'',6''-Dimethylpyrano[2'',3'':7,8]flavone [46] | 3297595 | 56639448 |
| 39 a | 23.80 | C17H10O4 | 279.0641 | 279.0652 | −1.1 | 251.0669, 177.0142, 149.0236, 121.0246 | Pongaglabol [15] | 4677607 | - |
| 40 a | 24.54 | C21H18O4 | 335.1270 | 335.1278 | −0.8 | 317.1153, 305.0808, 274.0978, 187.0387, 159.0401, 131.0489 | Karanjachromene [15] | 75652383 | 734535 |
| 41 a | 26.33 | C21H22O3 | 323.1617 | 323.1642 | −2.5 | 179.0712, 163.0395, 145.1034,133.0274 | Isoderricin A [47] | 40392988 | - |
3. Experimental
3.1. Reagents and Materials
3.2. Plant Material
3.3. Preparation of Sample Solutions
3.4. HPLC-DAD-ESI-IT-TOF-MSn
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- The health department of Guangxi. Guangxi Bencao Compiled; Guangxi People’s Publishing House: Nanning, China, 1974; Volume 2, p. 1622. [Google Scholar]
- Liang, Q.C.; Zhong, M. Chinese Zhuang Medicine; Guangxi Nationalities Publishing House: Nanning, China, 2005; p. 499. [Google Scholar]
- Dai, B. Chinese Modern Yao Medicine; Guangxi Science and Technology Press: Nanning, China, 2009; pp. 314–320. [Google Scholar]
- Li, Z.Q. Study on effecte of Fordia caulifolra on mouse acquired memory disorder. Acad. J. Guangdong Coll. Pharm. 2002, 18, 124–126. [Google Scholar]
- Zhou, Z.; Wei, Q.Z.; Li, Z.Q.; Chen, B.S.; Wu, Z.Q.; Dai, B. Effect of Fordia cauliflora extracts on learning and memory ability. Guangxi J. Tradit. Chin. Med. 2003, 26, 47–48. [Google Scholar]
- Wei, Q.Z.; Wu, Z.Q.; Zhou, Z.; Chen, B.S.; Dai, B. Researches on the acuity, toxicity and antisenility of the abstracts of Fordia caulifora Hemsl. J. Guangxi Tradit. Chin. Med. Univ. 2003, 6, 37–40. [Google Scholar]
- Tang, Z.Q.; Chen, B.S.; Zhou, Z.; Wu, Z.Q.; Qiu, C.C.; Chen, S.F.; Dai, B. Anti-inflammatory effect of various extracts of Fordia cauliflora. Chin. J. Ethnomed. Ethnopharm. 2003, 223–225. [Google Scholar]
- Wu, Z.Q.; Zhou, Z.; Wei, Q.Z. Protective effects of Abstracts of Fordia cauliflora Hemsl on bromobenzene-induced oxidative liver damage in mice and antioxidative capability in old mice. Chin. Pharmacol. Bull. 2004, 20, 1221–1223. [Google Scholar]
- The health department of Guangxi. Guangxi Herb Journal; Guangxi People’s Publishing House: Nanning, China, 1963; p. 74. [Google Scholar]
- Jian, J.; Qing, F.; Zhang, S.; Huang, J.; Huang, R.B. The effect of 17-methoxyl-7-hydroxy-benzene-furanchalcone isolated from Millettia pulchra on myocardial ischemia in vitro and in vivo. Planta Med. 2012, 78, 1324–1331. [Google Scholar] [CrossRef]
- Duan, X.Q.; Jiao, Y.; Huang, R.B.; Chen, J.H.; Jiang, W.Z.; Kong, X.L. Effect of YLS on blood pressure in spontaneous hypertension rats. J. Guangxi Med. Univ. 2003, 20, 18–20. [Google Scholar]
- Huang, Z.S.; Huang, R.B.; Li, J.; Zhang, S.J. The effects of LongYanShen polysaccharide on mouse memory function in different mouse dementia models. J. Youjiang Med. Coll. Natl. 2004, 26, 463–465. [Google Scholar]
- Jiang, W.Z.; Kong, X.L.; Huang, R.B.; Duan, X.Q.; Jiao, Y. Protective effects and mechanisms of longyanshen on acute chemical liver injury in mice. Chin. Pharm. 2004, 15, 398–400. [Google Scholar]
- Jiang, W.Z.; Kong, X.L.; Duan, X.Q.; Jiao, Y.; Huang, R.B. Study of scavenging action of longyanshen on oxygen free radicals. Chin. Pharm. 2001, 12, 451–452. [Google Scholar]
- Fan, L.L.; Zhang, Y.Z.; Huang, R.B.; Qin, S.D.; Yi, T.; Xu, F.; Tang, Y.N.; Qu, X.S.; Chen, H.B.; Miao, J.H. Determination of five flavonoids in different parts of Fordia cauliflora by ultra performance liquid chromatography/triple-quadrupole mass spectrometry and chemical comparison with the root of Millettia pulchra var. laxior. Chem. Cent. J. 2013, 7, 126–134. [Google Scholar] [CrossRef]
- Dai, B.; Cui, C.C.; Dai, X.D.; Xiang, S.F. Chemival constituents of Fordia cauliflora (I). Zhong Cao Yao 2003, 34, 21–22. [Google Scholar]
- Dai, X.D.; Yang, D.A.; Dai, B.; Cui, C.C. Chemival constituents of Fordia cauliflora (II). Zhong Cao Yao 2003, 34, 401–402. [Google Scholar]
- Dai, B.; Dai, X.D.; Yang, D.A.; Qiu, C.C. Chemival constituents of Fordia cauliflora (III). Zhong Cao Yao 2003, 34, 1063–1065. [Google Scholar]
- Zhao, W.Y.; Zhu, Y.F.; Guan, S.Y.; Zhong, S.Z.; Chen, F.T. Study on the chemical constituents of thickfruit Millettia root (Milllettia pachycarpa). Nat. Prod. Res. Dev. 2000, 13, 1–4. [Google Scholar]
- Malik, S.B.; Seshadri, T.R.; Sharma, P. Minor components of the leaves of Pongamia glabra. Indian J. Chem. B 1976, 14, 229–230. [Google Scholar]
- Liang, Z.Y.; Yang, X.S.; Zhu, H.Y.; Hao, X.J. Two new flavones from Fordia cauliflora of Yunnan. Yao Xue Xue Bao 2006, 41, 533–536. [Google Scholar]
- Liang, Z.Y.; Yang, X.S.; Wang, Y.; Hao, X.J.; Sun, Q.Y. Two new chalcones from Fordia cauliflora. Chin. Chem. Lett. 2010, 21, 818–820. [Google Scholar] [CrossRef]
- Jian, J.; Zhang, S.J.; Qiu, L.; Huang, J.C.; Huang, R.B. Study on chemical constituents of chalcones from Millettia Pulchra. Chin. J. Hosp. Pharm. 2010, 30, 1734–1737. [Google Scholar]
- Baruah, P.; Barua, N.C.; Sharma, R.P.; Baruah, J.N.; Kulanthaivel, P.; Herz, W. Flavonoids from Millettia pulchra. Phytochemistry 1984, 23, 443–447. [Google Scholar] [CrossRef]
- Gomes, C.M.R.; Gottlieb, O.R.; Bettolo, G.B.M.; Monache, F.D.; Polhill, R.M. Systematic significance of flavonoids in Derris and Lonchocarpus. Biochem. Syst. Ecol. 1981, 9, 129–147. [Google Scholar] [CrossRef]
- Mayer, R. Flavonoids from Leptospermum scoparium. Phytochemistry 1994, 29, 1340–1342. [Google Scholar] [CrossRef]
- Liu, J.L.; Pan, Z.H.; Su, T.; Yan, X.J.; Li, D.P. Chemical constituents from twigs and leaves of Fordia cauliflora. Zhong Cao Yao 2012, 43, 1071–1074. [Google Scholar]
- Simina, K.; Alia, Z.; Khaliq-Uz-Zamana, S.M.; Ahmada, V.U. Structure and biological activity of a new rotenoid from pongamia pinnata. Nat. Prod. Lett. 2002, 16, 351–357. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; To, J.T.; Li, H.; Wang, M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Mol. Nutr. Food Res. 2008, 52, 1530–1538. [Google Scholar] [CrossRef]
- Ghosh, A.; Mandal, S.; Banerji, A.; Kar, M.; Hazra, K.; Banerji, J. A novel biflavonyloxymethane from Pongamia pinnata and its radical Quenching activity. Nat. Prod. Commun. 2011, 6, 625–626. [Google Scholar]
- Koysomboon, S.; van Altena, I.; Kato, S.; Chantrapromma, K. Antimycobacterial flavonoids from Derris indica. Phytochemistry 2006, 67, 1034–1040. [Google Scholar] [CrossRef]
- Syah, Y.M.; Juliawaty, L.D.; Achmad, S.A.; Hakim, E.H.; Ghisalberti, E.L. Cytotoxic prenylated flavones from Artocarpus champeden. J. Nat. Med. 2006, 60, 308–312. [Google Scholar] [CrossRef]
- Yadav, P.P.; Ahmad, G.; Maurya, R. Furanoflavonoids from Pongamia pinnata fruits. Phytochemistry 2004, 65, 439–443. [Google Scholar] [CrossRef]
- Shen, X.W.; Zheng, S.Z.; Yin, Z.D.; Song, Z.W.; Wang, L. Five new compounds from Elsholtzia densa. Chem. J. Chin. Univ. 1994, 15, 540–542. [Google Scholar]
- Sritularak, B.; Likhitwitayawuid, K.; Conrad, J.; Vogler, B.; Reeb, S.; Klaiber, I.; Kraus, W. New Flavones from Millettia erythrocalyx. J. Nat. Prod. 2002, 65, 589–591. [Google Scholar] [CrossRef]
- Sritularak, B.; Likhitwitayawuid, K. Flavonoids from the pods of Millettia erythrocalyx. Phytochemistry 2006, 67, 812–817. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Rao, E.V.; Raju, N.R. 8-Substituted flavonoids and 3'-substituted 7-oxygenated chalcones from Tephrosia purpurea. J. Chem. Soc. Perkin Trans. 1 1981, 2491–2498. [Google Scholar] [CrossRef]
- Magalhães, A.F.; Tozzi, A.M.G.A.; Sales, B.H.L.N.; Magalhães, E.G. Twenty-three flavonoids from Lonchocarpus subglaucescens. Phytochemistry 1996, 42, 1459–1471. [Google Scholar] [CrossRef]
- Roy, D.; Khanna, R.N. Structure and synthesis of isopongaflavone, a new component of the seeds of Pongamia glabra. Indian J. Chem. B 1979, 18, 525–528. [Google Scholar]
- Kamperdick, C.; Phuong, N.M.; Sung, T.V.; Adam, G. Flavones and isoflavones from Millettia ichthyochtona. Phytochemistry 1998, 46, 577–579. [Google Scholar] [CrossRef]
- Carcache-Blanco, E.J.; Kang, Y.-H.; Park, E.J.; Su, B.-N.; Kardono, L.B.S.; Riswan, S.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of the stem bark of Pongamia pinnata with the potential to induce quinine reductase. J. Nat. Prod. 2003, 66, 1197–1202. [Google Scholar] [CrossRef]
- Gupta, R.K.; Krishnamurti, M. New dibenzoylmethane and chalcone derivatives from Milletia ovalifolia seeds. Phytochemistry 1977, 16, 1104–1105. [Google Scholar] [CrossRef]
- Magalhães, A.F.; Tozzi, A.M.A.; Magalhães, E.G.; Nogueira, M.A.; Queiroz, S.C.N. Flavonoids from Lonchocarpus latifolius roots. Phytochemistry 2000, 55, 787–792. [Google Scholar] [CrossRef]
- Pistelli, L.; Noccioli, C.; Appendino, G.; Bianchi, F.; Sterner, O.; Ballero, M. Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Phytochemistry 2003, 64, 595–598. [Google Scholar] [CrossRef]
- Ren, L.L. Study on Bioactivities and the Chemical Components of Alcohol Extract from Fordia Cauliflora Hemsl; Nanjing University of Technology: Nanjing, China, 2004. [Google Scholar]
- Liang, Z.Y.; Yang, X.S.; Zhu, H.Y.; Hao, X.J. Three Prenylflavanones from Fordia cauliflora. Nat. Prod. Res. Dev. 2005, 17, 592–594. [Google Scholar]
- Sample Availability: Samples of the compounds pachycarin A, 3',4'-dimethoxy[2",3":7,8]furanoflavone, karanjin, pongaglabol, karanjachromene and isoderricin A are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fan, L.-L.; Yi, T.; Xu, F.; Zhang, Y.-Z.; Zhang, J.-Y.; Li, D.-P.; Xie, Y.-J.; Qin, S.-D.; Chen, H.-B. Characterization of Flavonoids in the Ethomedicine Fordiae Cauliflorae Radix and Its Adulterant Millettiae Pulchrae Radix by HPLC-DAD-ESI-IT-TOF-MSn. Molecules 2013, 18, 15134-15152. https://doi.org/10.3390/molecules181215134
Fan L-L, Yi T, Xu F, Zhang Y-Z, Zhang J-Y, Li D-P, Xie Y-J, Qin S-D, Chen H-B. Characterization of Flavonoids in the Ethomedicine Fordiae Cauliflorae Radix and Its Adulterant Millettiae Pulchrae Radix by HPLC-DAD-ESI-IT-TOF-MSn. Molecules. 2013; 18(12):15134-15152. https://doi.org/10.3390/molecules181215134
Chicago/Turabian StyleFan, Lan-Lan, Tao Yi, Feng Xu, Ya-Zhou Zhang, Jian-Ye Zhang, Dian-Peng Li, Yang-Jiao Xie, Shan-Ding Qin, and Hu-Biao Chen. 2013. "Characterization of Flavonoids in the Ethomedicine Fordiae Cauliflorae Radix and Its Adulterant Millettiae Pulchrae Radix by HPLC-DAD-ESI-IT-TOF-MSn" Molecules 18, no. 12: 15134-15152. https://doi.org/10.3390/molecules181215134