The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5-Benzodiazepine from 1,2-Diaminobenzene in the Presence of Acetone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
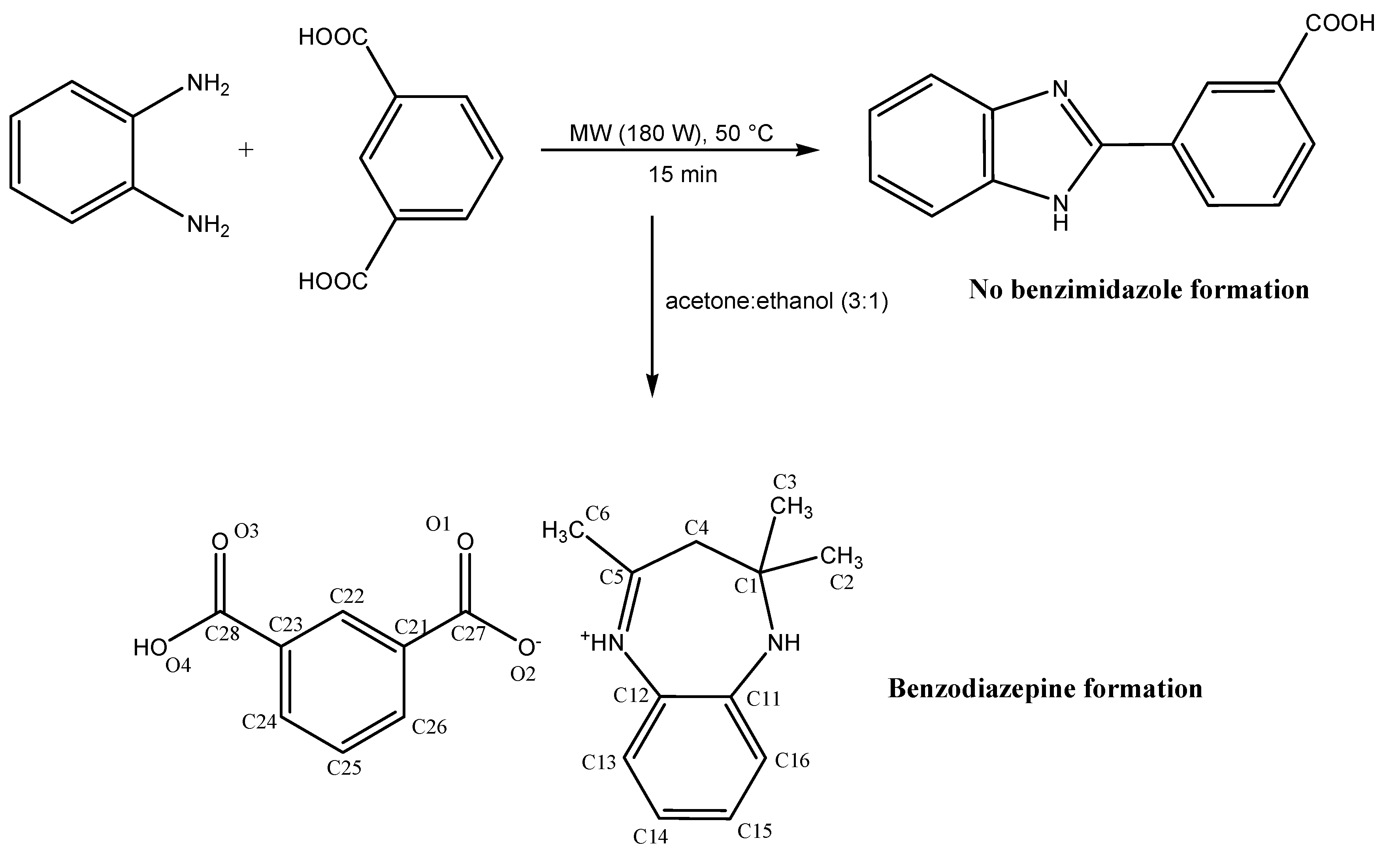
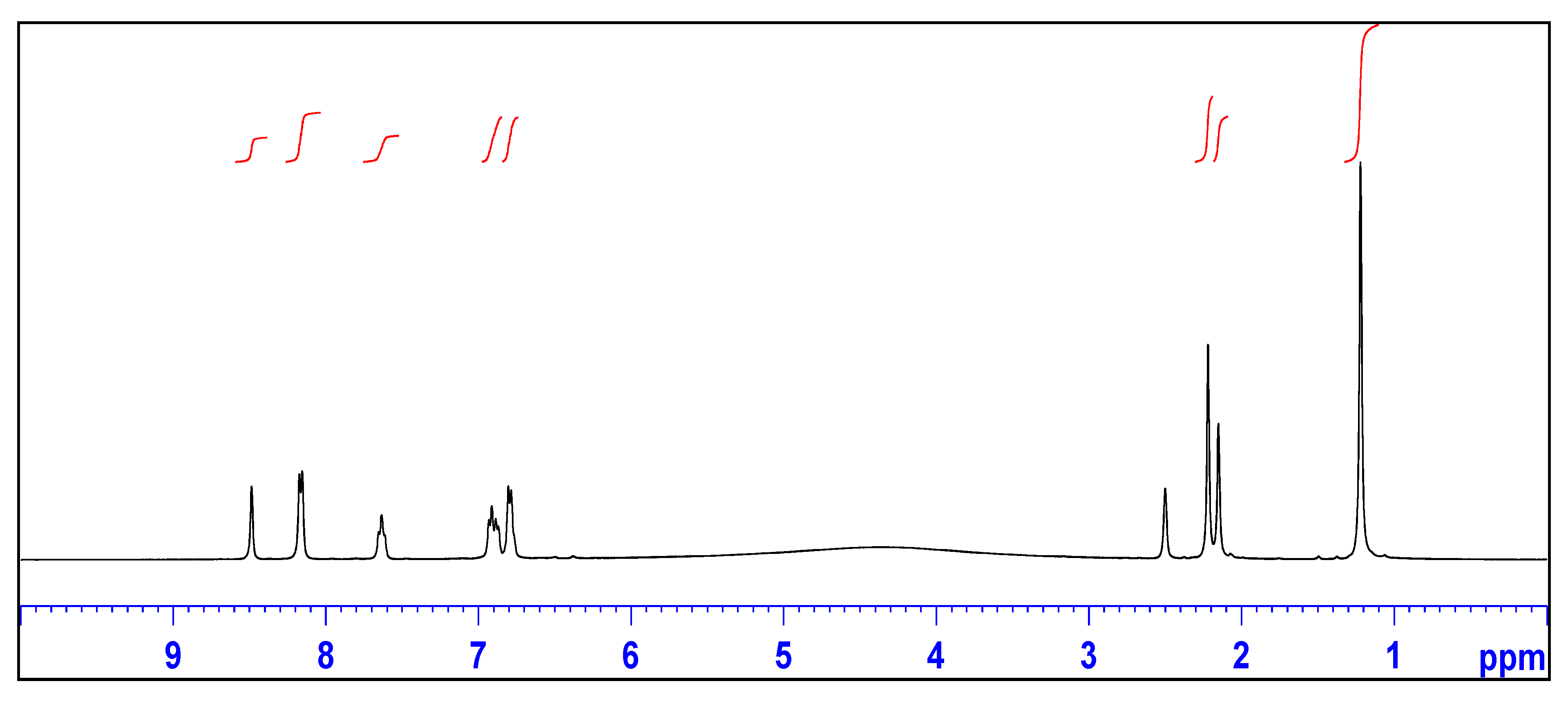
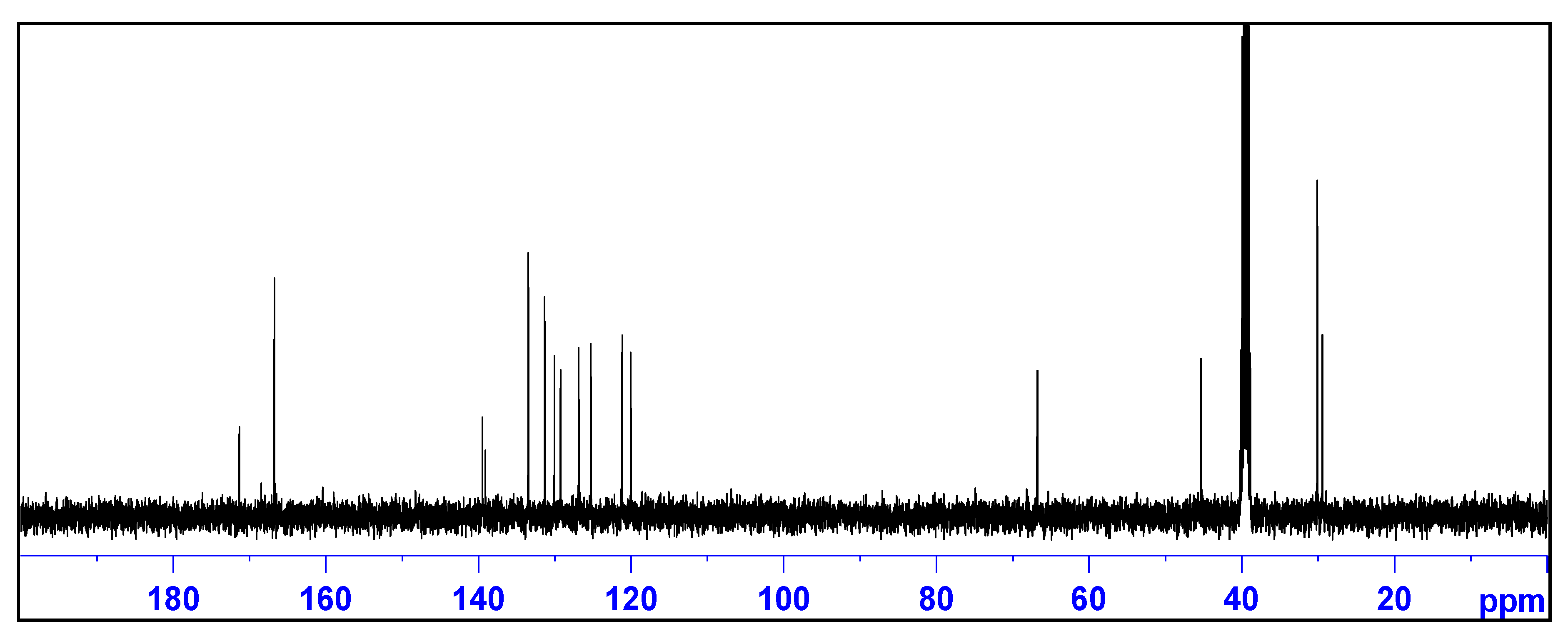
2.2. X-ray Crystallography
| Property | Compound I | Compound II |
|---|---|---|
| Formula | C12H17N2C8H5O2 | C12H16N2 |
| Formula Weight | 354.40 | 188.27 |
| Temperature (K) | 200 | 200 |
| Crystal System | triclinic | orthorhombic |
| Space group | P-1 | Pna 21 |
| a (Å) | 9.3608(4) | 12.1454(3) |
| b (Å) | 9.5706(3) | 7.2730(2) |
| c (Å) | 11.9881(4) | 11.9222(3) |
| α (˚) | 101.128(1) | 90 |
| β (˚) | 102.728(1) | 90 |
| γ (˚) | 114.297(1) | 90 |
| V (Å3) | 904.91(6) | 1053.13(5) |
| Z | 2 | 4 |
| D (calc) (g/cm3) | 1.301 | 1.187 |
| μ(MoKa) (mm) | 0.091 | 0.091 |
| F(000) | 376 | 408 |
| Crystal Size (mm) | 0.15 × 0.36 × 0.42 | 0.19 × 0.44 × 0.45 |
| Radiation (Å) | Mo Kα 0.71073 | Mo Kα 0.71073 |
| θ Min–Max (˚) | 2.5–28.3 | 3.3–28.3 |
| Data set | −12:12; −12:12, −15:15 | −15:16; −9:9;−10:15 |
| Tot. Uniq. Data R(int) | 16298, 4492, 0.015 | 9541, 2371, 0.015 |
| Observed data (I > 2.0 sigma (I)) | 3854 | 2285 |
| Nref, Npar | 4492, 240 | 2371, 134 |
| R, Wr2, S | 0.0385, 0.1045, 1.04 | 0.0306, 0.0802, 1.03 |
| Max and Av. Shift/Error | 0.00, 0.00 | 0.00, 0.00 |
| Min and Max, Resd Dens (e/Å3) | 0.20, 0.30 | −0.20, 0.18 |
| Property | I | II |
|---|---|---|
| Bond length | ||
| C(27)–O(1) | 1.24(2) | |
| C(27)–O(2) | 1.25(2) | |
| C(28)–O(3) | 1.20(2) | |
| C(28)–O(4) | 1.32(2) | |
| N(2)–C(5) | 1.28(2) | 1.28(2) |
| N(1)–C(1) | 1.47(2) | 1.48(2) |
| Bond angles | ||
| C(2)–C(1)–C(4) | 109.1(1) | 108.6(1) |
| C(4)–C(5)–C(6) | 121.2(2) | 117.5(1) |
| C(13)–C(12)–N(2) | 117.7(2) | 116.9(1) |
| C(16)–C(11)–N(1) | 121.2(1) | 119.7(1) |
| Torsion angles | ||
| C(12)–N(2)–C(5)–C(6) | 178.8(1) | 178.1(1) |
| C(6)–C(5)–C(4)–C(1) | −108.2(1) | −107.0(2) |
| C(2)–C(1)–N(1)–C(11) | 89.5(1) | 94.5(2) |
| N(1)-C(11)–C(12)–N(2) | −0.7(2) | −2.9(2) |
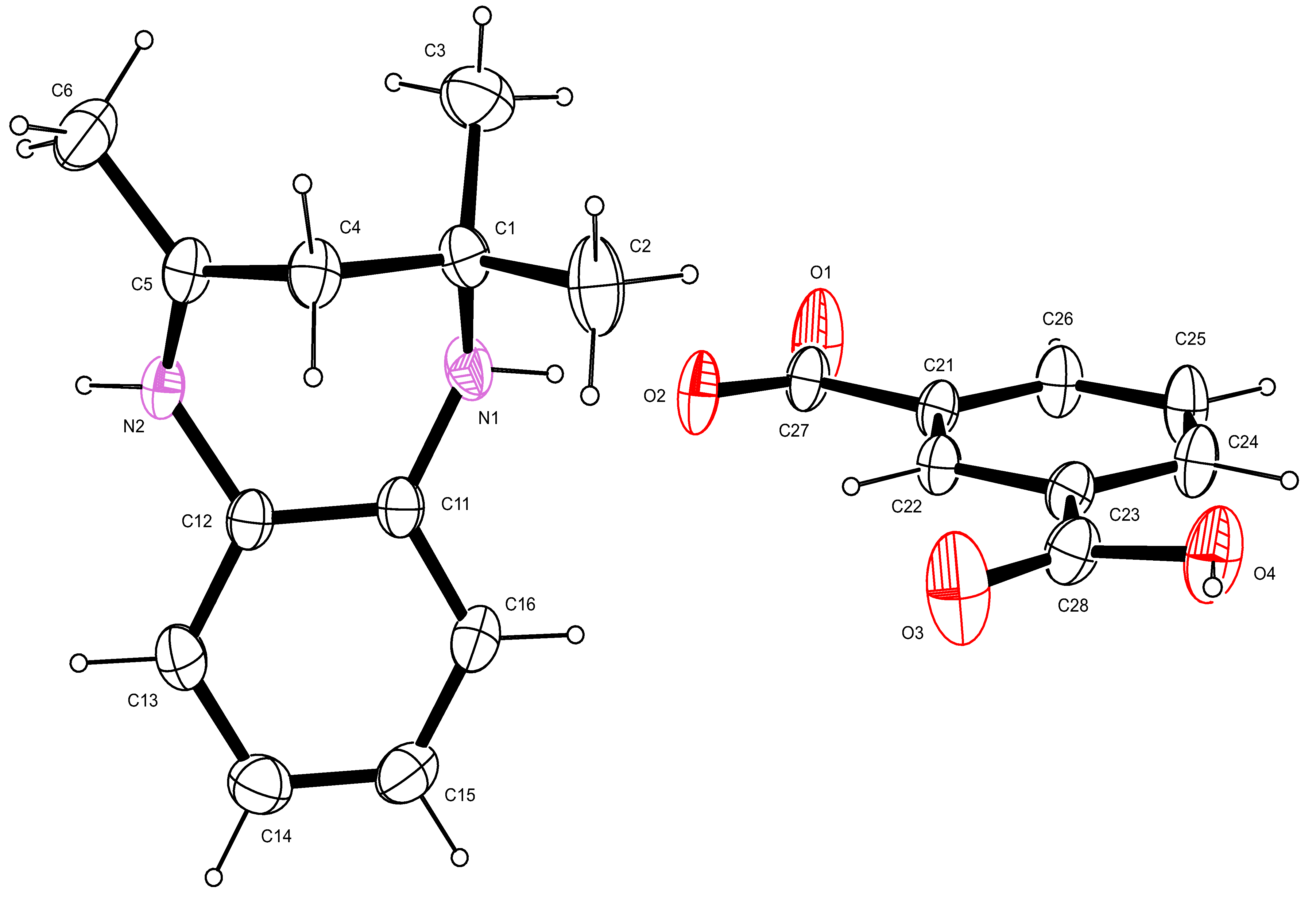
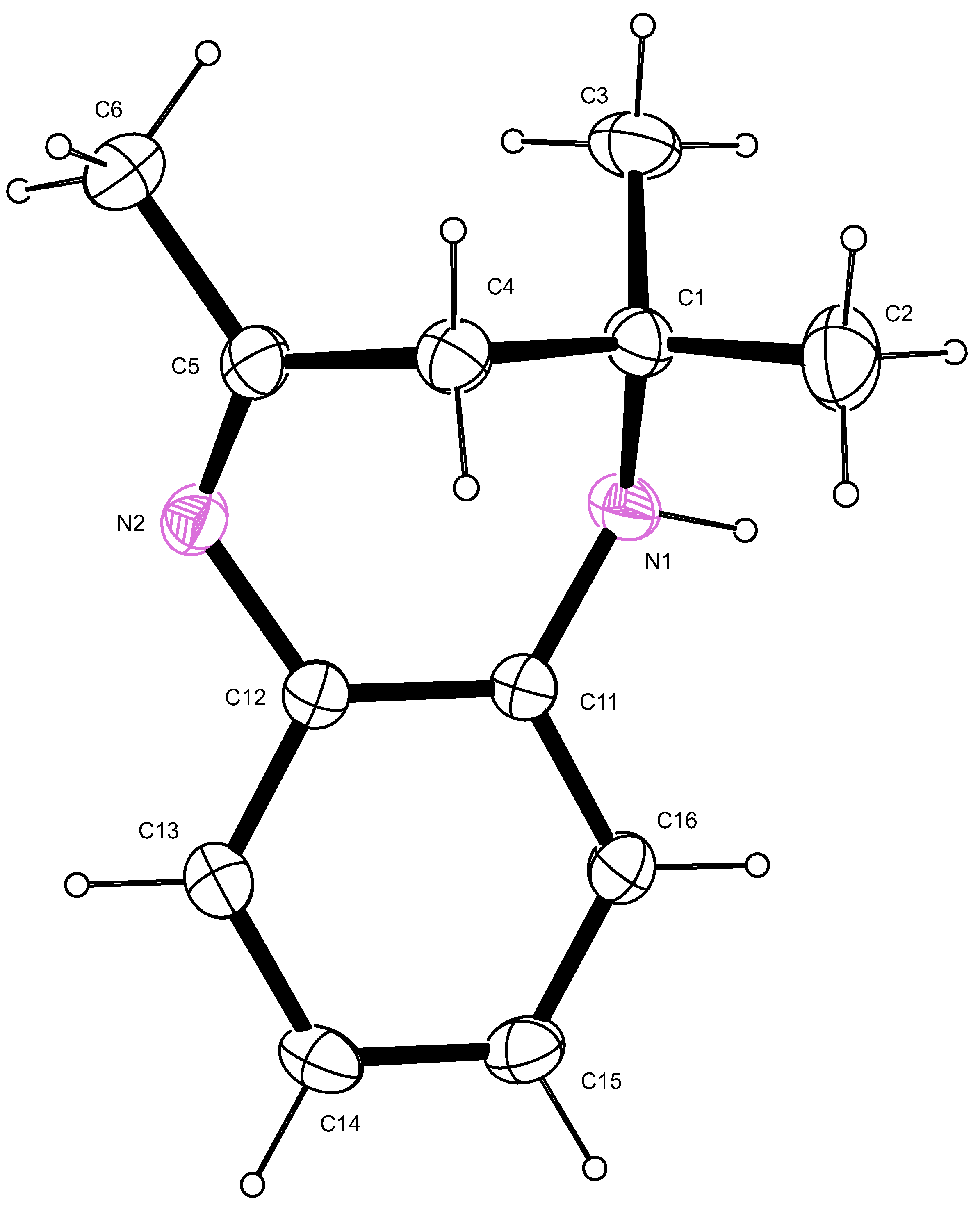
2.3. Proposed Reaction Mechanism
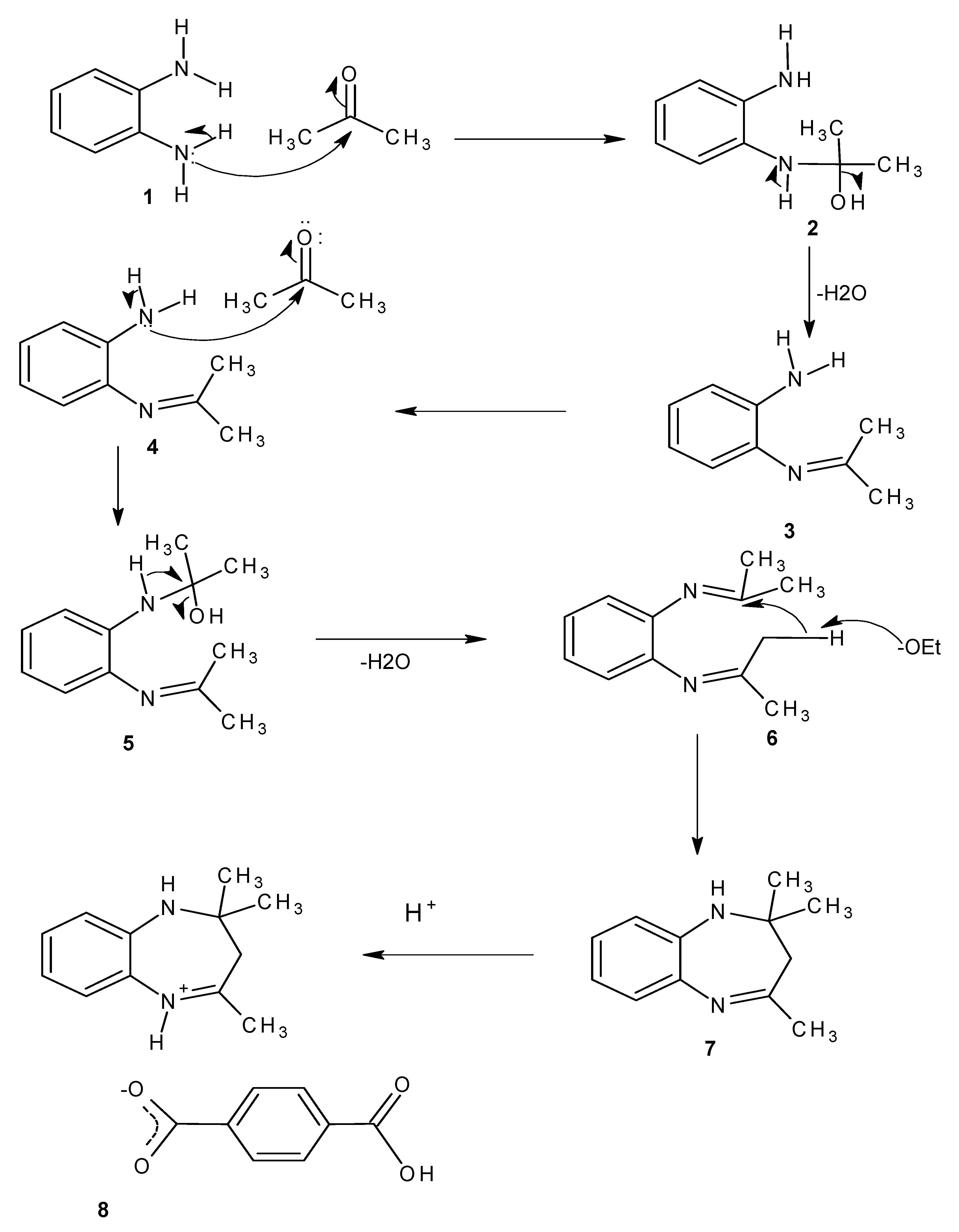
3. Experimental
3.1. Reagents and Instrumentation
3.2. Synthesis of Benzodiazepium Isophthalate Salt and Benzodiazepine
2,2,4-Trimethyl-2,3-dihydro-1H-1,5-benzodiazepin-5-ium isophthalate (I)
2,2,4-Trimethyl-2,3-dihydro-1H-1,5-benzodiazepine (II)
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Roth, T.; Morningstar, M.L.; Boyer, P.L.; Hughes, S.H.; Buckheit, R.W., Jr.; Michejde, C.J. Synthesis and biological activity of novel nonnucleoside inhibitors of HIV–1 reverse transcriptase, 2–aryl substituted benzimidazoles. J. Med. Chem. 1997, 40, 4199–4207. [Google Scholar] [CrossRef]
- Selvam, T.P.; Radhika, P.P.; Janagaraj, S.; Kumar, A.S. Synthesis of novel 2-substituted benzimidazole derivatives as potential antimicrobial agents. Res. Biotechnol. 2011, 2, 50–57. [Google Scholar]
- Vaidehi, K.G.; Satya, R.V.; Bangaramma, R.R.; Kumar, R.H.; Sudha, Y.R.; Kumar, T.R. Synthesis, Characterization and antibacterial activity of 2-substituted benzimidazole derivatives. Int. J. Res. Pharm. Chem. 2002, 2, 322–326. [Google Scholar]
- Abdullah, J.M.; Sulaiman, M.M.; Mohamed, S.J. Microwave-assisted one-pot synthesis of 2-aryl (1H) benzimidazoles without catalyst. J. Edu. & Sci. 2012, 25, 58–63. [Google Scholar]
- Karimi-Jaberi, Z.; Amiri, M. An efficient and inexpensive synthesis of 2-substituted benzimidazoles in water using boric acid at room temperature. J. Chem. 2012, 9, 167–170. [Google Scholar]
- Dhani, G.C.R.; Teja, S.C.; Mastanaiah, P.; Avinash, A.; Rathnam, P.R.; Nagina, S.K.; Sipa, C.V.; Dhana, L.K. Reactivity of novel substituted benzimidazole derivatives. Int. J. Adv. Pharm. Nano. 2011, 1, 114–120. [Google Scholar]
- Kushwaha, N.; Saini, R.K.; Kushwaha, S.K. Synthesis of some amide derivatives and their biological activity. Synthesis 2011, 3, 203–209. [Google Scholar]
- Zia-Ul-Haq, M.; Hameed, S.; Duddeck, H.; Ahmed, R. Synthesis of 1,4-diazepine nucleosides. Turk. J. Chem. 2002, 26, 807–813. [Google Scholar]
- Sapnakumari, M.; Narayana, B.; Samshuddin, S.; Sarojini, K. Synthesis and characterization of new 1,2-diazepine derivative. Der Pharma Chemica 2012, 4, 2198–2201. [Google Scholar]
- Kaoua, R.; Bennamane, N.; Bakhta, S.; Bennadji, S.; Rabia, C.; Nedjar-Kolli, B. Synthesis of substituted 1,4-diazepines and 1,5-benzodiazepines using an efficient heteropolyacid-catalysed procedure. Molecules 2011, 16, 92–99. [Google Scholar]
- Sabatie, A.; Veigh, D.; Loupy, A.; Floch, L. Synthesis of aromatic and heteroaromatic annelated [1,4] diazepine. ARKIVOC 2001, 6, 122–128. [Google Scholar] [CrossRef]
- Rekha, M.; Hamza, A.; Venugopal, B.R.; Nagaraju, N. Synthesis of 2-substituted benzimidazoles and 1,5–disubstituted benzodiazepines on alumina and zirconia catalysts. Chin. J. Catal. 2012, 33, 439–446. [Google Scholar] [CrossRef]
- Yadav, J.S.; Srivastava, Y.K. An efficient microwave-assisted synthesis of some novel 1,4–diazepine derivatives as possible antimicrobial agents. Rasayan J. Chem. 2010, 3, 726–730. [Google Scholar]
- Norman, J.R.; Michael, S.E.; Daryle, H.B. Complexes derived from the reaction of hexaamminenickel(II) ion with acetone. Inorg. Chem. 1967, 6, 1924–1926. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.T.; Guerrero, R.; Vicente-Hernandez, I.; Bautista, D. Synthesis and reactivity of Ir(I) and Ir(III) complexes with MeNH2, Me2C=NR (R=H, Me), C,N-C6H4{(CMe)=N(Me)}-2, and N,N'-RN=C(Me)CH2C(Me2)NHR(R=H,Me) ligands. Inorg. Chem. 2008, 47, 9592–9605. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, M.T.; Guerrero, R.; Vicente-Hernandez, I.; Alvarez-Falcon, M.M. Metal–assisted aldol-type condensation of two acetimino ligands to give a 4-imino-2-methylpentane-2-amino rhodium(III) complex. Organometallics 2005, 24, 4506–4508. [Google Scholar] [CrossRef]
- Fairlie, D.P.; Turner, M.; Byriel, K.A.; Mckweon, J.A.; Jackson, W.G. Facile and stereoselective condensation of acetone with ammonia ligands on cobalt(III): Structure of a N-bonded cyanate complex containing the 2-methyl-2-amino-4-imino-pentane ligand. Inorg. Chim. Acta 1999, 290, 133–138. [Google Scholar] [CrossRef]
- Perova, E.V.; Miloserdov, F.M.; Yakovlova, M.A.; Stolyarov, I.P.; Nefedov, S. E Effect of the nature of carboxylate anion on the features of intramolecular hydrogen bonding in [Pd(Hdmpz)4](OOCR)2 (R=Me,But,Ph). Russ. J. Inorg. Chem. 2009, 54, 1378–1389. [Google Scholar] [CrossRef]
- Ichikawa, M. The effect of hydrogen bonding on the bond length and angles in the carboxyl group. J. Cryst. Mol. Struct. 1979, 9, 87–105. [Google Scholar] [CrossRef]
- Gomez, J.A.P.S. The reductive amination of aldehyes and ketones and the hydrogenation of nitriles: Mechanistic aspects and selectivty control. Adv. Synth. Catal. 2002, 344, 1037–1057. [Google Scholar] [CrossRef]
- Bailey P.S., Jr.; Bailey, C.A. Organic Chemistry — A Brief Survey of Concepts and Applications, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; pp. 208–210. [Google Scholar]
- APEX2, SADABS and SAINT. Bruker AXS Inc.: Madison, WI, USA, 2010.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds I and II are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Odame, F.; Kleyi, P.; Hosten, E.; Betz, R.; Lobb, K.; Tshentu, Z. The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5-Benzodiazepine from 1,2-Diaminobenzene in the Presence of Acetone. Molecules 2013, 18, 14293-14305. https://doi.org/10.3390/molecules181114293
Odame F, Kleyi P, Hosten E, Betz R, Lobb K, Tshentu Z. The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5-Benzodiazepine from 1,2-Diaminobenzene in the Presence of Acetone. Molecules. 2013; 18(11):14293-14305. https://doi.org/10.3390/molecules181114293
Chicago/Turabian StyleOdame, Felix, Phumelele Kleyi, Eric Hosten, Richard Betz, Kevin Lobb, and Zenixole Tshentu. 2013. "The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5-Benzodiazepine from 1,2-Diaminobenzene in the Presence of Acetone" Molecules 18, no. 11: 14293-14305. https://doi.org/10.3390/molecules181114293





