Secondary Metabolites in Durian Seeds: Oligomeric Proanthocyanidins
Abstract
:1. Introduction
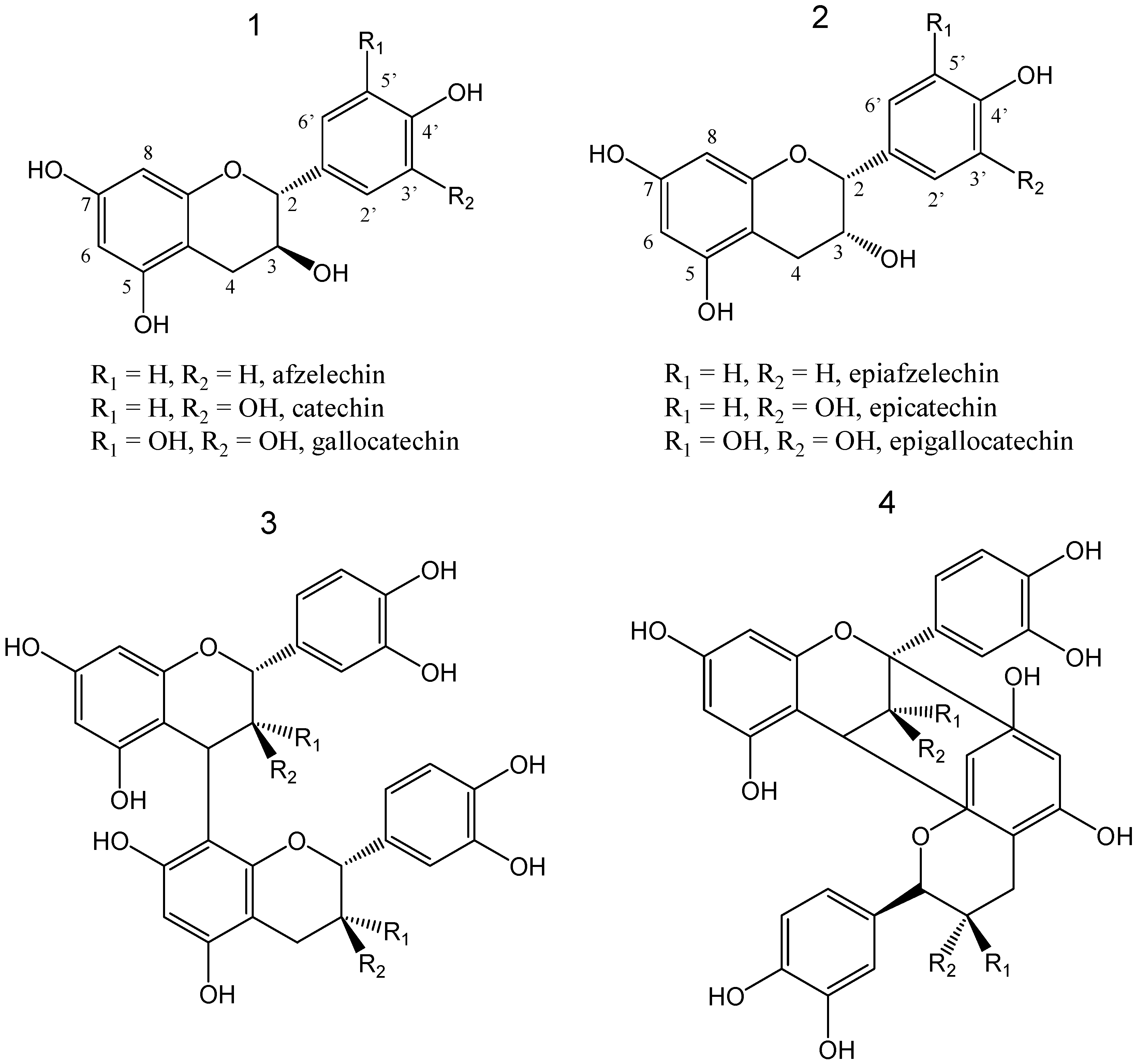
2. Results and Discussion
2.1. Chromatographic Fractionation of Durian Seed Extracts
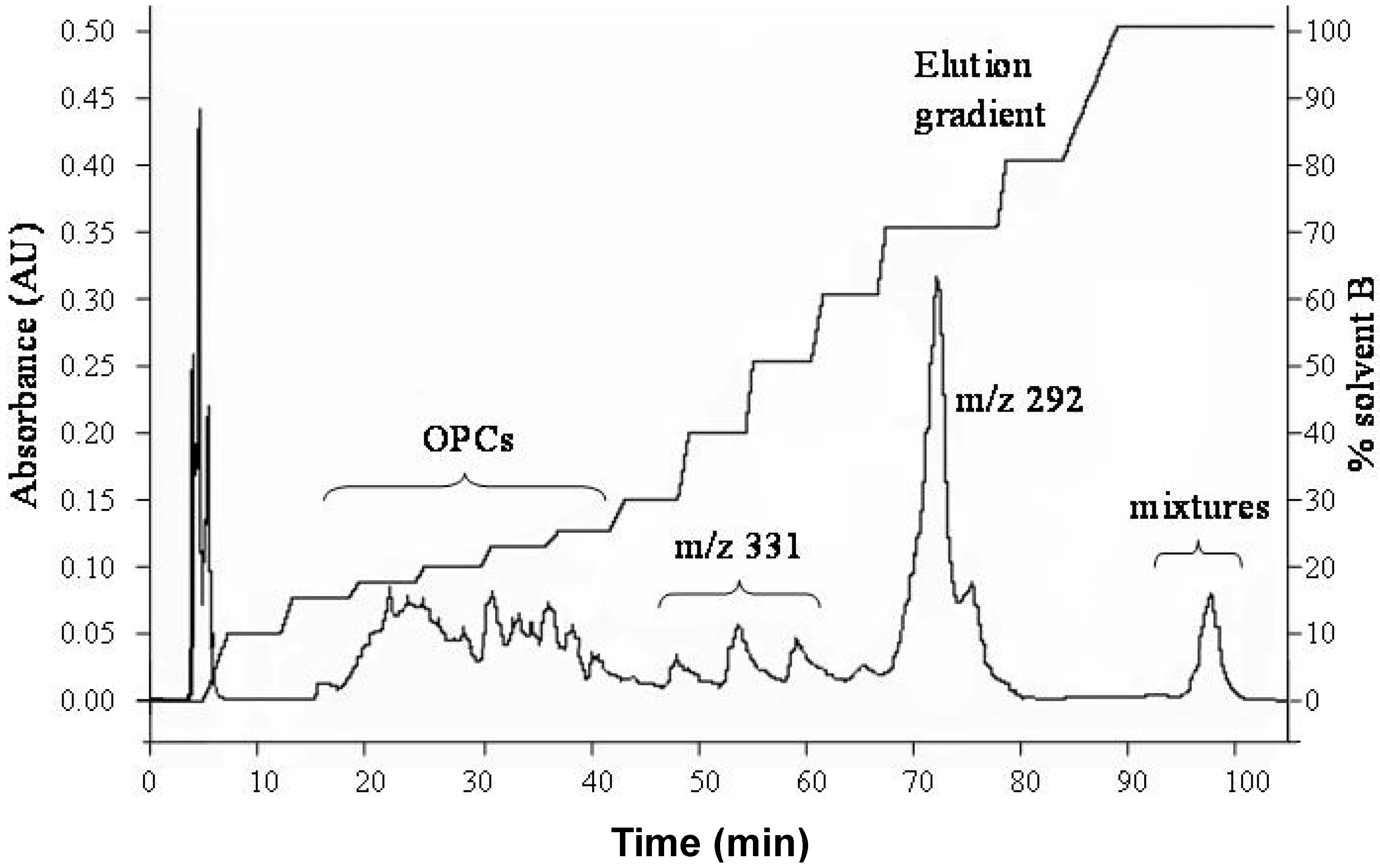
2.2. Extraction and Structural Elucidation of OPCs
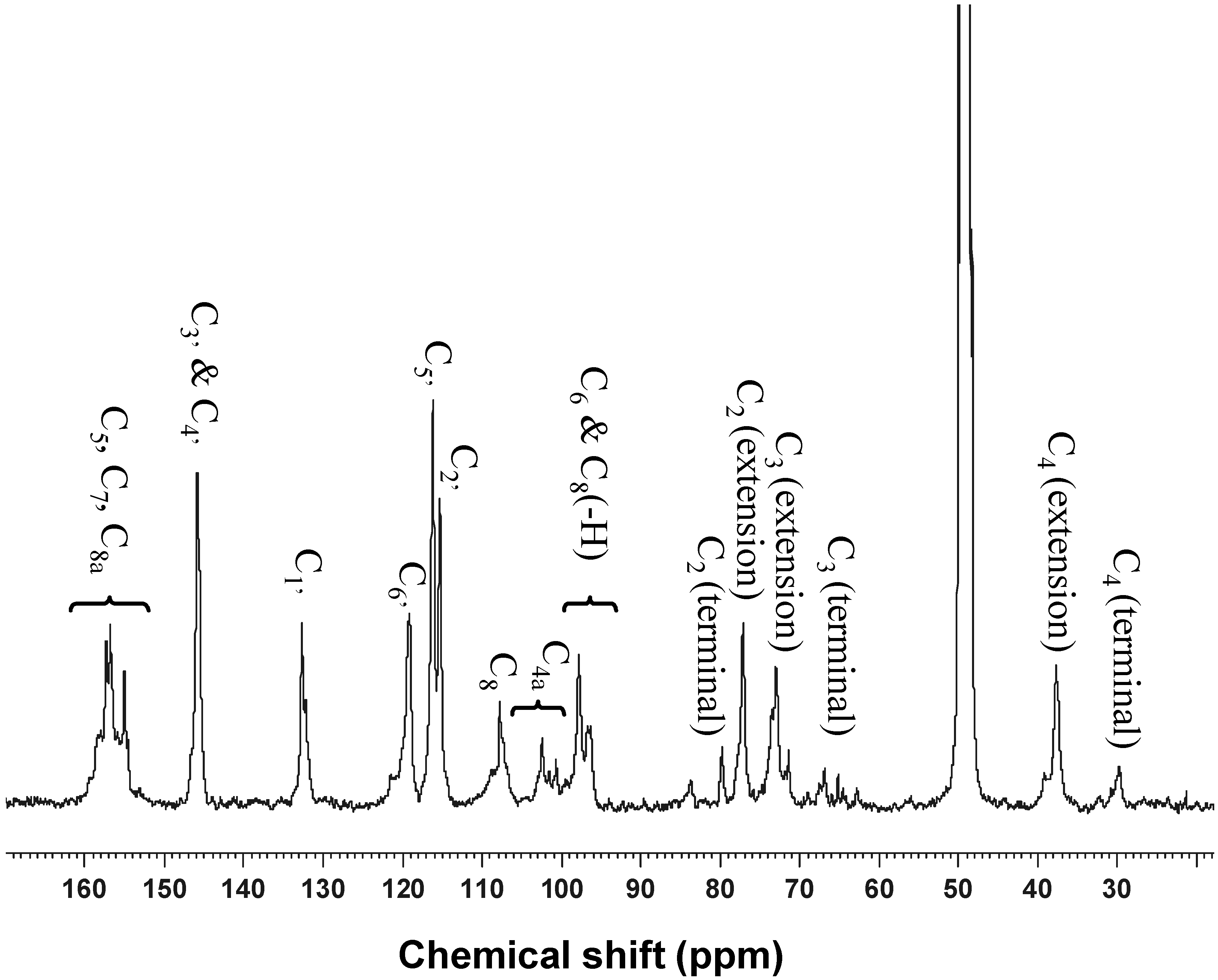

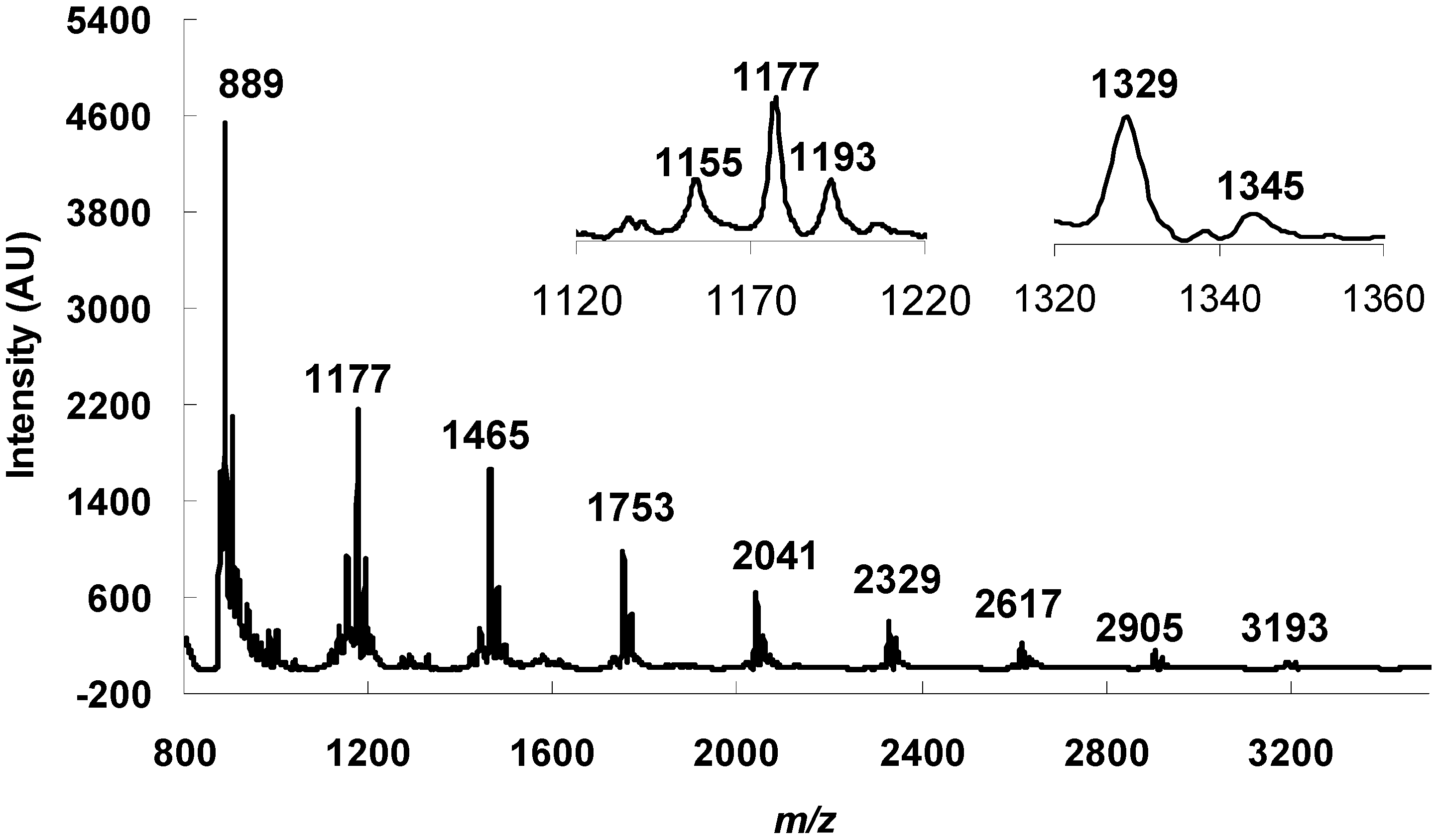
2.3. Determination of the Degree of Polymerization Procyanidins by Thiolysis
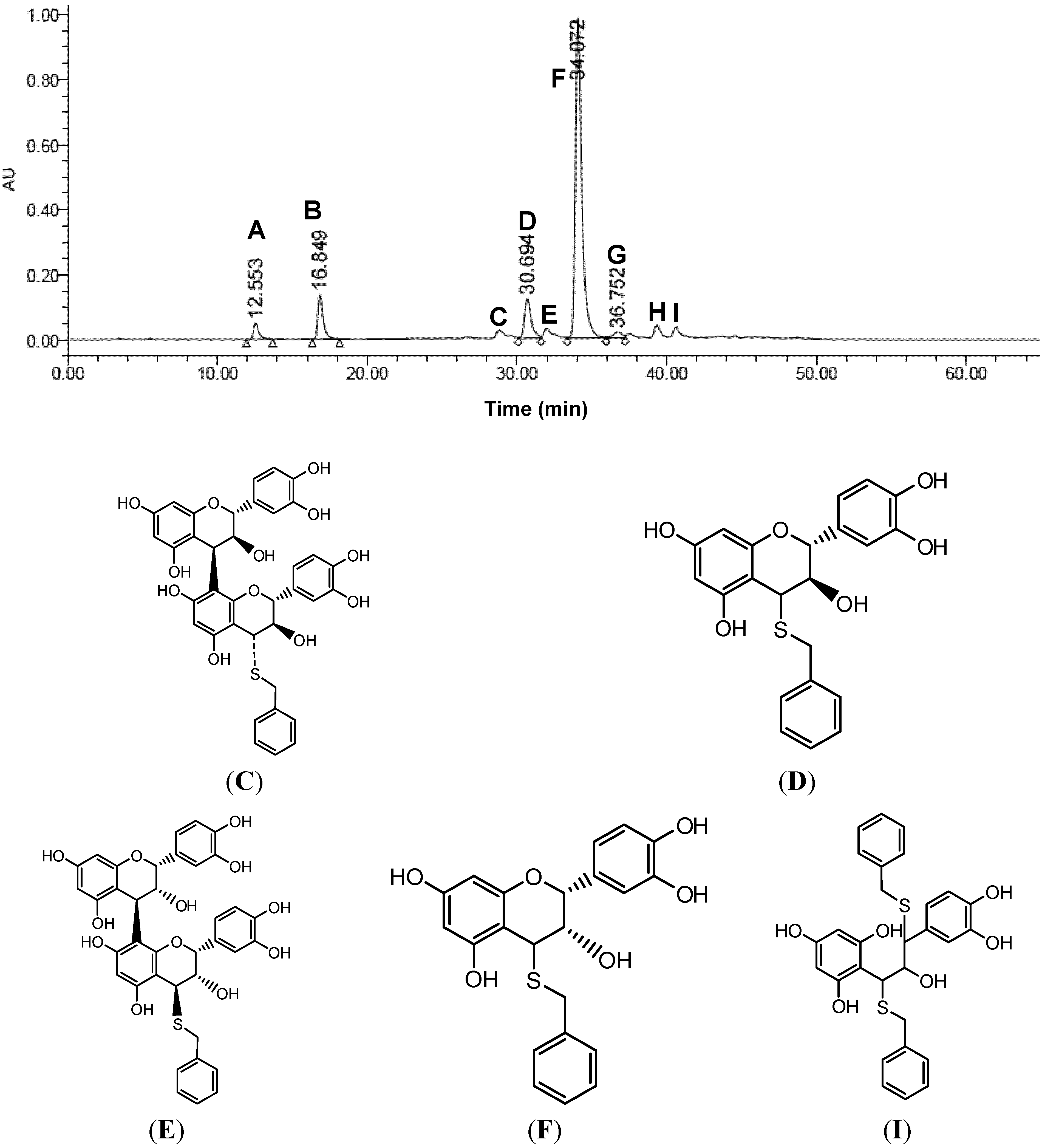
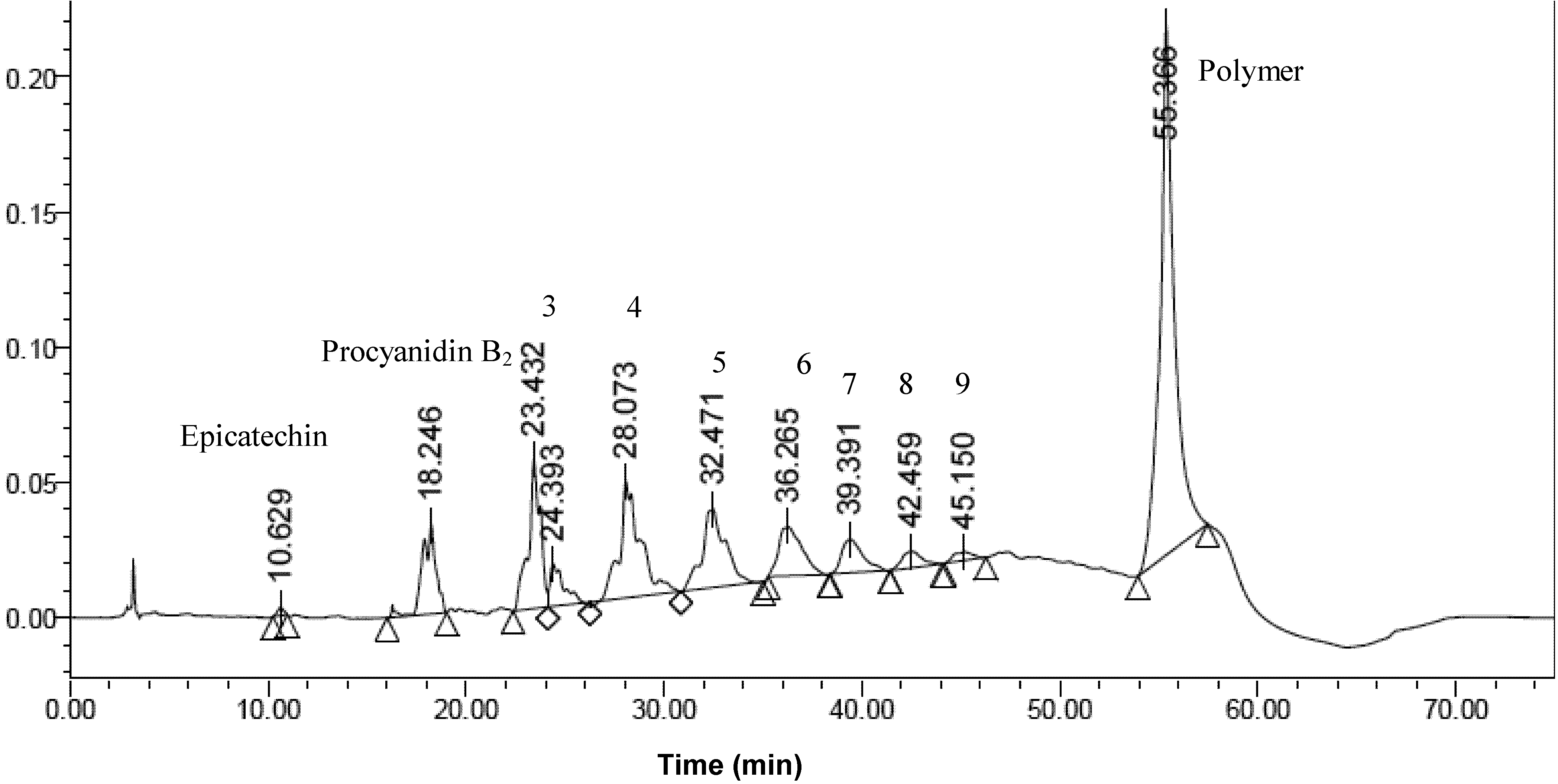
| Peak | Retention Time (min) | Concentration of procyanidins (μg/mL) | Content of procyanidins (mg ECE/g dry matter) |
|---|---|---|---|
| Monomers | 10.63 | 1.06 | 0.000958 |
| Dimers | 18.25 | 86.24 | 0.0780 |
| Trimers | 23.43 | 137.74 | 0.125 |
| Tetramers | 28.07 | 199.39 | 0.180 |
| Pentamers | 32.47 | 155.39 | 0.141 |
| Hexamers | 36.27 | 104.56 | 0.0946 |
| Heptamers | 39.39 | 57.72 | 0.0522 |
| Octamers | 42.46 | 31.97 | 0.0289 |
| Nonamers | 45.15 | 4.96 | 0.00449 |
| Polymers | 55.37 | 763.38 | 0.690 |
| Total | 1.395 |
3. Experimental
3.1. Instruments
3.2. Reagents
3.3. Solvent Extraction and Fractionation of Durian Seeds
3.4. Extraction and Purification of Oligomeric Proanthocyanidins from Durian Seeds
3.5. Oligomeric Proanthocyanidins Thiolysis and Identification
3.6. Quantitative Analysis of OPCs using Normal Phase HPLC
4. Conclusions
Abbreviations
| PC | Procyanidins |
| OPCs | Oligomeric proanthocyanidins |
| ESI-MS | electron spray ionization-mass spectrometry |
| MALDI-TOF MS | matrix assisted laser desorption/ionization–time of flight mass spectrometry |
| ECE | epicatechin equivalent |
Acknowledgments
Conflicts of Interest
References
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef]
- Fu, C.; Wang, H.; Ng, W.L.; Song, L.; Huang, D. Antioxidant activity and proanthocyanidin profile of Selliguea feei Rhizomes. Molecules 2013, 18, 4282–4292. [Google Scholar] [CrossRef]
- Skerget, M.; Kotnik, P.; Hadolin, M.; Hras, A.R.; Simonic, M.; Knez, Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Chen, M.; Tu, P. Fatty acids, tocopherols and proanthocyanidins in bramble seeds. Food Chem. 2006, 99, 586–590. [Google Scholar] [CrossRef]
- Wang, H.; Song, L.; Feng, S.; Liu, Y.; Zuo, G.; Lai, F.; He, G.; Chen, J.; Huang, D. Characterization of proanthocyanidins in stems of Polygonum multiflorum Thunb as strong starch hydrolase inhibitors. Molecules 2013, 18, 2255–2265. [Google Scholar]
- Cos, P.; de Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 10, 1345–1359. [Google Scholar]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar]
- Doijode, S.D. Seed Storage of Horticultural Crops; Haworth Press: New York, NY, USA, 2001; p. 339. [Google Scholar]
- Fischer, N.; Hammerschmidt, F.J.; Brunke, E.J. Studies on the biogeneration of sulfur-containing flavor components of the durian fruit. Abstr. Paper Am. Chem. Soc. 1994, 208, 101–107. [Google Scholar]
- Weenen, H.; Koolhaas, W.E.; Apriyantono, A. Sulfur-containing volatiles of durian fruits (Durio zibethinus Murr). J. Agric. Food Chem. 1996, 44, 3291–3293. [Google Scholar] [CrossRef]
- Haruenkit, R.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Sajewicz, M.; Kowalska, T.; Delgado-Licon, E.; Rocha-Guzman, N.E.; Gallegos-Infante, J.A.; Trakhtenberg, S.; et al. Comparative study of health properties and nutritional value of durian, mangosteen, and snake fruit: Experiments in vitro and in vivo. J. Agric. Food Chem. 2007, 55, 5842–5849. [Google Scholar] [CrossRef]
- Voon, Y.Y.; Hamid, N.S.A.; Rusul, G.; Osman, A.; Quek, S.Y. Characterisation of Malaysian durian (Durio zibethinus Murr.) cultivars: Relationship of physicochemical and flavour properties with sensory properties. Food Chem. 2007, 103, 1217–1227. [Google Scholar]
- Toledo, F.; Arancibia-Avila, P.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.G.; Drzewiecki, J.; Zachwieja, Z.; Zagrodzki, P.; Pasko, P.; et al. Screening of the antioxidant and nutritional properties, phenolic contents and proteins of five durian cultivars. Int. J. Food Sci. Nutr. 2008, 59, 415–427. [Google Scholar] [CrossRef]
- Brown, M.J. In Durio: A Bibliographic Review; IPGRI: New Delhi, India, 1997; pp. 188–224. [Google Scholar]
- Amin, A.M.; Ahmad, A.S.; Yin, Y.Y.; Yahya, N.; Ibrahim, N. Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocolloids 2007, 21, 273–279. [Google Scholar] [CrossRef]
- Eni, S.R. Fatty acid content in durian (Durio zibethinus Murr.) seed. Indones. J. Pharm. 2001, 12, 66–72. [Google Scholar]
- Zhang, Z.; Li, C.; Davies; Evan, G.R.; Liu, Y. Agricultural Waste. Water Environ. Res. 2013, 75, 1377–1451. [Google Scholar]
- Es-Safi, N.E.; Guyot, S.; Ducrot, P.H. NMR, ESI/MS, and MALDI-TOF/MS analysis of pear juice polymeric proanthocyanidins with potent free radical scavenging activity. J. Agric. Food Chem. 2006, 54, 6969–6977. [Google Scholar] [CrossRef]
- Guo, C.J.; Yang, J.J.; Wei, J.Y.; Li, Y.F.; Xu, J.; Jiang, Y.G. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Behrens, A.; Maie, N.; Knicker, H.; Kogel-Knabner, I. MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry 2003, 62, 1159–1170. [Google Scholar] [CrossRef]
- Fu, C.; Loo, A.E.K.; Chia, F.P.P.; Huang, D. Oligomeric proanthocyanidins from mangosteen pericarps. J. Agric. Food Chem. 2007, 55, 7689–7694. [Google Scholar] [CrossRef]
- Tarascou, I.; Barathieu, K.; Simon, C.; Ducasse, M.A.; Andre, Y.; Fouquet, E.; Dufourc, E.J.; Freitas, V.; Laguerre, M.; Pianet, I. A 3D structural and conformational study of procyanidin dimers in water and hydro-alcoholic media as viewed by NMR and molecular modeling. Magn. Reson. Chem. 2006, 44, 868–880. [Google Scholar] [CrossRef]
- Rigaud, J.; Perez-Ilzarbe, J.; da Silva, J.M.R.; Cheynier, V. Micro method for the identification of proanthocyanidin using thiolysis monitored by high-performance liquid chromatography. J. Chromatogr. A 1991, 540, 401–405. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.; Hammerstone, J.F.; Beecher, G.; Cunningham, D.; Vannozzi, S.; Prior, R.L. Fractionation of polymeric procyanidins from Lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J. Agric. Food Chem. 2002, 50, 4852–4860. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Feng, S.; Song, L.; He, G.; Chen, M.; Huang, D. Secondary Metabolites in Durian Seeds: Oligomeric Proanthocyanidins. Molecules 2013, 18, 14172-14185. https://doi.org/10.3390/molecules181114172
Liu Y, Feng S, Song L, He G, Chen M, Huang D. Secondary Metabolites in Durian Seeds: Oligomeric Proanthocyanidins. Molecules. 2013; 18(11):14172-14185. https://doi.org/10.3390/molecules181114172
Chicago/Turabian StyleLiu, Yuancai, Shengbao Feng, Lixia Song, Guangyuan He, Mingjie Chen, and Dejian Huang. 2013. "Secondary Metabolites in Durian Seeds: Oligomeric Proanthocyanidins" Molecules 18, no. 11: 14172-14185. https://doi.org/10.3390/molecules181114172




