‘One-pot’ Synthesis of Dihydrobenzo[4,5][1,3]oxazino[2,3-a] isoquinolines via a Silver(I)-Catalyzed Cascade Approach
Abstract
:1. Introduction
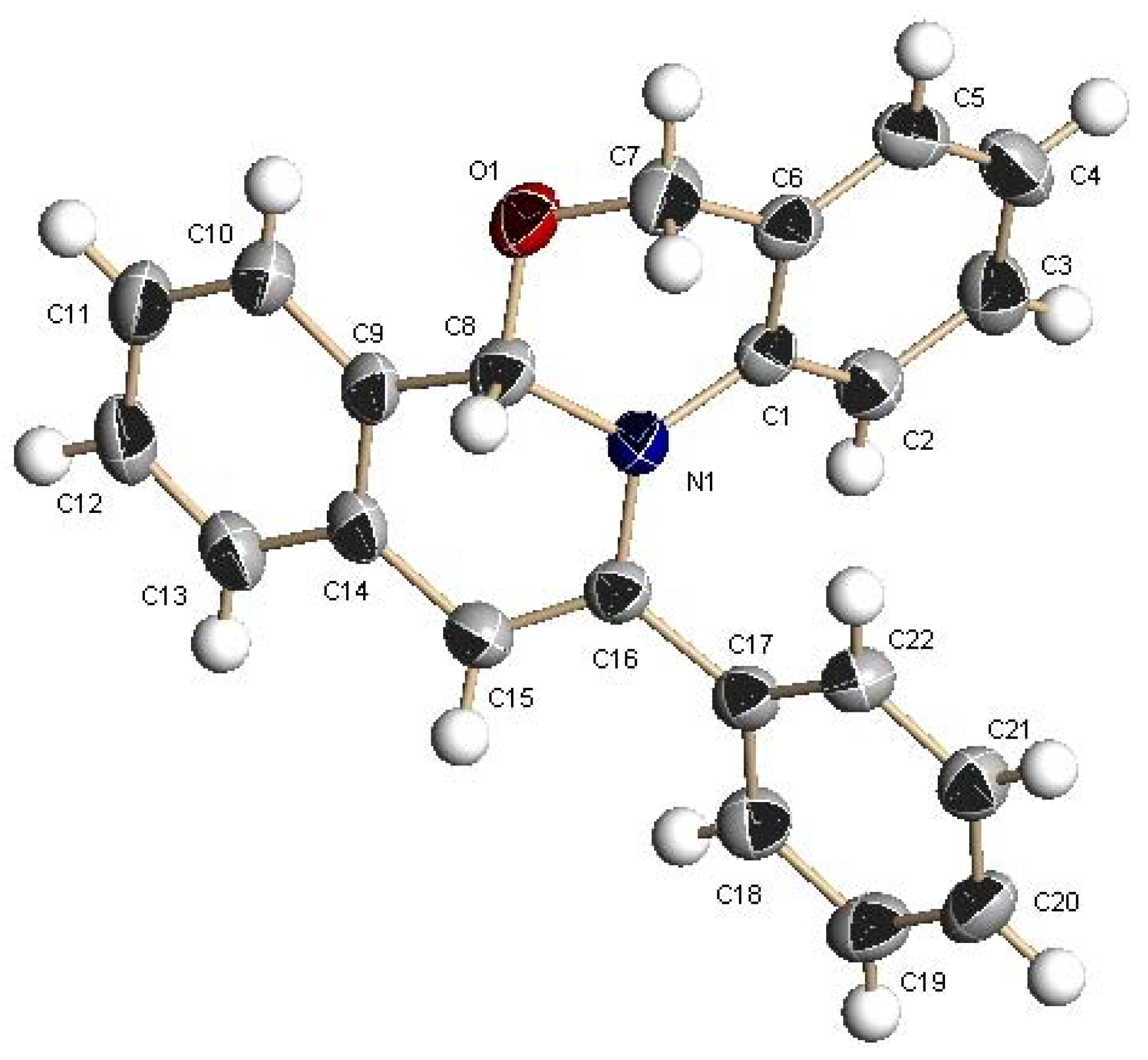
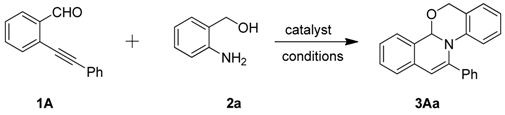
2. Results and Discussion
3. Experimental
3.1. General
3.2. General Procedure for Synthesis of 2-Substituted-ethynyl Benzaldehyde Derivatives 1C–H (1C as an Example)
3.3. General Procedure for Synthesis of Dihydrobenzo [4,5][1,3]oxazino[2,3-a]isoquinolines (3Aa as an Example)
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Parenty, A.D.; Smith, L.V.; Guthrie, K.M.; Long, D.L.; Plumb, J.; Brown, R.; Cronin, L. Highly stable phenanthridinium frameworks as a new class of tunable DNA binding agents with cytotoxic properties. J. Med. Chem. 2005, 48, 4504–4506. [Google Scholar] [CrossRef] [PubMed]
- Weinkauf, R.L.; Chen, A.Y.; Yu, C.; Liu, L.; Barrows, L.; LaVoie, E.J. Antineoplastic activity of benzimidazo[1,2-b]-isoquinolines, indolo[2,3-b]quinolines, and pyridocarbazoles. Bioorg. Med. Chem. 1994, 2, 781–786. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J. Efficient generation of biologically active H-pyrazolo[5,1-a]isoquinolines via multicomponent reaction. Org. Lett. 2010, 12, 4856–4859. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Moriyasu, M.; Tachibana, Y.; Kim, H.S.; Wataya, Y.; Wiegrebe, W.; Bastow, K.F.; Cosentino, L.M.; Kozuka, M.; Lee, K.H. Simple isoquinoline and benzylisoquinoline alkaloids as potential antimicrobial, antimalarial, cytotoxic, and anti-HIV agents. Bioorg. Med. Chem. 2001, 9, 2871–2884. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, M.R.; Rhodes, D.; Hansen, M.S.; Rubins, K.; Bushman, F.D.; Venkateswarlu, Y.; Faulkner, D.J. Lamellarin alpha 20-sulfate, an inhibitor of HIV-1 integrase active against HIV-1 virus in cell culture. J. Med. Chem. 1999, 42, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Petrignet, J.; Boudhar, A.; Blond, G.; Suffert, J. Step-economical synthesis of taxol-like tricycles through a palladium-catalyzed domino reaction. Angew. Chem. Int. Ed. 2011, 50, 3285–3289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Li, C.F.; An, X.L.; Zhang, J.J.; Zhu, X.Y.; Xiao, W.J. Ru-catalyzed tandem cross-metathesis/intramolecular-hydroarylation sequence. Angew. Chem. Int. Ed. 2008, 47, 2489–2492. [Google Scholar] [CrossRef] [PubMed]
- Wasilke, J.C.; Obrey, S.J.; Baker, R.T.; Bazan, G.C. Concurrent tandem catalysis. Chem. Rev. 2005, 105, 1001–1020. [Google Scholar] [CrossRef] [PubMed]
- Velcicky, J.; Soicke, A.; Steiner, R.; Schmalz, H.G. Palladium-catalyzed cyanomethylation of aryl halides through domino Suzuki coupling-isoxazole fragmentation. J. Am. Chem. Soc. 2011, 133, 6948–6951. [Google Scholar] [CrossRef] [PubMed]
- Malakar, C.C.; Schmidt, D.; Conrad, J.; Beifuss, U. Cu(I)-catalyzed domino reactions: efficient and selective synthesis of 4H-chromenes and naphthalenes. Org. Lett. 2011, 13, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, T.; Tsukano, C.; Takemoto, Y. Palladium-catalyzed cascade process consisting of isocyanide insertion and benzylic C(sp3)-H activation: concise synthesis of indole derivatives. Org. Lett. 2012, 14, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Kumar, P.M.; Reddy, M.A.; Ferozuddin, M.; Sreenivasulu, M.; Jafar, A.A.; Krishna, G.R.; Reddy, C.M.; Rambabu, D.; Kumar, K.S.; et al. Yb(OTf)3 catalyzed new cascade reaction: A facile assembly of fused quinazolinones. Chem. Commun. 2011, 47, 10263–10265. [Google Scholar] [CrossRef] [PubMed]
- Gigant, N.; Gillaizeau, I. Palladium(II)-catalyzed direct alkenylation of nonaromatic enamides. Org. Lett. 2012, 14, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Mutyala, A.K.; Konala, A.; Tella, R.B. Tuning the reactivity of Au-complexes in an Au(I)/chiral Bronsted acid cooperative catalytic system: an approach to optically active fused 1,2-dihydroisoquinolines. Chem. Commun. 2012, 48, 3094–3096. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.C.; Tang, R.Y.; Zhong, P.; Zhang, X.G.; Li, J.H. CuI/I2-promoted electrophilic tandem cyclization of 2-ethynylbenzaldehydes with ortho-benzenediamines: Synthesis of iodoisoquinoline-fused benzimidazoles. J. Org. Chem. 2011, 76, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Mutyala, A.K.; Lakshmi, P.G.V.V.; Raju, P.V.K.; Sridhar, B. Facile Assembly of Fused Isoquinolines by Gold(I)-Catalyzed-Coupling Cyclization Reactions between o-Alkynylbenzaldehydes and Aromatic Amines Containing Tethered Nucleophiles. Eur. J. Org. Chem. 2010, 1999–2007. [Google Scholar] [CrossRef]
- Yanada, R.; Hashimoto, K.; Tokizane, R.; Miwa, Y.; Minami, H.; Yanada, K.; Ishikura, M.; Takemoto, Y. Indium(III)-catalyzed tandem reaction with alkynylbenzaldehydes and alkynylanilines to heteroaromatic compounds. J. Org. Chem. 2008, 73, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Yanada, R.; Obika, S.; Kono, H.; Takemoto, Y. In(OTf)3-catalyzed tandem nucleophilic addition and cyclization of ortho-alkynylarylaldimines to 1,2-dihydroisoquinolines. Angew. Chem. Int. Ed. 2006, 45, 3822–3825. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, Y.; Ye, D.; Zhang, D.; Ding, X.; Jiang, H.; Liu, H. Silver-Catalyzed Intramolecular Cyclization of o-(1-Alkynyl)benzamides: Efficient Synthesis of (1H)-Isochromen-1-imines. Adv. Synth. Catal. 2009, 351, 2605–2610. [Google Scholar] [CrossRef]
- Patil, N.T.; Pahadi, N.K.; Yamamoto, Y. Silver(I)-catalyzed novel cascade cyclization reactions: incorporation of allenes into the isochromenes. J. Org. Chem. 2005, 70, 10096–10098. [Google Scholar] [CrossRef] [PubMed]
- Blanc, A.; Tenbrink, K.; Weibel, J.M.; Pale, P. Silver(I)-catalyzed cascade: Direct access to furans from alkynyloxiranes. J. Org. Chem. 2009, 74, 4360–4363. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Porco, J.A., Jr. Synthesis of pyrrolo-isoquinolines related to the lamellarins using silver-catalyzed cycloisomerization/dipolar cycloaddition. J. Am. Chem. Soc. 2007, 129, 7744–7745. [Google Scholar] [CrossRef] [PubMed]
- Weibel, J.M.; Blanc, A.; Pale, P. Ag-mediated reactions: Coupling and heterocyclization reactions. Chem. Rev. 2008, 108, 3149–3173. [Google Scholar] [CrossRef] [PubMed]
- Huple, D.B.; Chen, C.H.; Das, A.; Liu, R.S. Silver-catalyzed exo-dig-azacyclization/[3+2] cycloaddition cascades on 1-tosylhydrazon-4-oxy-5-yne substrates: Applicability to diverse alkenes. Adv. Synth. Catal. 2011, 353, 1877–1882. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Lu, T. Facile synthesis of 1-aminoisoquinolines via the tandem reactions of 2-alkynylbenzaldoximes with isothiocyanates. Tetrahedron 2012, 68, 6843–6848. [Google Scholar] [CrossRef]
- Huang, P.; Yang, Q.; Chen, Z.; Ding, Q.; Xu, J.; Peng, Y. Metal cocatalyzed tandem alkynylative cyclization reaction of in situ formed N-iminoisoquinolinium ylides with bromoalkynes via C–H bond activation. J. Org. Chem. 2012, 77, 8092–8098. [Google Scholar] [CrossRef] [PubMed]
- Rustagi, V.; Aggarwal, T.; Verma, A.K. Highly efficient Ag(I)-catalyzed regioselective tandem synthesis of diversely substituted quinoxalines and benzimidazoles in water. Green Chem. 2011, 13, 1640–1643. [Google Scholar] [CrossRef]
- Rustagi, V.; Tiwari, R.; Verma, A.K. AgI-Catalyzed Cascade Strategy: Regioselective Access to Diversely Substituted Fused Benzimidazo[2,1-a]isoquinolines, Naphthyridines, Thienopyridines, and Quinoxalines in Water. Eur. J. Org. Chem. 2012, 4590–4602. [Google Scholar] [CrossRef]
- Yu, X.X.; Yang, Q.; Lou, H.L.; Peng, Y.Y.; Wu, J. An efficient approach to pyrazolo[5,1-a]isoquinolin-2-amines via a silver(I)-catalyzed three-component reaction of 2-alkynylbenzaldehyde, sulfonohydrazide, and nitrile. Org. Biomol. Chem. 2011, 9, 7033–7037. [Google Scholar] [CrossRef] [PubMed]
- Markina, N.A.; Mancuso, R.; Neuenswander, B.; Lushington, G.H.; Larock, R.C. Solution-Phase Parallel Synthesis of a Diverse Library of 1,2-Dihydroisoquinolines. ACS Comb. Sci. 2011, 13, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.P.; Wu, J. Lewis acid- and organocatalyst-cocatalyzed multicomponent reactions of 2-alkynylbenzaldehydes, amines, and ketones. Org. Lett. 2007, 9, 4959–4962. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.X.; Wu, J. Synthesis of 1-(1H-Indol-3-yl)-1,2-dihydroisoquinolines via AgOTf-Catalyzed Three-Component Reactions of 2-Alkynylbenzaldehydes, Amines, and Indoles. J. Comb. Chem. 2010, 12, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Asao, N.; Yudha, S.S.; Nogami, T.; Yamamoto, Y. Direct Mannich and nitro-Mannich reactions with non-activated imines: AgOTf-catalyzed addition of pronucleophiles to ortho-alkynylaryl aldimines leading to 1,2-dihydroisoquinolines. Angew. Chem. Int. Ed. 2005, 44, 5526–5528. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.N.; Zhou, Y.; Lin, D.Z.; Wang, J.F.; Zhang, L.; Jiang, H.L.; Liu, H. Synthesis of Pyrrolo[1,2-a]quinoxalines via Gold(I)-Mediated Cascade Reactions. ACS Comb. Sci. 2011, 13, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Ji, X.; Zhou, W.; Zhang, X.; Qian, W.; Jiang, H.; Liu, H. Silver- and gold-mediated domino transformation: A strategy for synthesizing benzo[e]indolo[1,2-a]pyrrolo/pyrido[2,1-c][1,4]diazepine-3,9-diones. J. Org. Chem. 2011, 76, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Zhou, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-catalyzed tandem transformation: A simple approach for the synthesis of pyrrolo/pyrido[2,1-a][1,3]benzoxazinones and pyrrolo/pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.G.; Zhou, Y.; Zhao, F.; Chen, X.J.; Zhang, L.; Jiang, H.L.; Liu, H. Gold-catalyzed tandem reaction in water: An efficient and convenient synthesis of fused polycyclic indoles. Green Chem. 2012, 14, 1888–1895. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, E.G.; Liu, G.N.; Ye, D.J.; Li, J.; Jiang, H.L.; Liu, H.; Liu, G. Gold-Catalyzed One-Pot Cascade Construction of Highly Functionalized Pyrrolo[1,2-a]quinolin-1(2H)-ones. J. Org. Chem. 2009, 74, 7344–7348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhai, Y.; Ji, X.; Liu, G.N.; Feng, E.G.; Ye, D.J.; Zhao, L.X.; Jiang, H.L.; Liu, H. Gold(I)-Catalyzed One-Pot Tandem Coupling/Cyclization: An Efficient Synthesis of Pyrrolo-/Pyrido[2,1-b]benzo[d][1,3]oxazin-1-ones. Adv. Synth. Catal. 2010, 352, 373–378. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, X.; Liu, G.N.; Zhang, D.Y.; Zhao, L.X.; Jiang, H.L.; Liu, H. Gold(I)-Catalyzed Cascade for Synthesis of Pyrrolo[1,2-a:2′,1′-c]-/Pyrido[2,1-c]pyrrolo[1,2-a]quinoxalinones. Adv. Synth. Catal. 2010, 352, 1711–1717. [Google Scholar] [CrossRef]
- Waldmann, H.; Eberhardt, L.; Wittstein, K.; Kumar, K. Silver catalyzed cascade synthesis of alkaloid ring systems: Concise total synthesis of fascaplysin, homofascaplysin C and analogues. Chem. Commun. 2010, 46, 4622–4624. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all target compounds are available from the authors. |

| Entry | Catalyst system b | Solvent | Temp (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | AuCl(PPh3) | Toluene | 100 | 75 |
| 2 | AuCl | Toluene | 100 | 49 |
| 3 | Au catalyst I | Toluene | 100 | 58 |
| 4 | Au catalyst II | Toluene | 100 | 28 |
| 5 | AgOOCCF3 | Toluene | 100 | 60 |
| 6 | AgNO3 | Toluene | 100 | 93 |
| 7 | AgBF4 | Toluene | 100 | 57 |
| 8 | AgOTf | Toluene | 100 | 92 |
| 9 | AgNO3 | MeOH | reflux | 77 |
| 10 | AgNO3 | THF | reflux | 60 |
| 11 | AgNO3 | DMF | 100 | 71 |
| 12 | AgNO3 | DMSO | 100 | 92 |
| 13 | AgNO3 | CH3CN | reflux | 87 |
| 14 | AgNO3 | Dioxane | 100 | 83 |
| 15 | AgNO3 | Toluene | 80 | 94 |
| 16 | AgNO3 | DMSO | 80 | 75 |
| 17 | AgNO3 | CH3CN | 80 | 78 |
| 18 | AgNO3 | Dioxane | 80 | 77 |
| 19 | AgNO3 | Toluene | 50 | 65 |
| 20 | AgNO3 | Toluene | RT | 30 |
| 21 | AgNO3 | Toluene | 80 | 94 c |
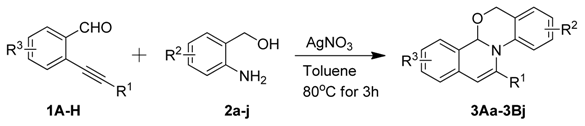
| Entry | Product | Yield (%) | Entry | Product | Yield (%) | ||
|---|---|---|---|---|---|---|---|
| 1 | 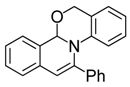 | 3Aa | 94 | 18 | 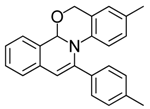 | 3Df | 65 |
| 2 | 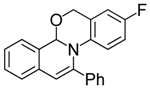 | 3Ab | 92 | 19 | 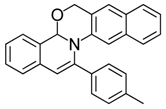 | 3Dh | 70 |
| 3 |  | 3Ac | 85 | 20 |  | 3Ea | 87 |
| 4 |  | 3Ad | 81 | 21 | 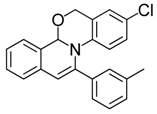 | 3Ec | 85 |
| 5 | 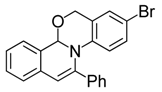 | 3Ae | 77 | 22 | 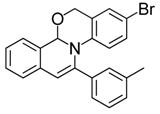 | 3Ee | 68 |
| 6 |  | 3Bb | 85 | 23 | 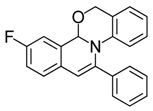 | 3Fa | 90 |
| 7 | 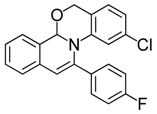 | 3Bd | 82 | 24 | 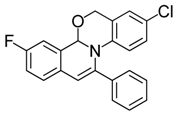 | 3Fc | 70 |
| 8 | 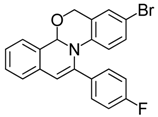 | 3Be | 69 | 25 | 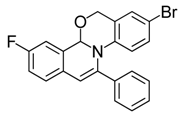 | 3Fe | 85 |
| 9 | 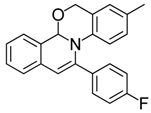 | 3Bf | 68 | 26 |  | 3Ff | 45 |
| 10 | 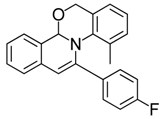 | 3Bg | 65 | 27 |  | 3Ga | 90 |
| 11 | 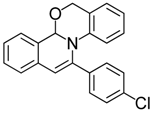 | 3Ca | 88 | 28 | 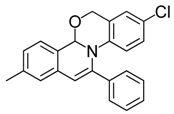 | 3Gc | 77 |
| 12 | 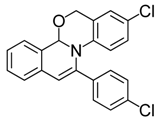 | 3Cc | 82 | 29 | 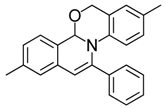 | 3Gf | 85 |
| 13 |  | 3Cd | 80 | 30 |  | 3Gh | 55 |
| 14 | 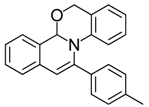 | 3Da | 97 | 31 | 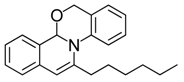 | 3Ha | 82 |
| 15 | 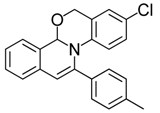 | 3Dc | 76 | 32 | 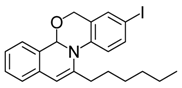 | 3Hi | 80 |
| 16 | 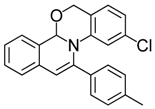 | 3Dd | 80 | 33 | 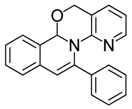 | 3Aj | 47 |
| 17 |  | 3De | 75 | 34 | 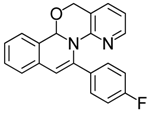 | 3Bj | 45 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, B.; Zhou, Y.; Kong, Q.; Jiang, H.; Liu, H.; Li, J. ‘One-pot’ Synthesis of Dihydrobenzo[4,5][1,3]oxazino[2,3-a] isoquinolines via a Silver(I)-Catalyzed Cascade Approach. Molecules 2013, 18, 814-831. https://doi.org/10.3390/molecules18010814
Jiang B, Zhou Y, Kong Q, Jiang H, Liu H, Li J. ‘One-pot’ Synthesis of Dihydrobenzo[4,5][1,3]oxazino[2,3-a] isoquinolines via a Silver(I)-Catalyzed Cascade Approach. Molecules. 2013; 18(1):814-831. https://doi.org/10.3390/molecules18010814
Chicago/Turabian StyleJiang, Baifeng, Yu Zhou, Qingya Kong, Hualiang Jiang, Hong Liu, and Jian Li. 2013. "‘One-pot’ Synthesis of Dihydrobenzo[4,5][1,3]oxazino[2,3-a] isoquinolines via a Silver(I)-Catalyzed Cascade Approach" Molecules 18, no. 1: 814-831. https://doi.org/10.3390/molecules18010814




