In Vitro Antioxidant Properties of Flavonoids and Polysaccharides Extract from Tobacco (Nicotiana tabacum L.) Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Flavonoids and Polysaccharides from Tobacco Leaves
2.2. Scavenging Ability on Hydroxyl Radicals
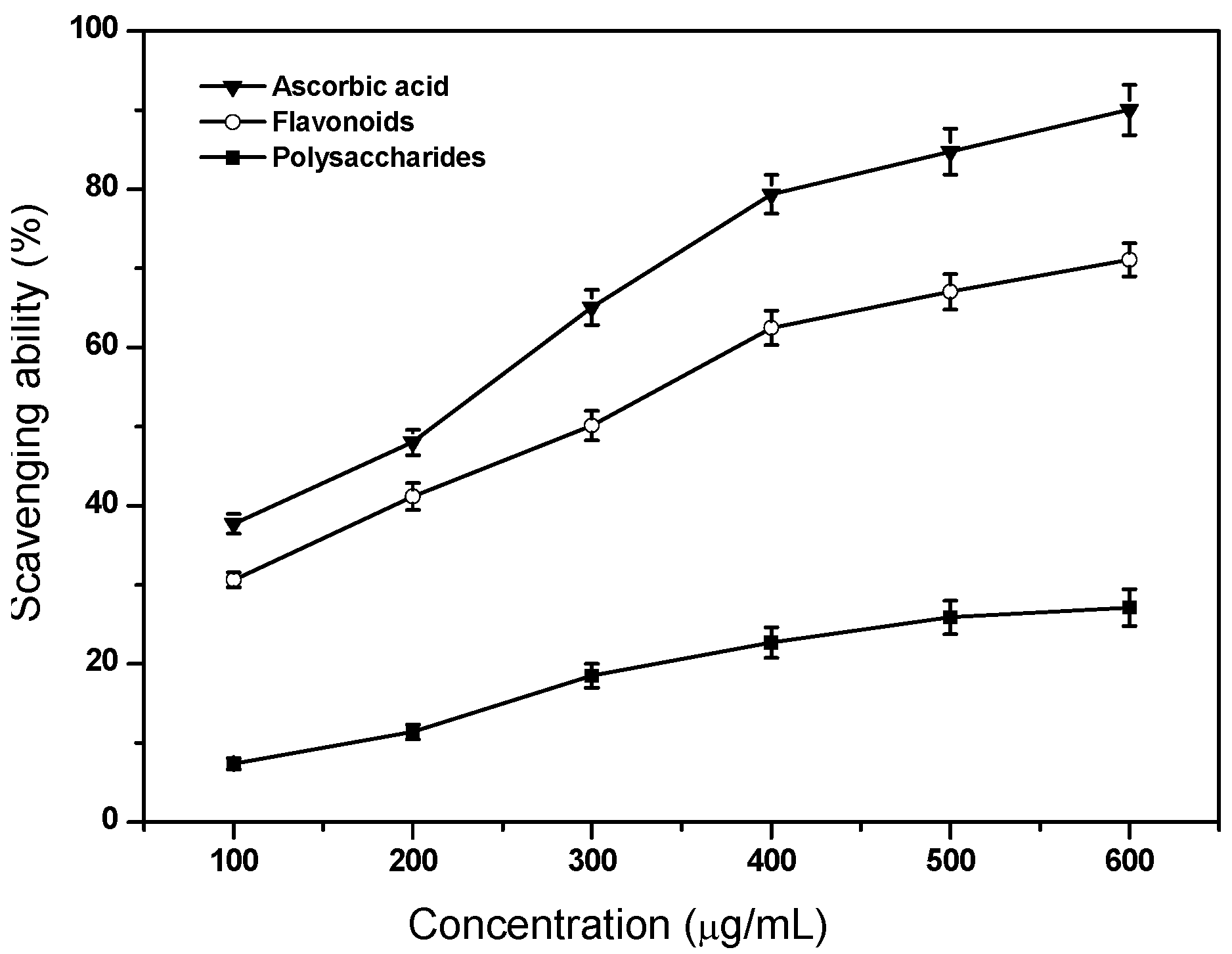
2.3. Scavenging Ability on Superoxide Anion Radicals
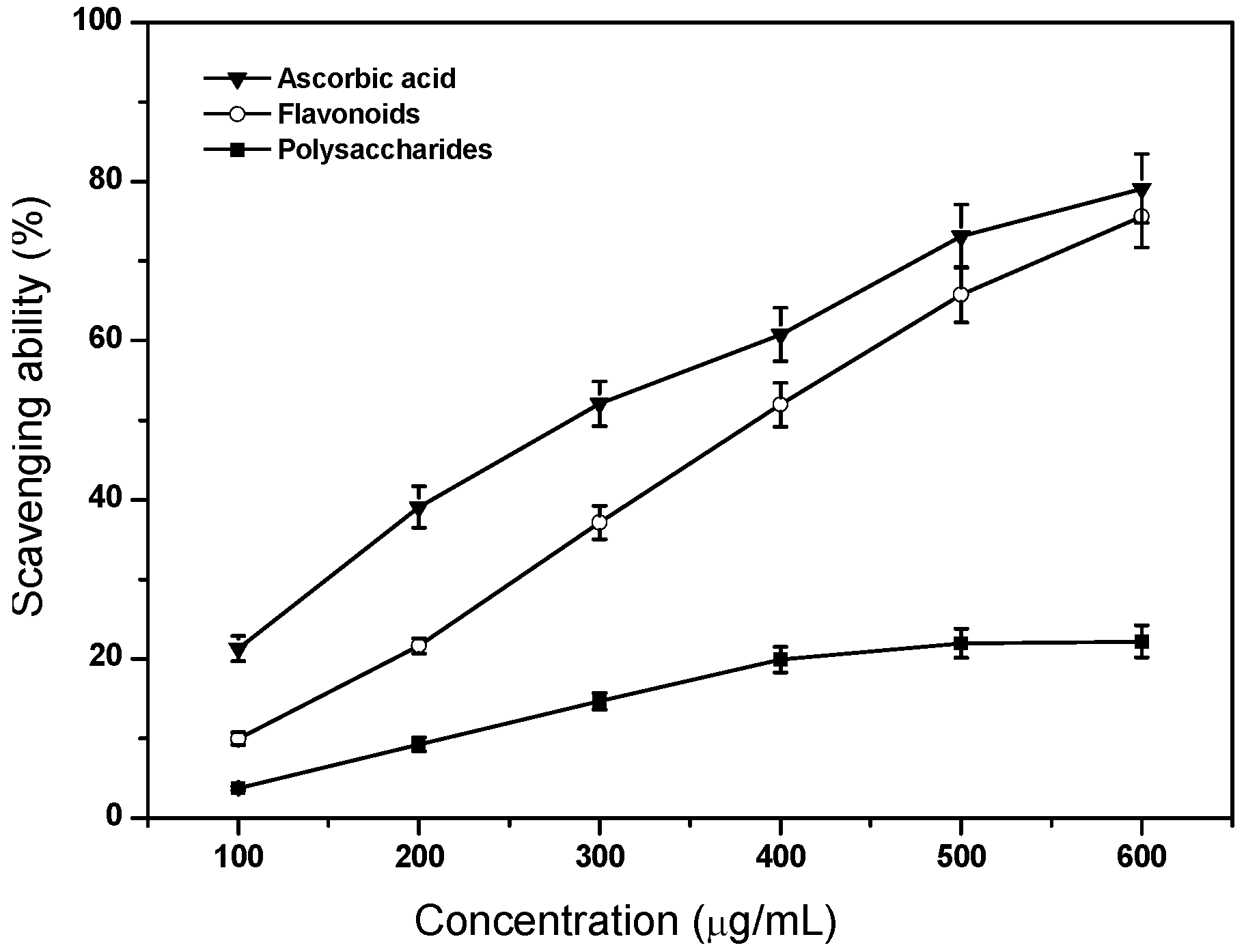
2.4. Scavenging Ability on DPPH Radicals

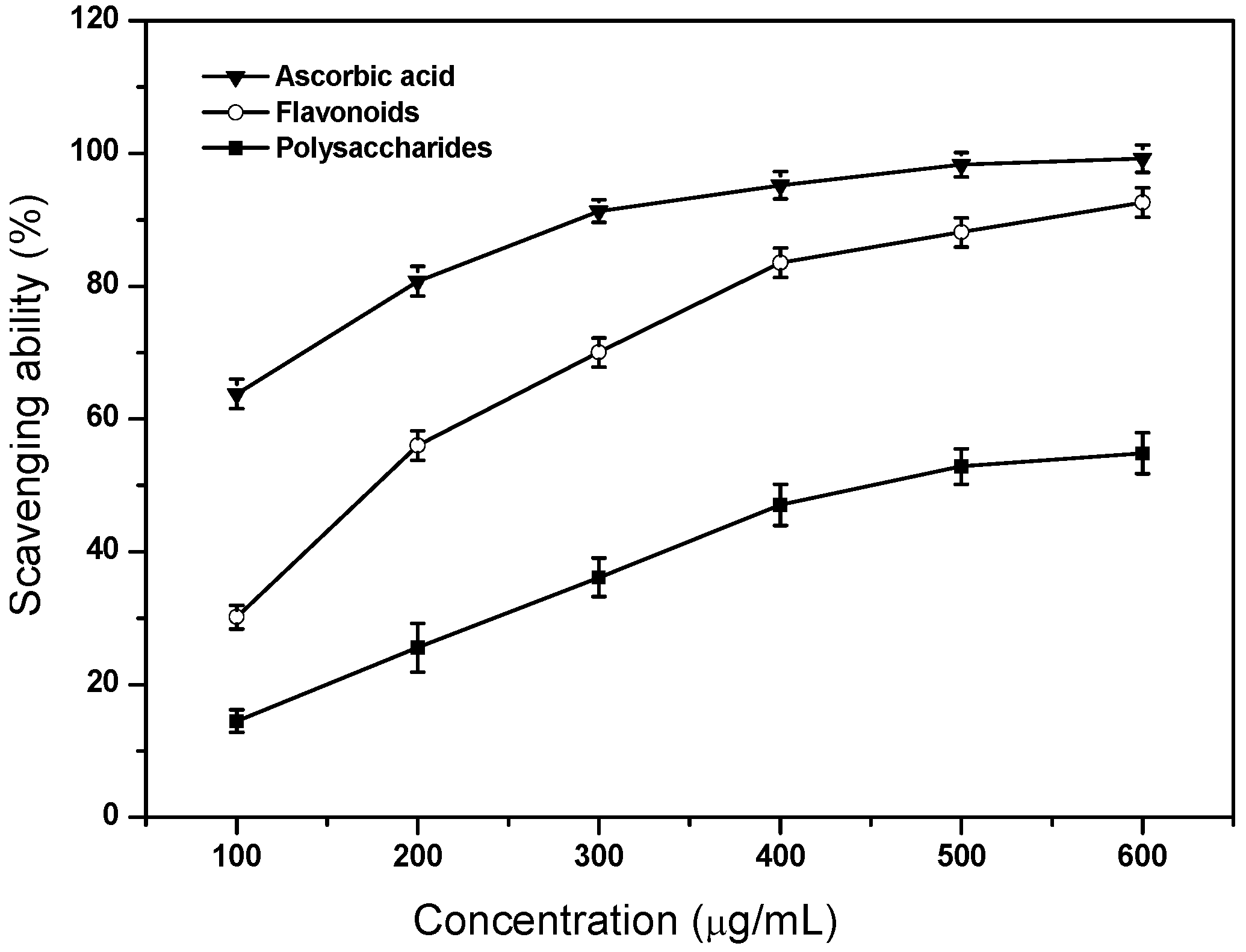
2.5. Scavenging Ability on ABTS Radicals
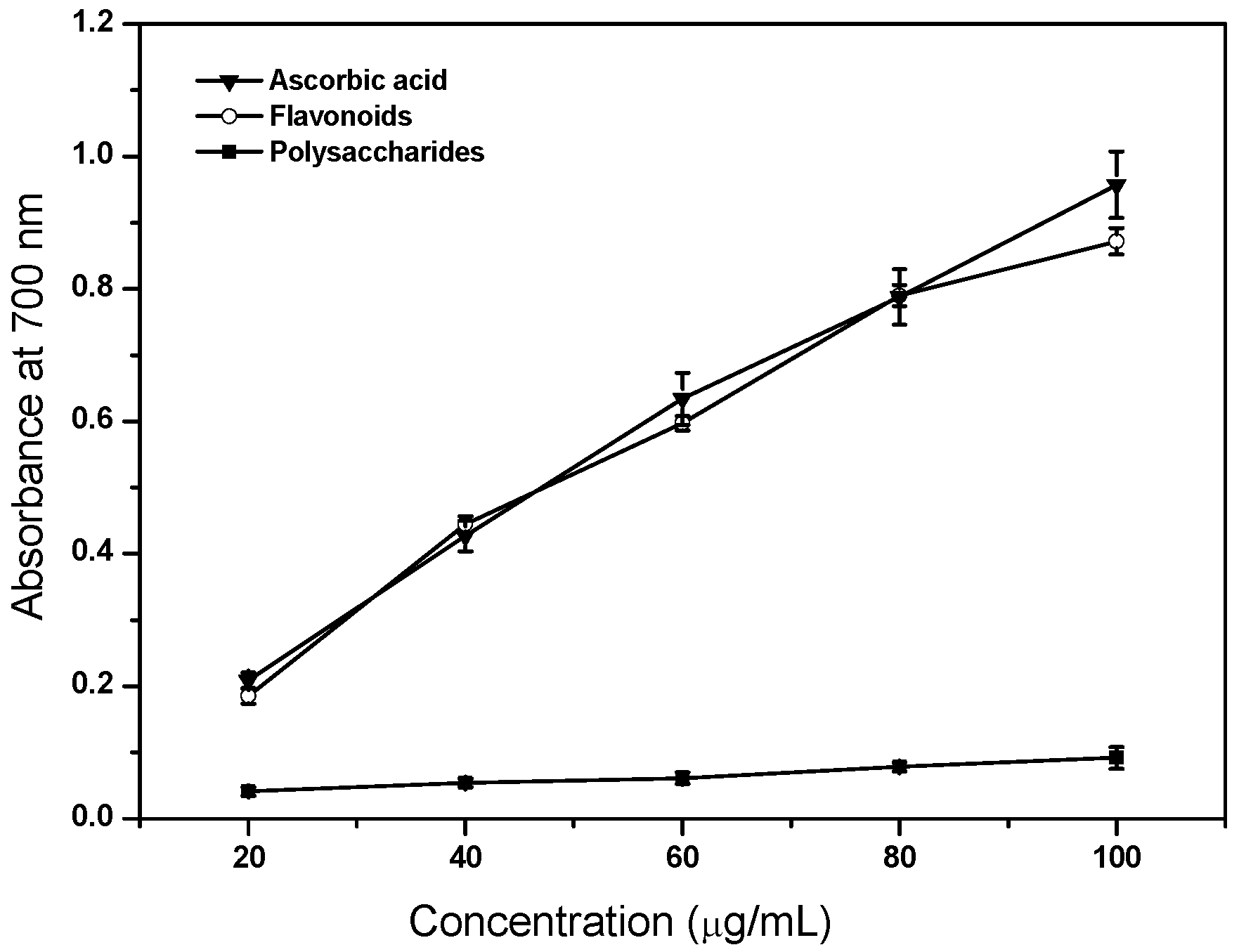
2.6. Reducing Power Ability
2.7. EC50 Values for Antioxidant Properties
| Test | EC50 value (μg/mL) | ||
|---|---|---|---|
| Flavonoids | Polysaccharides | Ascorbic acid | |
| Scavenging ability on hydroxyl radicals | 305 | >600 | 210 |
| Scavenging ability on superoxide radicals | 390 | >600 | 285 |
| Scavenging ability on DPPH radicals | 235 | >600 | >100 |
| Scavenging ability on ABTS radicals | 175 | >600 | >100 |
| Reducing power | 47 | >100 | 47 |
3. Experimental
3.1. Materials and Chemicals
3.2. Isolation of Polysaccharides
3.3. Quantitation of Polysaccharide Content
3.4. Isolation of Flavonoids
3.5. Determination of Flavonoid Content
3.6. Determination of Total Phenolic Content
3.7. Assay of Antioxidant Property
3.7.1. Scavenging Activity on Hydroxyl Radicals
3.7.2. Scavenging Activity on Superoxide Anion Radicals
3.7.3. Scavenging Activity on DPPH Radicals
3.7.4. Scavenging Activity on ABTS Radicals
3.7.5. Reducing Power Ability
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Zhang, X.; Gao, H.; Zhang, L.; Liu, D.; Ye, X. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Ind. Crop Prod. 2012, 39, 162–169. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Bretones, G.; Baghour, M.; Ragala, L.; Belakbir, A.; Romero, L. Relationship between boron and phenolic metabolism in tobacco leaves. Phytochemistry 1998, 48, 269–272. [Google Scholar]
- Teng, Z.; Wang, Q. Extraction, identification and characterization of the water-insoluble proteins from tobacco biomass. J. Sci. Food Agric. 2012, 92, 1368–1374. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Han, X.-Q.; Wu, X.-M.; Chai, X.-Y.; Chen, D.; Dai, H.; Dong, H.-L.; Ma, Z.-Z.; Gao, X.-M.; Tu, P.-F. Isolation, characterization and immunological activity of a polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk.) S. Ito. Food Res. Int. 2011, 44, 489–493. [Google Scholar]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Kao, T.-H.; Chen, B.-H. Functional components in soybean cake and their effects on antioxidant activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; van den Berg, D.; Tromp, M.N.J.L.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dziri, S.; Hassen, I.; Fatnassi, S.; Mrabet, Y.; Casabianca, H.; Hanchi, B.; Hosni, K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J. Funct. Foods 2012, 4, 423–432. [Google Scholar]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Asker, M.M.S.; Mahmoud, M.G.; Ibrahim, G.S. Structural characterization and biological activity of acidic polysaccharide fractions isolated from Bacillus polymyxa NRC-A. J. Appl. Sci. Res. 2007, 3, 1170–1177. [Google Scholar]
- Oszmianski, J.; Wolniak, M.; Wojdylo, A.; Wawer, I. Comparative study of polyphenolic content and antiradical activity of cloudy and clear apple juices. J. Sci. Food Agric. 2007, 87, 573–579. [Google Scholar] [CrossRef]
- Zha, X.-Q.; Wang, J.-H.; Yang, X.-F.; Liang, H.; Zhao, L.-L.; Bao, S.-H.; Luo, J.-P.; Xu, Y.-Y.; Zhou, B.-B. Antioxidant properties of polysaccharide fractions with different molecular mass extracted with hot-water from rice bran. Carbohyd. Polym. 2009, 78, 570–575. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of sweet potato (Ipomoea batatas L.) leaves flavonoid. Food Bioprod. Process. 2012, in press. [Google Scholar]
- He, J.-Z.; Ru, Q.-M.; Dong, D.-D.; Sun, P.-L. Chemical characteristics and antioxidant properties of crude water soluble polysaccharides from four common edible mushrooms. Molecules 2012, 17, 4373–4387. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Fu, L.; Xu, M.; Sun, P.-L.; Zhang, J.-S. Structure of a water-soluble heteropolysaccharide from fruiting bodies of Hericium erinaceus. Carbohyd. Polym. 2012, 88, 558–561. [Google Scholar] [CrossRef]
- Navarini, L.; Gilli, R.; Gombac, V.; Abatangelo, A.; Bosco, M.; Toffanin, R. Polysaccharides from hot water extracts of roasted Coffea arabica beans: Isolation and characterization. Carbohyd. Polym. 1999, 40, 71–81. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities In Vitro. Innov. Food Sci. Emerg. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Yi, Z.; Yu, Y.; Liang, Y.; Zeng, B. In vitro antioxidant and antimicrobial activities of the extract of Pericarpium Citri Reticulatae of a new Citrus cultivar and its main flavonoids. LWT-Food Sci. Technol. 2008, 41, 597–603. [Google Scholar]
- Li, J.-E.; Nie, S.-P.; Qiu, Z.-H.; Che, M.-J.; Li, C.; Xie, M.-Y. Antimicrobial and antioxidant activities of the essential oil from Herba Moslae. J. Sci. Food Agric. 2010, 90, 1347–1352. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant Principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Fellegrini, N.; Ke, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2'-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. Method. Enzymol. 1999, 299, 379–389. [Google Scholar]
- Dorman, H.J.D.; Hiltunen, R. Fe(III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions. Food Chem. 2004, 88, 193–199. [Google Scholar]
- Sample Availability: Samples of the compounds (Flavonoids and Polysaccharides) are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ru, Q.-M.; Wang, L.-J.; Li, W.-M.; Wang, J.-L.; Ding, Y.-T. In Vitro Antioxidant Properties of Flavonoids and Polysaccharides Extract from Tobacco (Nicotiana tabacum L.) Leaves. Molecules 2012, 17, 11281-11291. https://doi.org/10.3390/molecules170911281
Ru Q-M, Wang L-J, Li W-M, Wang J-L, Ding Y-T. In Vitro Antioxidant Properties of Flavonoids and Polysaccharides Extract from Tobacco (Nicotiana tabacum L.) Leaves. Molecules. 2012; 17(9):11281-11291. https://doi.org/10.3390/molecules170911281
Chicago/Turabian StyleRu, Qiao-Mei, Li-Juan Wang, Wei-Ming Li, Jing-Lu Wang, and Yu-Ting Ding. 2012. "In Vitro Antioxidant Properties of Flavonoids and Polysaccharides Extract from Tobacco (Nicotiana tabacum L.) Leaves" Molecules 17, no. 9: 11281-11291. https://doi.org/10.3390/molecules170911281




