A 3-D Metal-Organic Framework Constructed with Manganese(ΙΙ), 4,4'-Oxybis(benzoic acid) and 2,2'-Biphenyl: Synthesis, Crystal Structure and Photoelectric Property Characterization
Abstract
:1. Introduction
2. Results and Discussion
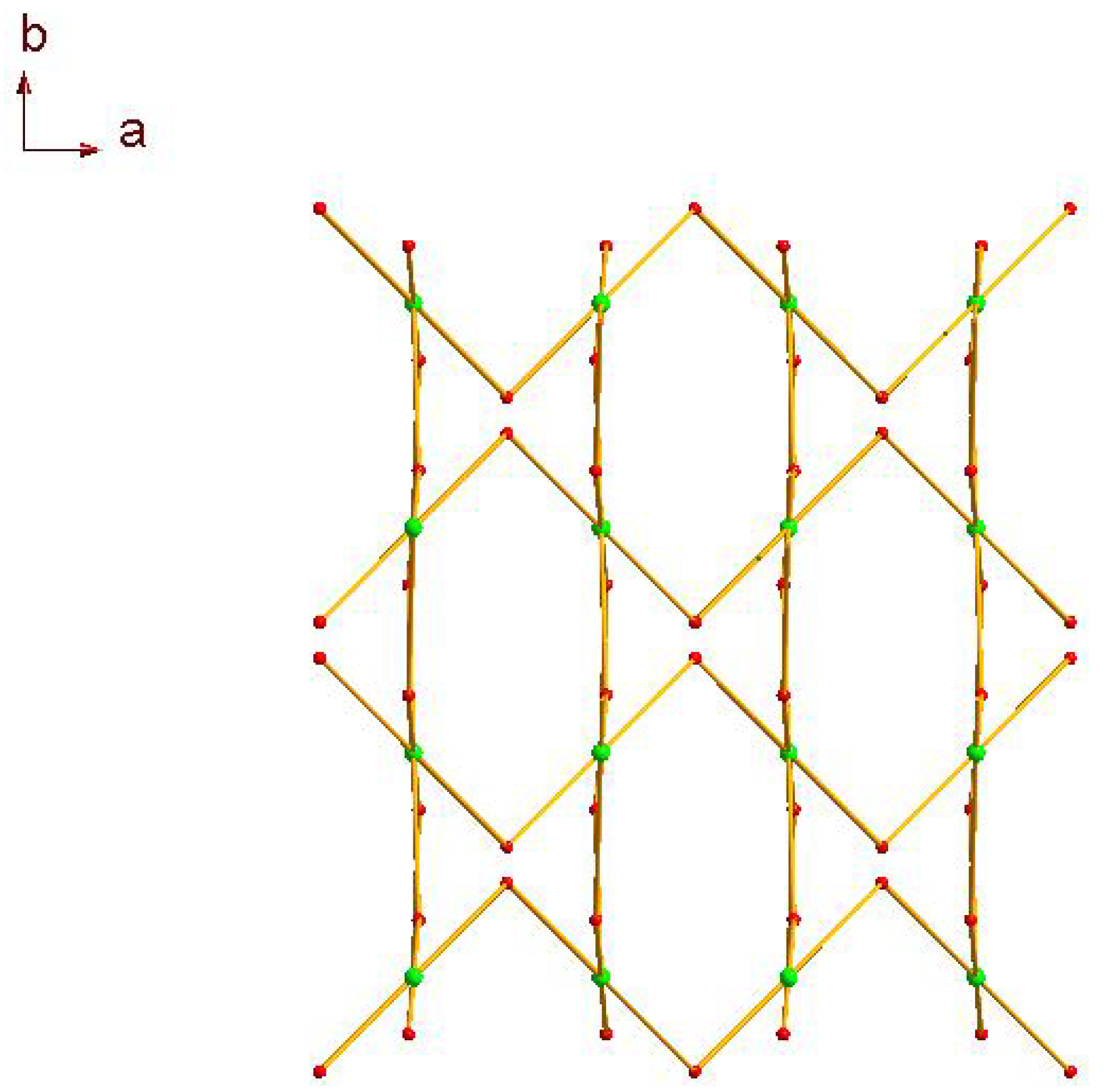
2.1. Crystal Structure Descriptions
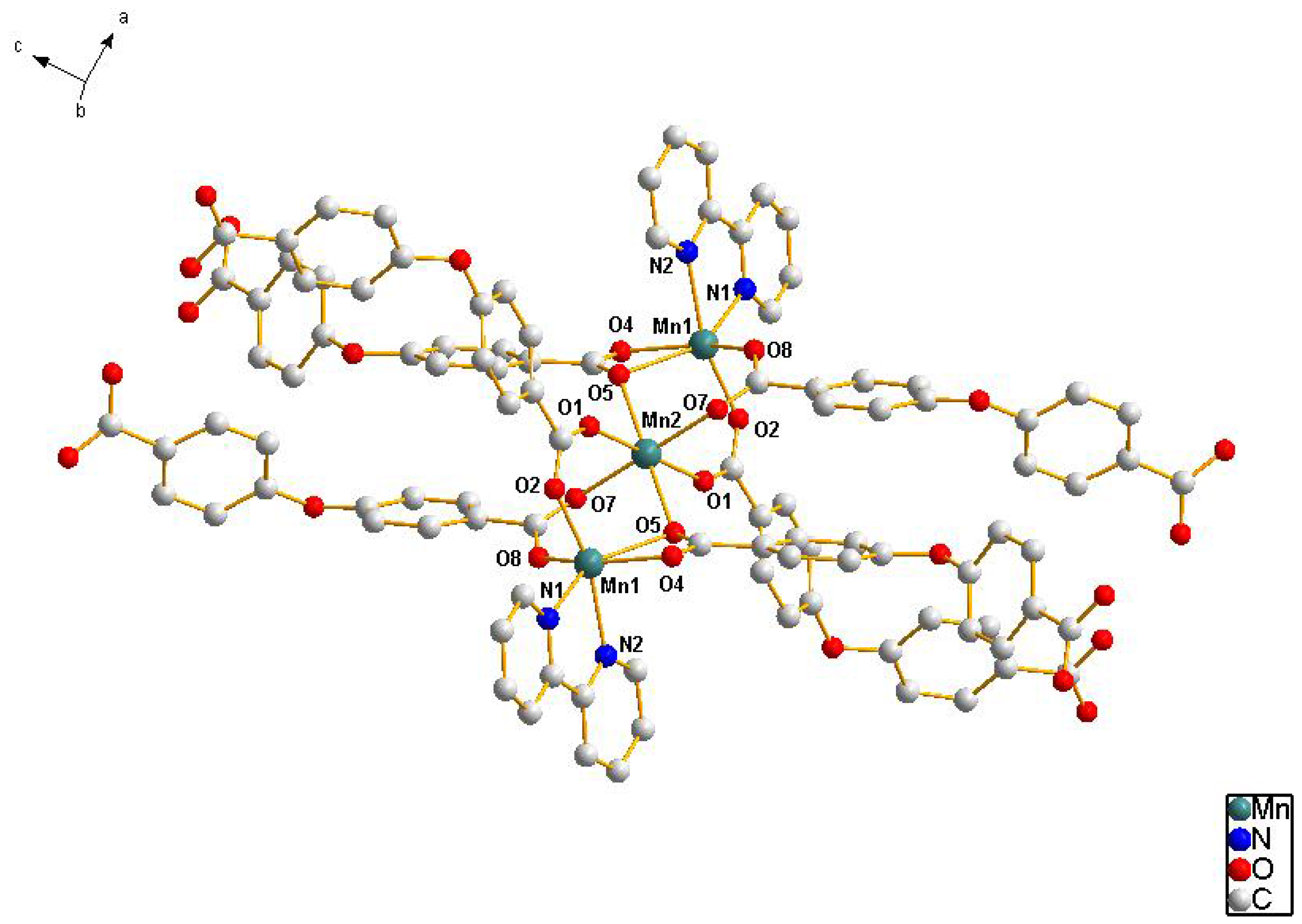
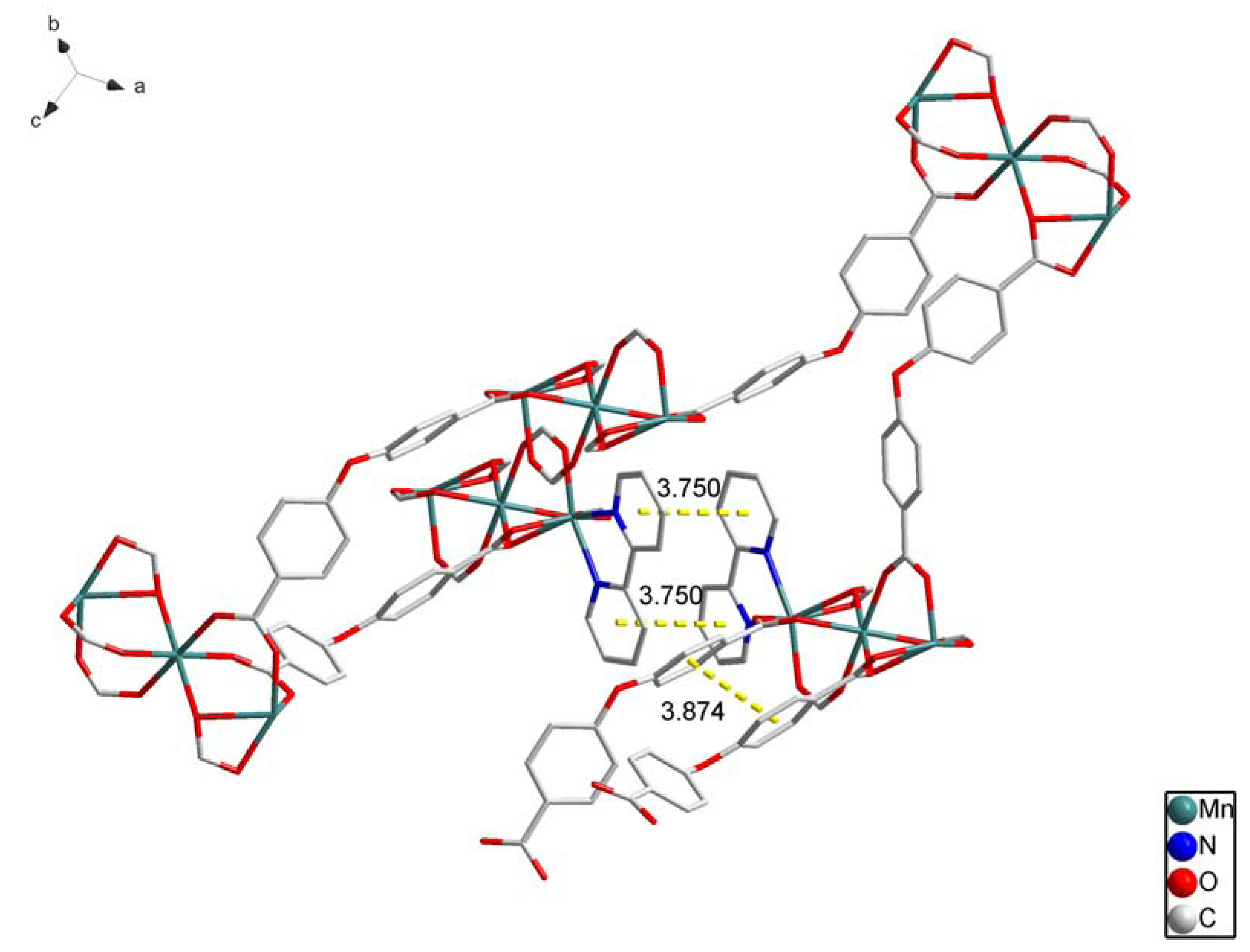
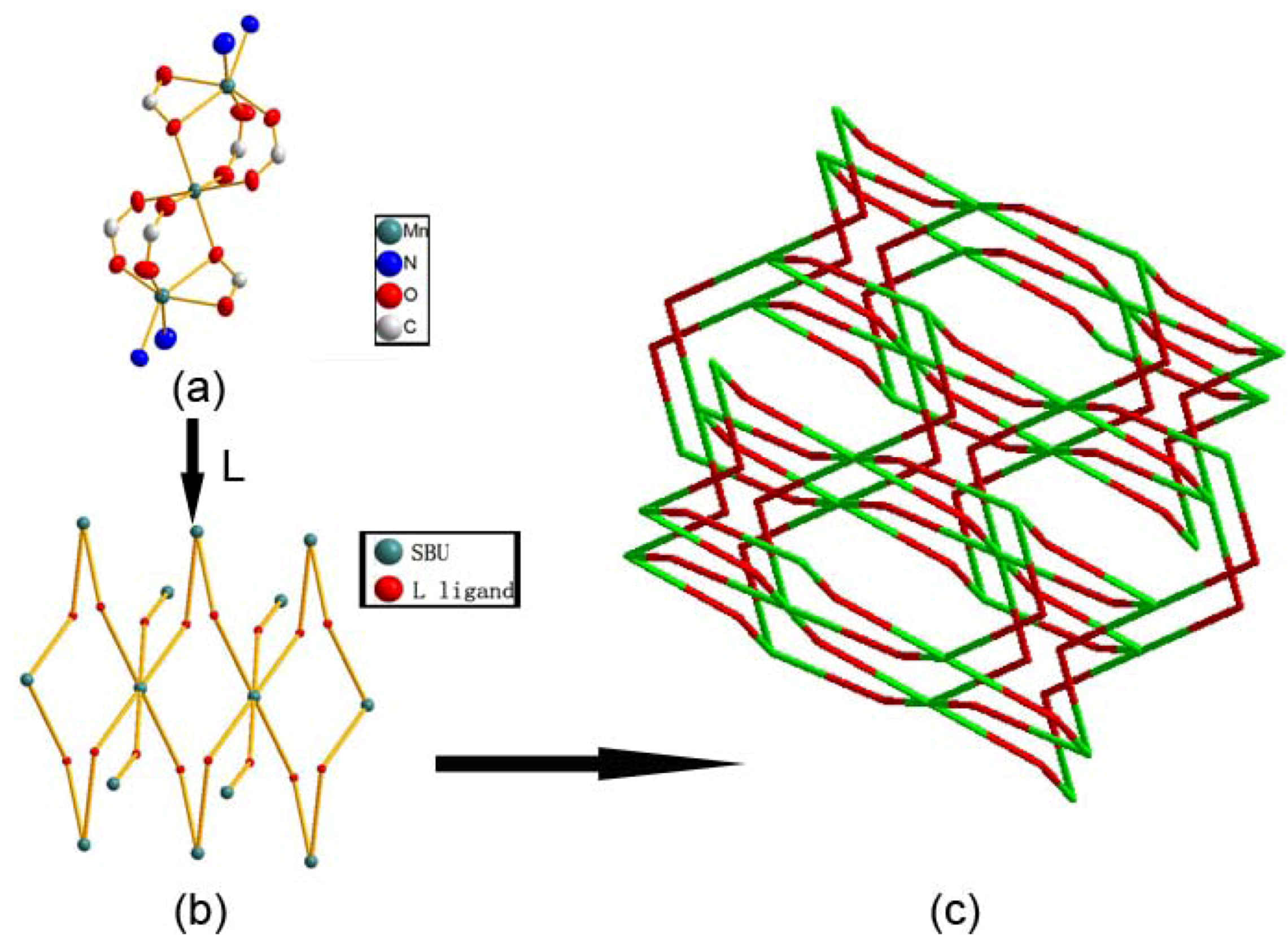

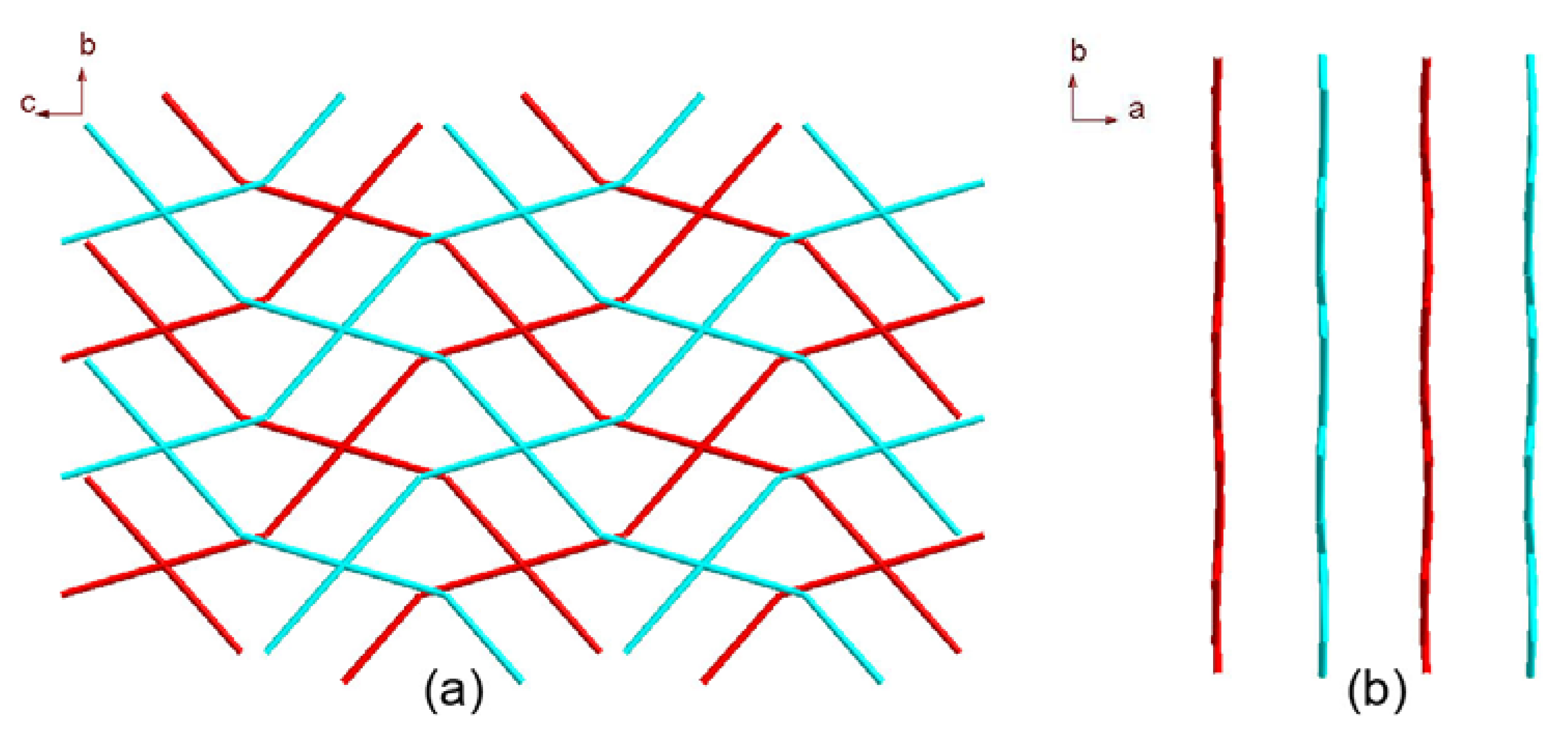

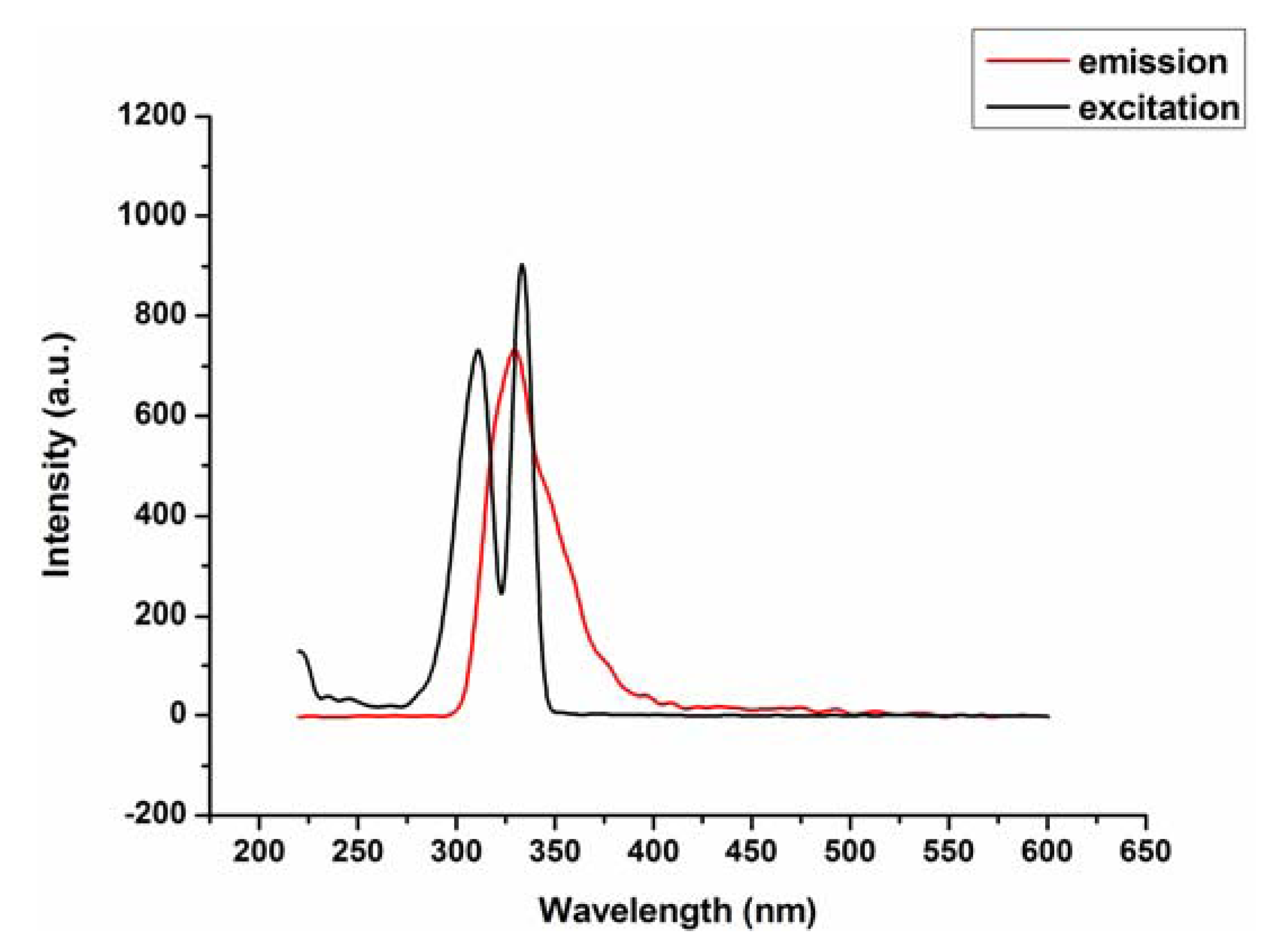
2.2. Fluorescence Emission Properties
2.3. Electrochemical Properties

3. Experimental
3.1. Materials and Instrumentation
3.2. Synthesis of (3-D) Coordination Framework 1
3.3. X-ray Structure Determination
| Empirical formula | C62H40Mn3N4O15 |
|---|---|
| Temperature (K) | 1245.80 |
| Wavelength (Å) | 296(2) |
| Crystal system | 0.71073 |
| space group | Monoclinic |
| a (Å) | C2/c |
| b (Å) | 14.230(4) |
| c (Å) | 17.019(2) |
| α (°) | 25.805(3) |
| β (°) | 90 |
| γ (°) | 92.932(2) |
| V (Å3) | 90 |
| Z | 6241.5(9) |
| Dc (Mg/m3) | 4 |
| µ (mm−1) | 1.326 |
| F (000) | 0.664 |
| Crystal size (mm) | 2540 |
| θ range | 0.35 × 0.34 × 0.32 |
| Reflections collected | 1.87–25.00 |
| Independent reflections | 5493 |
| Completeness to θ = 25.00 | 5493 [R(int) = 0.0012] |
| Absorption correction | 0.999 |
| Max. and min. transmission | Multi-scan |
| Data/restraints/parameters | 0.8156 and 0.8008 |
| Goodness-of-fit on F | 5493/0/381 |
| R indices [I > 2σ(I)] | 1.07 |
| R indices (all data) | R1 = 0.0288, wR2 = 0.0732 |
| Mn(1)-O(8) 2.0740(13) | O(1)#3-Mn(2)-O(5)#2 93.51(5) |
|---|---|
| Mn(1)-O(2)#1 2.0820(13) | O(5)-Mn(2)-O(5)#2 180.0 |
| Mn(1)-O(4) 2.1794(13) | O(4)-Mn(1)-N(2) 90.99(6) |
| Mn(1)-N(1) 2.2612(15) | N(1)-Mn(1)-N(2) 71.66(6) |
| Mn(1)-N(2) 2.2625(17) | O(8)-Mn(1)-O(5) 93.82(5) |
| Mn(1)-O(5) 2.4032(12) | O(2)#1-Mn(1)-O(5) 105.45(5) |
| Mn(2)-O(7) 2.1782(12) | O(4)-Mn(1)-O(5) 56.89(4) |
| Mn(2)-O(7)#2 2.1782(12) | N(1)-Mn(1)-O(5) 148.94(5) |
| Mn(2)-O(1)#1 2.1801(12) | N(2)-Mn(1)-O(5) 91.70(6) |
| Mn(2)-O(1)#3 2.1801(12) | O(7)-Mn(2)-O(7)#2 180.00(6) |
| Mn(2)-O(5)#2.2451(12) | O(7)-Mn(2)-O(1)#1 90.51(5) |
| Mn(2)-O(5)#2 2.2451(12) | O(7)#2-Mn(2)-O(1)#1 89.49(5) |
| O(1)-Mn(2)#4 2.1801(12) | O(7)-Mn(2)-O(1)#3 89.49(5) |
| O(2)-Mn(1)#5 2.0821(13) | O(7)#2-Mn(2)-O(1)#3 90.51(5) |
| O(8)-Mn(1)-O(2)#1 92.42(6) | O(1)#1-Mn(2)-O(1)#3 180.0 |
| O(8)-Mn(1)-O(4) 150.68(5) | O(7)-Mn(2)-O(5) 87.41(5) |
| O(2)#1-Mn(1)-O(4) 96.77(6) | O(7)#2-Mn(2)-O(5) 92.59(5) |
| O(8)-Mn(1)-N(1) 111.11(6) | O(1)#1-Mn(2)-O(5) 93.51(5) |
| O(2)#1-Mn(1)-N(1) 92.13(5) | O(1)#3-Mn(2)-O(5) 86.49(5) |
| O(4)-Mn(1)-N(1) 96.35(5) | O(7)-Mn(2)-O(5)#2 92.59(5) |
| O(8)-Mn(1)-N(2) 88.19(6) | O(7)#2-Mn(2)-O(5)#2 87.41(5) |
| O(2)#1-Mn(1)-N(2) 162.76(6) | O(1)#1-Mn(2)-O(5)#2 86.49(5) |
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Pan, L.; Sander, M.B.; Huang, X.; Li, J.; Smith, M.; Bittner, E.; Bockrath, B.; Johnson, J.K. Microporous metal organic materials: Promising candidates as sorbents for hydrogen storage. J. Am. Chem. Soc. 2004, 126, 1308–1309. [Google Scholar]
- Batten, S.R.; Robson, R. Interpenetrating nets: Ordered, periodic entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Bu, X.H.; Tong, M.L.; Chang, H.C.; Kitagawa, S.; Batten, S.R. A neutral 3d copper coordination polymer showing 1D open channels and the first interpenetrating NbO-Type network. Angew. Chem. Int. Ed. 2004, 43, 192–195. [Google Scholar] [CrossRef]
- Tong, X.L.; Hu, T.L.; Zhao, J.P.; Wang, Y.K.; Zhang, H.; Bu, X.H. Chiral magnetic metal-organic frameworks of MnII with achiral tetrazolate-based ligands by spontaneous resolution. Chem. Commun. 2010, 46, 8543–8545. [Google Scholar]
- Bu, X.H.; Tong, M.L.; Xie, Y.B.; Li, J.R.; Chang, H.C.; Kitagawa, S.; Ribas, J. Synthesis, structures, and magnetic properties of the Copper(II), Cobalt(II), and Manganese(II) complexes with 9-Acridinecarboxylate and 4-Quinolinecarboxylate Ligands. Inorg. Chem. 2005, 44, 9837–9846. [Google Scholar] [CrossRef]
- Liu, F.C.; Zeng, Y.F.; Zhao, J.P.; Hu, B.W.; Bu, X.H.; Ribas, J.; Cano, J. An unusual 1D manganese Azido complex with novel EO/EO/EO/EE coordination mode: Synthesis, structure, and magnetic properties. Inorg. Chem. 2007, 46, 1520–1522. [Google Scholar]
- Zhao, J.P.; Hu, B.W.; Yang, Q.; Hu, T.L.; Bu, X.H. Single-Crystal-to-Single-Crystal transformation in unusual three-dimensional Manganese(II) frameworks exhibiting unprecedented topology and homospin ferrimagnet. Inorg. Chem. 2009, 48, 7111–7116. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.P.; Hu, B.W.; Zhang, X.F.; Bu, X.H. New Manganese(II) azido coordination polymers with nicotinic/isonicotinic acids as coligands: Synthesis, structure, and magnetic properties. Inorg. Chem. 2010, 49, 3746–3751. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, M.N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar]
- Moulton, B.; Zaworotko, M. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar]
- Wang, X.L.; Qin, C.; Wang, E.B.; Xu, L.; Su, Z.M.; Hu, C.W. Interlocked and interdigitated architectures from self-assembly of long flexible ligands and cadmium salts. Angew. Chem. Int. Ed. 2004, 43, 5036–5040. [Google Scholar] [CrossRef]
- Lu, Y.J. Crystal engineering of Cu-containing metal-organic coordination polymers under hydrothermal conditions. Coord. Chem. Rev. 2003, 246, 327–347. [Google Scholar]
- Mukherjee, P.S.; Dalai, S.; Mostafa, G.; Zangrando, E.T.; Lu, H.T.; Rogez, G.; Mallah, T.; Chaudhuri, N.R. A three component fully interlocked 3-D network: Crystal structure and magnetic properties. Chem. Commun. 2001, 1346–1347. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Crystallogr. 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL97, Program for Refining Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Diao, K.-S.; Li, L.; Ding, Y.-Q.; Lei, F.-H. A 3-D Metal-Organic Framework Constructed with Manganese(ΙΙ), 4,4'-Oxybis(benzoic acid) and 2,2'-Biphenyl: Synthesis, Crystal Structure and Photoelectric Property Characterization. Molecules 2012, 17, 11103-11112. https://doi.org/10.3390/molecules170911103
Diao K-S, Li L, Ding Y-Q, Lei F-H. A 3-D Metal-Organic Framework Constructed with Manganese(ΙΙ), 4,4'-Oxybis(benzoic acid) and 2,2'-Biphenyl: Synthesis, Crystal Structure and Photoelectric Property Characterization. Molecules. 2012; 17(9):11103-11112. https://doi.org/10.3390/molecules170911103
Chicago/Turabian StyleDiao, Kai-Sheng, Long Li, Yu-Qiu Ding, and Fu-Hou Lei. 2012. "A 3-D Metal-Organic Framework Constructed with Manganese(ΙΙ), 4,4'-Oxybis(benzoic acid) and 2,2'-Biphenyl: Synthesis, Crystal Structure and Photoelectric Property Characterization" Molecules 17, no. 9: 11103-11112. https://doi.org/10.3390/molecules170911103




