3.2. Syntheses
8-Amino-7-cyano-1-oxo-6-aryl-3-phenyl-1,2-dihydroisoquinolines (2a,b). A solution of compound 1 (10 mmol) in pyridine (30 mL) was treated with an arylidene malononitrile (10 mmol). The reaction mixture was refluxed for 6 h, left to cool to room temperature, poured into ice-cold water, and neutralized with HCl (10%). The solid product was filtered off and recrystallized from ethanol. 2a: Yield 60%; m.p. > 360 °C; IR cm–1: 3434, 3329 (NH2) 3169 (NH), 3050 (CH–Ar), 2221 (C≡N), 1672 (C=O); 1H-NMR: δ 4.22 (s, 2H, NH2), 6.80 (s, 1H, pyridine-H) 7.22–7.72 (m, 9H, Ar–H), 9.80 (s, 1H, NH); 13C-NMR: δ 115.00 (C≡N), 188.00 (C=O), 111.00–142.90 (Ar–C); MS m/z (%): 327 (M+, 100). Anal. For: C20H13N3O2: Calcd. C, 73.39; H, 3.97; N, 12.84 Found: C, 73.40, H, 3.88; N, 13.00. 2b: Yield 62%; m.p. 280 °C; IR cm–1: 3430, 3333 (NH2) 3199 (NH), 3050 (CH–Ar), 2215(CN), 1666 (C=O); 1H-NMR: 4.82 (s, 2H, NH2), 6.64 (s, 1H, pyridine-H-3) 7.88 (d, 2H, Ar–H, J = 7.00 Hz) 9.12 (d, 2H, Ar–H, J = 8.22 Hz), 7.64 (m, 6H, Ar–H), 9.30 (s, 1H, NH); 13C-NMR: δ 118.00 (C≡N), 188.00 (C=O), 101.00-139.63 (Ar-C); MS m/z (%): 382 (M+, 8.11), (77, 100). Anal. For: C22H14N4O3: Calcd. C, 69.10; H, 3.66; N, 14.65; Found: C, 69.30, H, 3.80; N, 14.80.
6-Chloro-4-methyl-2-phenyl-5-pyridinecarbonitrile (3). A mixture of 1 (116 mmol) and phosphorus oxychloride (55.9 mL, 600 mmol) in the presence of triethylamine (3 mL) was stirred at 105 °C for 3 h. The mixture was poured onto crushed ice (800 g) and neutralized with 10% sodium hydroxide. The extract was washed with water 5% aqueous sodium bicarbonate and again with water and recrystallized from ethanol. 3: Yield 72%, m.p. 180–182 °C, IR cm–1: 3033 (CH–Ar), 2999 (CH–aliph.), 2222 (C≡N), 1644 (C=N); 1H-NMR: δ 2.44 (s, 3H, CH3), 6.80 (s, 1H, pyridine-H-3), 7.22–7.48 (m, 5H, Ar–H); 13C-NMR: δ 116.22 (C≡N), 128.34–148.00 (Ar–C); MS m/z (%): 228.5 (3.28), 230.5 (1.11), 57 (100). Anal. For: C13H9ClN2: Calcd. C, 68.27, H, 3.93, N, 12.25, Cl, 15.53. Found. C, 68.30; H, 2.00; N, 12.50; Cl, 15.98.
6-Amino-4-methyl-2-phenyl-5-pyridinecarbonitrile (4). Method A: A mixture of compound 1 (0.005 mL) and ammonium acetate (0.005 mol) in pyridine (30 mL) was refluxed for 8 h. The reaction mixture was cooled at room temperature, poured into ice water, and neutralized with dilute HCl. The isolated solid was filtered off and recrystallized from ethanol to afford 4: Yield 75%; m.p. 250–252 °C; IR cm–1: 3350, 3292 (NH2), 3100 (CH–Ar), 2900 (CH–aliph.), 2220 (C≡N), 1644 (C=N); 1H-NMR: δ 2.48 (s, 3H, CH3), 4.44 (s, 2H, NH2), 6.80 (s, 1H, pyridine-H-3), 7.22–7.68 (m, 5H, Ar–H); 13C-NMR: δ 115.72 (C≡N), 122.42–152.23 (Ar–C); MS m/z (%): 209 (M+, 3.18), 75 (100). Anal. For: C13H11N3: Calcd. C, 74.64; H, 5.26; N, 20.09; Found: C, 74.71, H, 5.22, N, 20.38. Method B: To a solution of compound 3 (9.58 mmL) in DMF (25 mL) was added NH3 (50 mL, 25% in water) and the resulting mixture was stirred at room temperature for 12 h. The mixture was extracted with ethylacetate (3 × 50 mL) and the combined organic layer was dried over Na2SO4, filtered and concentrated under vacuum and the resulting solid was recrystallized from ethanol to afford 4 in a low yield.
6-Hydrazide-4-methyl-2-phenyl-5-pyridinecarbonitrile (5). A mixture of compound 3 (0.01 mmol) and hydrazine hydrate (98%, 0.01 mmol) in ethanol (20 mL) was heated under reflux for 6 h. The excess of solvent was removed under reduced pressure, and the resulting precipitate was filtered off, washed with ethanol and recrystallized from methanol to give 5, yield 62%; m.p. 100–102 °C; IR cm–1; 3428–3298 (NH, NH2), 3046 (CH–Ar), 2921 (CH–aliph.), 2220 (C≡N), 1632 (C=N); 1H-NMR: δ 2.43 (s, 3H, CH3), 5.14 (br. S, 2H, NHNH2), 6.71 (s, 1H, pyridine-H-3), 7.33–7.80 (m, 5H, Ar–H), 9.12 (s, 1H, NH), 13C-NMR: δ 118.64 (C≡N), 126.88–155.43 (Ar–C); MS m/z (%): 244 (M+, 2.11), 75 (100). Anal. For: C13H12N4: Calcd. C, 69.64; H, 5.35; N, 25.00; Found: 69.70, H, 5.50; N, 24.80.
2-(3-Cyano-4-methyl-6-phenyl-pyridine)-2,4-dioxo butanamide (
6). A mixture of compound
4 (5.62 mmol) and (8.00 mL) of ethyl acetoacetate was heated for 6 h at 140–150 °C. Excess ethyl aceto- acetate was distilled off under reduced pressure, and the residue was purified by recrystallization from ethanol.
6: Yield 75%; m.p. 110–112 °C; IR cm
–1: 3210 (NH), 3032 (CH–Ar), 2990–2828 (CH–aliph.), 2215 (C≡N) 1710 (
![Molecules 17 10902 i001]()
), 1680 (
![Molecules 17 10902 i002]()
);
1H-NMR: δ 2.48 (s, 3H, CH
3), 3.80 (s, 3H, CH
3), 4.24 (s, 2H, CH
2), 7.12–7.48 (m, 6H, Ar–H), 11.55 (s, 1H, NH);
13C-NMR: δ 14.11 (CH
3), 64.19 (CH
2), 115.00 (C≡N), 112.88–156.09 (Ar–C), 168.50 (CO–CH
3), 182.41 (CO–NH); MS
m/z (%): 293 (9.80), 75 (100). Anal. For: C
17H
15N
3O
2: C, 69.62; H, 5.11; N, 14.33; Found: C, 69.60, H, 5.22, N, 14.50.
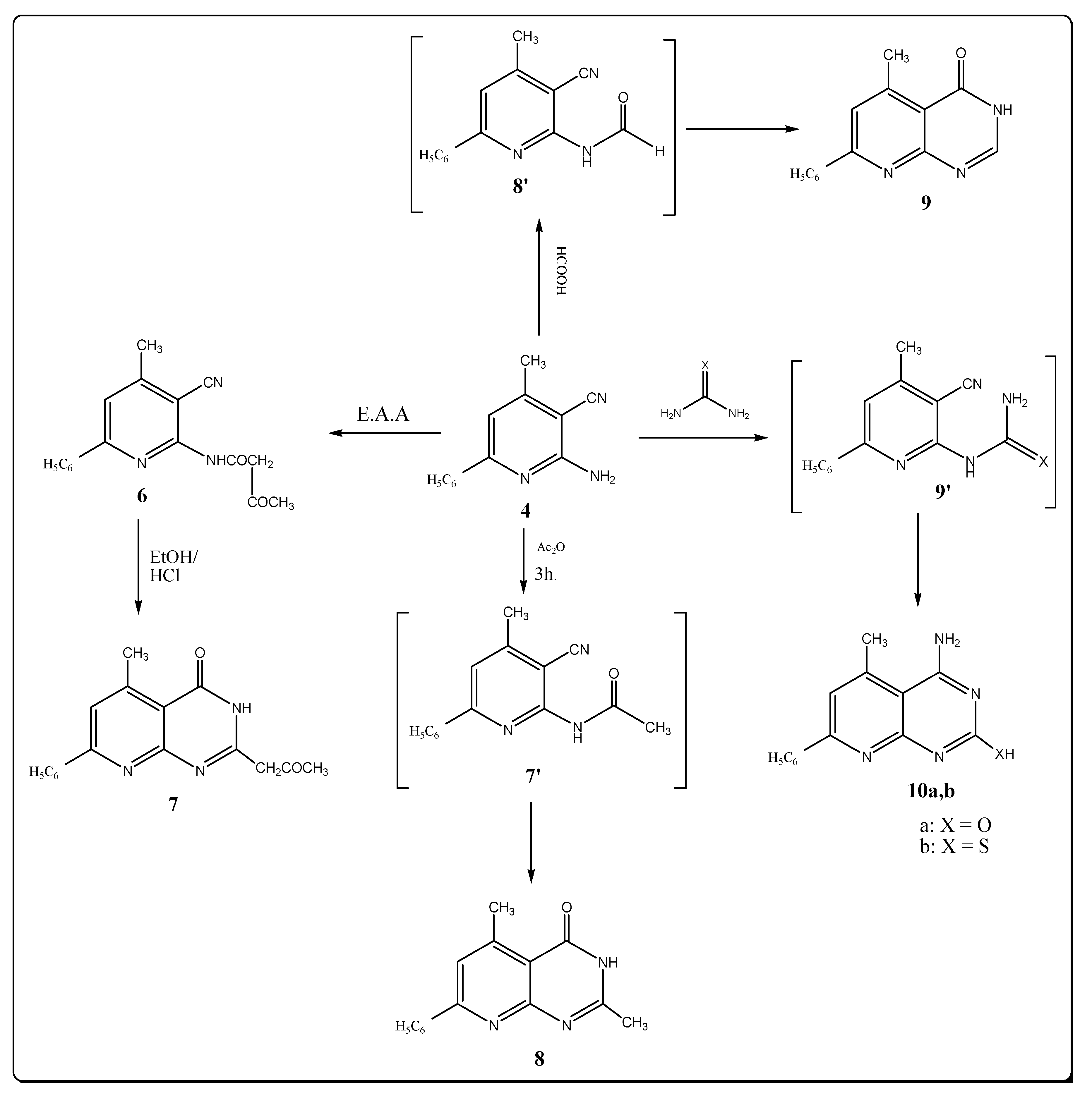
5-Methyl-7-phenyl-2-propanone-3H-pyrido [2,3-d] pyrimidine-4-one (7). Compound 6 (0.01 mol) was treated with concentrated hydrochloric acid (3 drops) in ethanol (30 mL) and refluxed for 6 h. The reaction mixture was left to cool, and then poured into ice-cold water. The precipitate was collected and recrystallized from ethanol. 7: Yield 55%; m.p. 222–224 °C, IR cm–1: 3299 (NH), 3112 (CH–Ar), 2989–2885 (CH–aliph.), 1686 (C=O) 1660 (C=O); 1H-NMR: δ 2.48 (s, 3H, CH3), 3.11 (s, 3H, COCH3), 4.17 (s, 2H, CH2), 7.22–7.78 (m, 6H, Ar–H), 12.34 (br s, 1H, NH); 13C–NMR: δ 14.36 (CH3), 16.50 (COCH3), 64.04 (CH2), 112.69–156.64 (Ar–C), 169.80 (C=O), 189.00 (C=O). MS m/z (%): 393 (M+, 4.12), (74, 100). Anal. For: C17H15N3O2: Calcd. C, 69.62; H, 5.11; N, 14.33; Found: C, 69.50, H, 5.10, N, 14.30.
2,5-Dimethyl-7-phenyl-3H-pyrido[2,3-d] pyrimidine-4-one (8). Compound 4 (0.002 mol) in acetic anhydride (10 mL) was heated under reflux for 3 h. After cooling, the solvent was concentrated under reduced pressure, then the reaction mixture was poured into ice-water (40 mL) to give a solid precipitate which was filtered off and recrystallized from petroleum ether 60/80 to furnish 8, yield 55%; m.p. 300–302 °C; IR cm–1: 3300 (NH), 3033 (CH–Ar), 2928 (CH–aliph.) 1680 (C=O), 1644 (C=N); 1H-NMR: δ 2.50, 2.44 (2s, 6H, 2CH3), 7.38–7.80 (m, 6H, Ar–H), 11.82 (s, 1H, NH); 13C-NMR: δ 13.02 (CH3), 14.22 (CH3), 112.70–148.12 (Ar–C), 170.80 (C=O); MS m/z (%): 251 (2.11), 77 (100). Anal. For: C15H13N3O: Calcd. C, 71.71; H, 5.17, N, 16.73; Found: C, 71.50, H, 5.20, N, 16.80.
5-Methyl-7-phenyl-3H-pyrido [2,3-d] pyrimidin-4-one (9). Compound 4 (0.002 mol) in formic acid (10 mL) was heated under reflux for 6 h. After cooling the reaction mixture was poured into ice-water (40 mL) to give a solid precipitate which was filtered off and recrystallized from methanol to furnish 9, yield 45%; m.p. 180–182 °C; IR cm–1: 3330 (NH), 3100 (CH–Ar), 2900 (CH–aliph.) 1688 (C=O), 1640 (C=N); 1H-NMR: δ 2.55 (s, 3H, CH3), 6.80 (s, 1H, pyridine-H-6), 7.28-7.84 (m, 5H, Ar-H), 8.28 (s, 1H, pyrimidine-H-2). 13C-NMR: δ 14.62 (CH3), 110.18–155.80 (C–Ar), 188.01 (C=O); MS m/z (%): 237 (5.18) 75 (100). Anal. For: C14H11N3O: Calcd. C, 70.88; H, 4.64; N, 17.72. Found: C, 70.80; H, 4.70, N, 17.80.
4-Amino-5-methyl-7-phenyl-1H-pyrido [2,3-d] pyrimidine-2-one(or thione) (10a,b). A mixture of 4 (0.01 mol) and urea (or thiourea) (0.01 mol) was fused in an oil bath at 180 C, for 1h. After cooling and dilution with ethanol (30 mL) the solid product formed was filtered off and recrystallized from ethanol. 10a: Yield 70%; m.p. 320–322 °C; IR cm–1: 3483, 3320, 3250 (OH) (NH2, NH), 3033 (CH–Ar), 2880 (CH–aliph.), 1683 (C=O), 1638 (C=N); 1H-NMR: δ 2.44 (s, 3H, CH3), 4.48 (s, 2H, NH2), 6.88–7.44 (m, 6H, Ar–H), 8.91 (s, 1H, NH); 13C-NMR: δ 11.12 (CH3), 202.32 (C=O), 112.43–148.91 (Ar–C); MS m/z (%): 252 (3.21) 75 (100). Anal. For: C14H12N4O: Calcd. C, 66.66; H, 4.76; N, 22.22; Found: C, 66.50; H, 4.80, N, 22.50. 10b: Yield 65%; 300–302 °C; IR cm–1: 3330, 3280, 3221 (NH2, NH), 3052 (CH–Ar), 2988 (CH–aliph.), 1222 (C=S); 1H-NMR: δ 2.50 (s, 3H, CH3), 4.22 (s, 2H, NH2) 6.88–7.22 (m, 6H, Ar–H), 10.68 (s, 1H, NH); 13C-NMR: δ 13.43 (CH3), 212.98 (C=S), 112.65–144.34 (Ar–C); MS m/z (%): 268 (1.11), 77 (100). Anal. For: C14H12N4S: Calcd., C, 62.68; H, 4.47; N, 20.89, S, 11.94. Found: C, 62.80; H, 4.50, N, 2.80, S, 12.12.
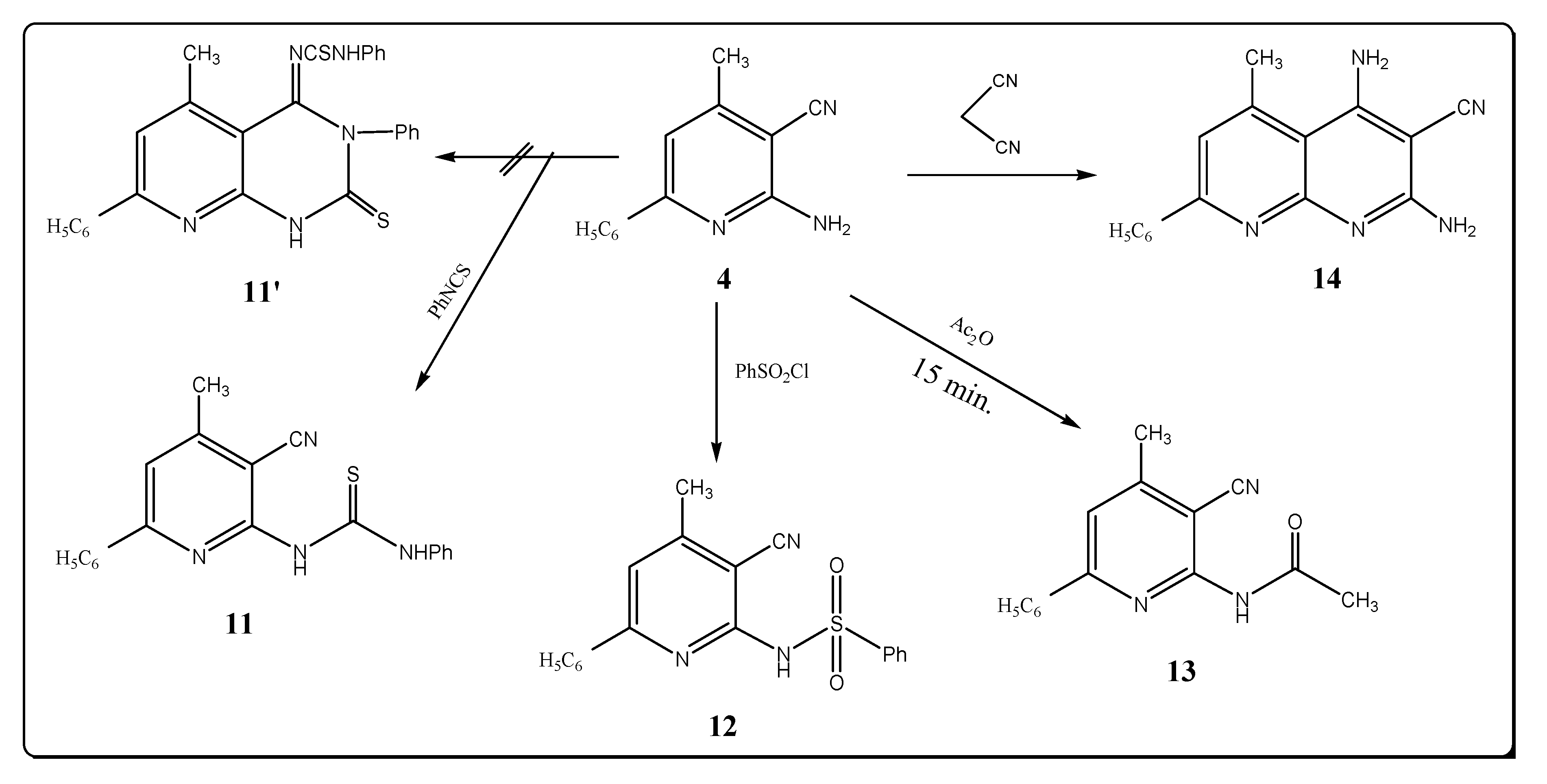
N-(Phenyl)-N′-[2(3-cyano-4-methylphenylpyridinyl] thiourea (11). A mixture of compound 4 (0.01 mol), finely divided sodium metal (0.01 mol) and phenyl isothiocyanate (0.01 mol) were refluxed for 6 h. in dry dioxane (50 mL). After cooling, the solvent was concentrated under pressure, then the reaction mixture was poured into ice-water (40 mL) to give a solid precipitate which was filtered off and recrystallized from petroleum ether 60/80 to furnish 11, yield 60%; m.p. 220–222 °C. IR cm–1: 3212 (NH), 2222 (CN); 1H-NMR: δ 2.55 (s, 3H, CH3), 11.08 (br. s, 1H, NH), 13.26 (br. s, 1H, NH), 7.58–7.69 (m, 5H, Ar–H) 13C-NMR: δ 115 (C≡N), 179.30 (C=S), 14.88 (CH3), 112.00–155.40 (C–Ar); MS m/z (%): 344 (M+, 2.18). Anal. For: C20H16N4S: Calcd. C, 69.76; H, 4.65; 16.27; S, 9.30; Found: C, 69.50; H, 4.70; N, 16.30, S, 9.50.
6-Benzenesulfonylamino-4-methyl-2-phenyl-5-pyridinecarbonitrile (12). Compound 4 was taken in a mixture of pyridine (4 mL) and acetic anhydride (20 mL). To the solution formed, p-benzenesulphonyl chloride was added (0.01 mol) and the reaction mixture was reflux for 6 h., filtered and poured onto acidic crushed ice. The solid product 12 obtained was recrystallized from ethanol. 12: Yield 55%; m.p. 200–202 °C; IR cm–1: 3250 (NH), 3100 (CH–Ar), 2215 (C≡N), 2995 (CH–aliph.), 1632 (C=N), 1360 (S=O, asym), 1150 (S=O, sym); 1H-NMR: δ 2.55 (s, 3H, CH3); 6.88–7.67 (m, 11H, Ar–H), 8.50 (s, 1H, SO2, NH); 13C-NMR: δ 14.17 (CH3), 117 (C≡N), 110.00–155.50 (Ar–C). Anal. For: C19H15N3O2S: calcd. C, 65.32; H, 4.29; N, 12.03; S, 9.16; Found: C, 65.00, H, 4.80; N, 12.00, 55, 9.40.
6-Acetyl amino-4-methyl-2-phenyl-5-pyridinecarbonitrile (13). A suspension of 4 (0.002 mol) in acetic anhydride (10 mL) was heated under reflux for 15 min. After cooling, the solvent was concentrated under reduced pressure, then the reaction mixture was poured into ice-water (40 mL) to give a solid precipitate which was filtered off and recrystallized from ethanol. 13: Yield 55%; m.p. 180–182 °C; IR cm–1: 4330 (OH), 3280 (NH), 3100 (CH–Ar), 2910 (CH–aliph.), 2220 (C≡N), 1720 (C=O); 1H-NMR: δ 2.50 (s, 3H, CH3), 3.33 (s, 3H, COCH3), 6.82–7.72 (m, 6H, Ar–H), 10.22 (s, 1H, NH); 13C-NMR: δ 14.13 (CH3), 17.22 (CH3), 117 (C≡N), 122.00–156.11 (Ar–C), 168.33 (C=O). Anal. For: C15H13N3O. Calcd.: C, 71.71; H, 5.17; N, 16.73; Found: C, 71: 80; H, 5.00, N, 16.90.
2,4-Diamino-3-cyano-5-methyl-7-phenyl-1,8-naphthyridine (14). A suspension of 4 (0.01 mol) in ethanol (20 mL) containing a catalytic amount of triethylamine (3 mL) was treated with malononitrile (0.01 mol). The reaction mixture was refluxed for 10 h. The separated solid was filtered off and recrystallized from ethanol. 14: Yield 72%; m.p. 300–302 °C; IR cm–1: 3450, 3400, 3340, 3250 (2NH2), 3100 (CH–Ar), 2990 (CH–aliph.), 2230 (C≡N); 1H-NMR: δ 2.48 (s, 3H, CH3), 4.42, 5.33 (2s, 4H, 2NH2) 6.84–7.88 (m, 6H, Ar–H); 13C-NMR: δ 13.42 (CH3), 118.95 (C≡N), 127.87–149.97 (Ar–C); MS: m/z (%): 275 (2.08), 77 (100). Anal. For: C16H13N5: C, 69.81; H, 4.72; N, 25.45; Found: C, 69.50; H, 4.80, N, 25.50.
3-Amino-4-methyl-6-phenyl-1H-pyrazolo-[3,4-b]-pyridine (15). Method A: A mixture of compound 5 (0.001 mol) in ethanol and few drops of glacial acetic acid was refluxed for 8 h. The reaction mixture was poured into ice-cold water and the solid product was filtered off, washed with petroleum ether and recrystallized from ethanol. Yield 62%; Method B: Compound 5 (0.005 mol) in DMF 20 mL was refluxed for 12 h. and then allowed to cool. The solid product that precipitated on cooling was filtered off, dried and recrystallized from DMF. 15: Yield 50%; m.p 200–202 °C; IR cm–1: 3320, 3300, 3221 (NH2, NH), 3090 (CH–Ar), 2980 (CH–aliph.), 1644 (C=N), 1H-NMR: δ 2.55 (s, 3H, CH3) 4.50 (s, 2H, NH2), 7.22–7.76 (m, 6, Ar–H), 12.11 (s, 1H, NH); 13C-NMR: δ 11.18(CH3), 124.62–149.06 (Ar–C); MS m/z (%): 224 (6.88), 75 (100). Anal. For: C13H12N4. Calcd. C, 69.64; H, 5.35; N, 25.00; Found: C, 69.70, H, 5.50, N, 25.32.
3-Amino-4-methyl-6-phenyl-1H-pyrazolo-[3,4-b]-pyridine-2-yl-phenylthioamide (16). A suspension of 5 (0.001 mol) and phenyl isothiocyanate (0.001 mol) in pyridine (10 mL) was heated under reflux for 6 h. After cooling the reaction mixture was poured into ice/water (30 mL) and neutralized with dilute 10% HCl to give a solid precipitate that was recrystallized from ethanol-DMF (3:1). 16: Yield 70%; m.p. > 360 °C; IR cm–1: 3330, 3280, 3261 (NH2, NH), 3055 (CH–Ar), 2990 (CH–aliph.), 1333 (C=S); 1H-NMR: δ 2.50 (s, 3H, CH3), 4.80 (s, 2H, NH2), 6.88–7.76 (m, 11H, Ar–H), 11.80 (br s, 1H, NH); 13C-NMR: δ 13.44 (CH3), 208.65 (C=S), 114.89–145.67 (Ar–C); MS m/z (%): 359 (8.11), 75 (100). Anal. For: C20H17N5S: Calcd. C, 66.85; H, 4.73; N, 19.49; S, 8.91. Found: C, 69.50; H, 5.00, N, 19.11.; S, 9.00.
3-Amino-4-methyl-6-phenyl-1H-pyrazolo-[3,4-b]-pyridine-2-yl-methyl propionate (17). A mixture of compound 5 (0.01 mol) and methyl methacrylate (0.01 mol) in acetic acid (20 mL) containing 3 drops of concentrated hydrochloric acid was refluxed for 5 h. The reaction mixture was concentrated and allowed to cool and poured onto H2O (100 mL). The solid obtained was recrystallized from ethanol. 17: Yield 45%; m.p. 140–142 °C; IR cm–1: 3384, 3280 (NH2), 3099 (CH–Ar), 2888–2990 (CH–aliph.), 1710 (C=O); 1H-NMR: δ 2.48 (s, 3H, CH3), 3.38 (s, 3H, OCH3) 4.50 (t, 2H, CH2, J = 10.11 Hz), 4.80 (t, 2H, CH2, J = 8.22 Hz) 5.80 (s, 2H, NH2), 6.82–7.78 (m, 6H, Ar–H); 13C-NMR: δ 14.13 (CH3), 17.80 (OCH3) 40.81 (CH2), 60.38 (CH2), 112.8–153.11 (Ar–C); MS m/z (%): 310 (M+, 0.80) 75 (100). Anal. For: C17H18N4O2: Calcd. C, 65.80; H, 5.80; N, 18.06, Found: C, 65.38; H, 9.13, N, 18.00.
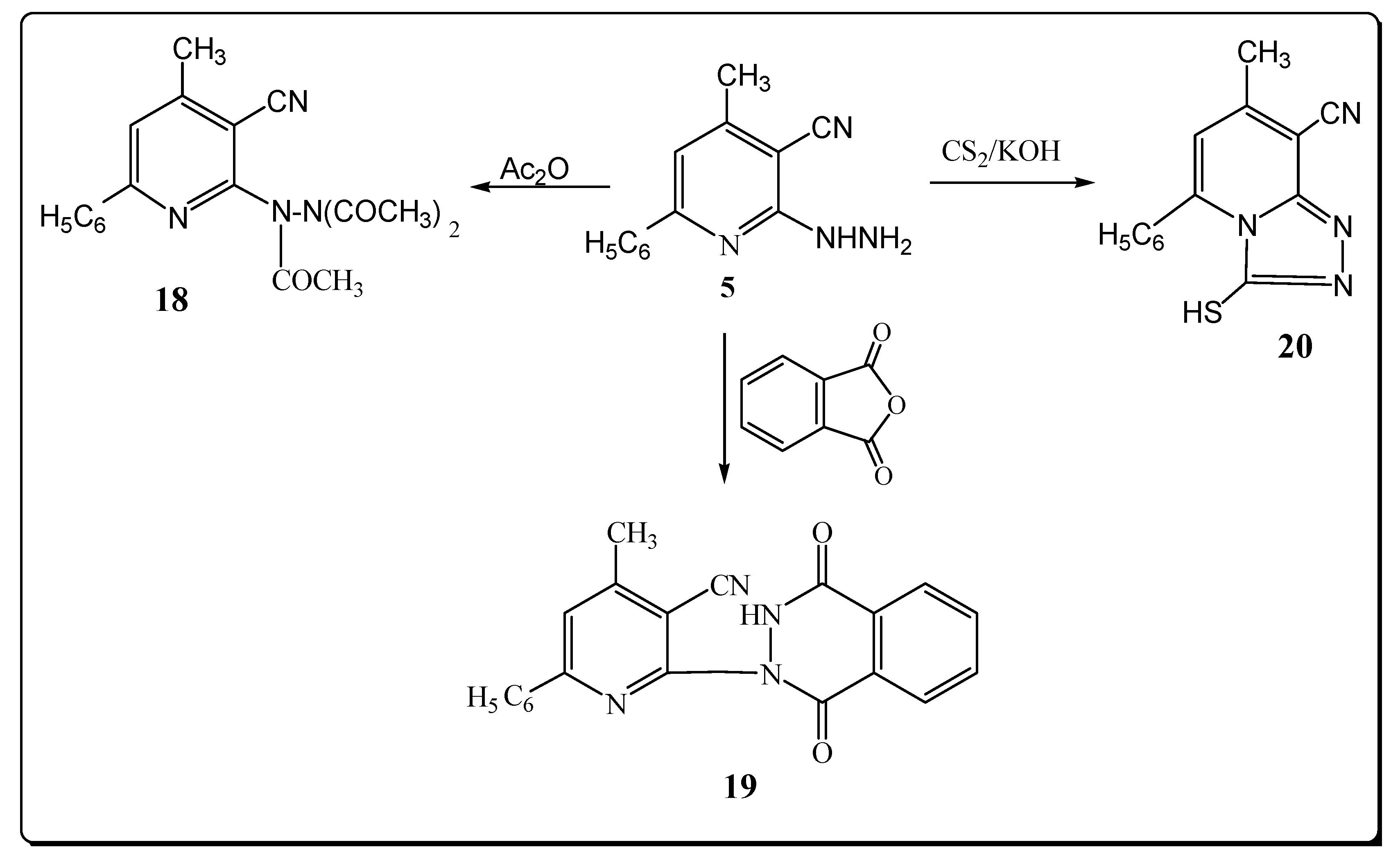
4-Methyl-2-phenyl-6-triacetylhydrazide-5-pyridinecarbonitrile (18). Compound 5 (0.002 mol) in acetic anhydride (10 mL) was heated under reflux for 1h. After cooling, the solvent was concentrated under reduced pressure, then the reaction mixture was poured into ice-water (40 mL) to give a solid precipitate which was filtered off an recrystallized from methanol. 18: Yield 60%; m.p. 180–182 °C; IR cm–1: 3100 (CH–Ar), 2910 (CH–aliph.), 2215 (C≡N), 1681 (C=O); 1H-NMR: δ 2.48 (s, 1H, CH3), 2.50, 2.80, 3.10 (3s, 9H, COCH3) 7.52–7.78 (m, 6H, Ar–H), 13C-NMR: δ 13.22 (CH3) 17.11 (COCH3), 19.28 (COCH3), 21.00 (COCH3) 115 (C≡N), 186.00 (C=O), 180.11 (C=O), 110.66–153.18 (Ar–C); MS m/z (%): 350 (M+, 0.82), 75 (100). Anal. For: C19H18N4O3. Calcd. C, 65.14; H, 5.14; Nm 16.00; Found: C, 65.30; H, 5.25; N, 16.30.
1-[3-Cyano-4-methyl-6-phenyl]-pyridine-2-yl-(2H)-phthalazine-3,8-dione (19). A mixture of compound 5 (0.01 mol) and phthalic anhydride (0.01 mol) in acetic acid (20 mL) was refluxed for 6 h. The reaction mixture was concentrated, allowed to cool and poured onto H2O (100 mL). The solid obtained was recrystallized from dioxane. 19: Yield 52%; m.p. 310–312 °C; IR cm–1: 3280 (NH), 3033 (CH–Ar), 2999 (CH–aliph.), 2222 (C≡N), 1739, 1714 (2C=O); 1H-NMR: δ 2.48 (s, 3H, CH3), 7.22–7.83 (m, 10H, Ar–H), 12.21 (brs,1H,NH); 13C-NMR: δ 11.82 (CH3), 186.98 (C=O), 202.65 (C=O), 118.76 (C≡N), 128.89–162.33 (Ar–C); MS m/z (%): 354 (M+, 1.08), 77 (100). Anal. For: C21H14N4O2: Calcd. C, 71.18; H, 3.95; Nm 15.81; Found: C, 71.30; H, 4.18, N, 15.50.
8-Cyano-7-methyl-5-phenyl-2,3-dihydro-2-thioxo-1,2,4,triazolo-[4,3-a]-pyridine (20). To a stirred suspension of compound 5 (22 mmol) in ethanol (20 mL), ethanolic potassium hydroxide (30 mL, 0.01 mol) and CS2 (2 mL) were added dropwise. The reaction mixture was then heated under reflux for 6 h. After cooling and evaporation of the solvent, the potassium salt obtained was dissolved in water and acidified with 2N aqueous HCl. The solid product formed was collected and recrystallized from ethanol. 20: Yield 72%; m.p. 160–162 °C. IR cm–1: 3220 (NH), 3055 (CH–Ar) 2880 (CH–aliph.), 2220 (C≡N), 1644 (C=N); 1H-NMR: δ 2.48 (s, 3H, CH3), 6.88–7.45 (m, 6H, Ar–H) 11.33 (s, 1H, NH); 13C-NMR: δ 14.58 (CH3), 118.67 (C≡N), 212.00 (C=S), 125.78–148.79 (Ar–C); MS m/z (%) 266 (M+, 3.11) 75 (100). Anal. For: C14H10N4S. Calcd. C, 63.15; H, 3.75; N, 21.05; S, 12.03; Found: C, 63.30; H, 4.00; N, 21.30; S, 12.11.


 ), 1680 (
), 1680 (  ); 1H-NMR: δ 2.48 (s, 3H, CH3), 3.80 (s, 3H, CH3), 4.24 (s, 2H, CH2), 7.12–7.48 (m, 6H, Ar–H), 11.55 (s, 1H, NH); 13C-NMR: δ 14.11 (CH3), 64.19 (CH2), 115.00 (C≡N), 112.88–156.09 (Ar–C), 168.50 (CO–CH3), 182.41 (CO–NH); MS m/z (%): 293 (9.80), 75 (100). Anal. For: C17H15N3O2: C, 69.62; H, 5.11; N, 14.33; Found: C, 69.60, H, 5.22, N, 14.50.
); 1H-NMR: δ 2.48 (s, 3H, CH3), 3.80 (s, 3H, CH3), 4.24 (s, 2H, CH2), 7.12–7.48 (m, 6H, Ar–H), 11.55 (s, 1H, NH); 13C-NMR: δ 14.11 (CH3), 64.19 (CH2), 115.00 (C≡N), 112.88–156.09 (Ar–C), 168.50 (CO–CH3), 182.41 (CO–NH); MS m/z (%): 293 (9.80), 75 (100). Anal. For: C17H15N3O2: C, 69.62; H, 5.11; N, 14.33; Found: C, 69.60, H, 5.22, N, 14.50.


