Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses
Abstract
:1. Introduction

2. Results and Discussion
2.1. Volatile Oil Characterization in Mature N. sativa Seeds
2.2. Changes in the N. sativa Seed Volatiles during Seed Maturation
| Seed source | Ein-Harod | Naan | |||||
|---|---|---|---|---|---|---|---|
| Compound | µg/g F.W. | % (W/W) | µg/g F.W. | % (W/W) | |||
| Monoterpene hydrocarbons | I.M. * | R.I. | |||||
| α-thujene | MS,RI,AS | 925 | 43.8 ± 6.8 | 9.7 ± 0.3 | 51.7 ± 11.4 | 10.4 ± 0.3 | |
| α-pinene | MS,RI,AS | 940 | 21.9 ± 4.2 | 4.8 ± 0.2 | 24.3 ± 4.5 | 4.9 ± 0.2 | |
| sabinene | MS,RI,AS | 970 | 3.9 ± 0.7 | 0.9 ± 0.04 | 4.7 ± 1.1 | 1 ± 0.04 | |
| β-pinene | MS,RI,AS | 975 | 11 ± 2 | 2.4 ± 0.1 | 12.7 ± 2.8 | 2.6 ± 0.1 | |
| myrcene | MS,RI,AS | 985 | 2.1 ± 0.2 | 0.5 ± 0.1 | 1.9 ± 0.5 | 0.4 ± 0.04 | |
| α-terpinene | MS,RI,AS | 1014 | 5.5 ± 1.8 | 1.2 ± 0.4 | 1.5 ± 0.3 | 0.3 ± 0.1 | |
| p-cymene | MS,RI,AS | 1024 | 113 ± 17.3 | 25 ± 0.5 | 128 ± 25.9 | 25.9 ± 0.02 | |
| limonene | MS,RI,AS | 1030 | 3.1 ± 0.6 | 0.7 ± 0.03 | 3.1 ± 0.6 | 0.6 ± 0.04 | |
| γ-terpinene | MS,RI,AS | 1055 | 52.1 ± 22.4 | 11.6 ± 5.5 | 4.5 ± 0.2 | 0.9 ± 0.2 | |
| terpinolene | MS,RI,AS | 1086 | 1.3 ± 0.1 | 0.3 ± 0.1 | 1 ± 0.2 | 0.2 ± 0.02 | |
| total | 257 | 57.1 | 233.33 | 47.3 | |||
| Monoterpene alcohols | |||||||
| terpinene 4-ol | MS,RI,AS | 1181 | 2 ± 0.4 | 0.4 ± 0.01 | 2.6 ± 0.7 | 0.5 ± 0.1 | |
| carvacrol | MS,RI,AS | 1298 | 26.1 ± 3.9 | 5.8 ± 1 | 16.9 ± 4.1 | 3.4 ± 0.9 | |
| thymohydroquinone | MS,RI | 1550 | 73.0 ± 15.7 | 16.2 ± 1.9 | 114.5 ± 15.1 | 23.2 ± 2.4 | |
| total | 101.1 | 22.5 | 134 | 27.1 | |||
| Monoterpene ethers | |||||||
| cis-4-methoxythujane | MS,RI | 1094 | 3 ± 0.6 | 0.7 ± 0.04 | 3.5 ± 0.9 | 0.7 ± 0.04 | |
| trans-4-methoxythujane | MS,RI | 1118 | 17 ± 3 | 3.8 ± 0.2 | 19.9 ± 4.6 | 4 ± 0.1 | |
| 4,5-epoxy-1-isopropyl-4-methyl-1-cyclohexene | MS | 1201 | 4.1 ± 0.9 | 0.9 ± 0.1 | 4.7 ± 1.5 | 0.9 ± 0.1 | |
| total | 24.1 | 5.4 | 28.2 | 5.7 | |||
| Monoterpene ketones | |||||||
| carvone | MS,RI,AS | 1245 | 0.2 ± 0.1 | 0.04 ± 0.01 | 0.3 ± 0.1 | 0.1 | |
| thymoquinone | MS,RI,AS | 1253 | 35.2 ± 17.5 | 7.8 ± 3.7 | 67.7 ± 23.7 | 13.7 ± 2.3 | |
| total | 35.4 | 7.9 | 68 | 13.8 | |||
| Monoterpene ester | |||||||
| bornyl acetate | MS,RI,AS | 1286 | 0.4 ± 0.1 | 0.1 ± 0.01 | 0.5 ± 0.1 | 0.1 ± 0.01 | |
| total | 0.4 | 0.1 | 0.5 | 0.1 | |||
| Aldehydes | |||||||
| 2 E,4Z- decadienal | MS,RI | 1295 | 0.3 ± 0.02 | 0.1 ± 0.01 | 0.3 ± 0.03 | 0.1 ± 0.01 | |
| 2 E,4E- decadienal | MS,RI | 1319 | 0.5 ± 0.1 | 0.1 ± 0.01 | 0.7 ± 0.1 | 0.1 ± 0.01 | |
| total | 0.8 | 0.2 | 0.9 | 0.2 | |||
| Sesquiterpenes | |||||||
| longipinene | MS,RI | 1355 | 3.4 ± 0.5 | 0.8 ± 0.04 | 2.7 ± 1.3 | 0.5 ± 0.2 | |
| longifolene | MS,RI | 1415 | 17.1 ± 1.8 | 3.8 ± 0.20.04 ± 0.01 | 13.3 ± 5.8 | 2.7 ± 1 | |
| trans-caryophyllene | MS,RI,AS | 1423 | 0.2 ± 0.1 | 0.2 ± 0.03 | 0.03 | ||
| zonarene | MS,RI | 1523 | 0.6 ± 0.2 | 0.1 ± 0.04 | 0.6 ± 0.1 | 0.1 | |
| total | 21.3 | 4.7 | 16.7 | 3.4 | |||
| Unidentified | 14.1 ± 3.4 | 2.4 | 16.7 ± 5.3 | 2.3 | |||
| Total essential oil | 450.3 ± 69.5 | 100 | 493.7 ± 99.5 | 100 | |||
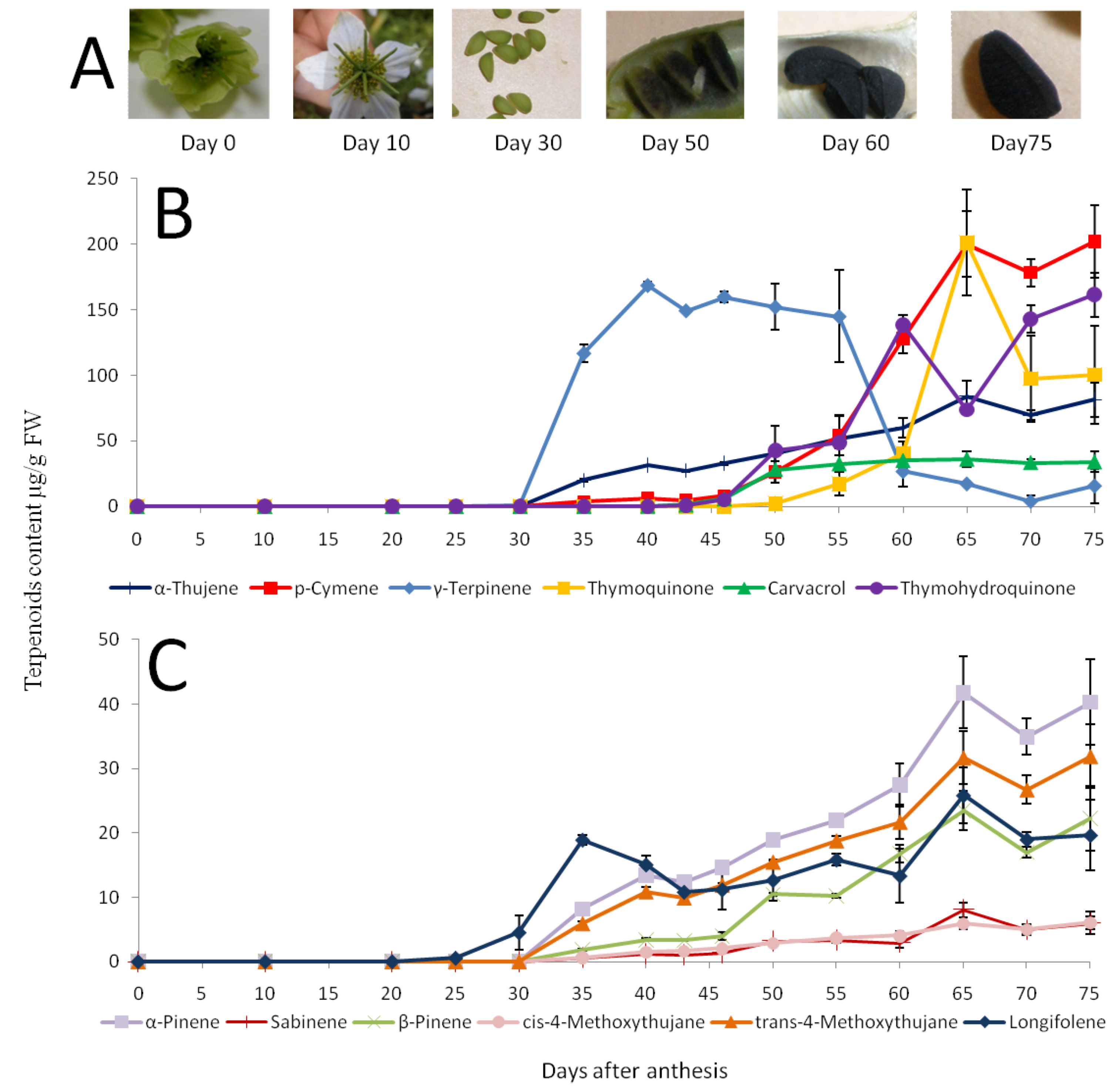

2.3. Distribution of Specialized and Other Metabolites within N. sativa Seed

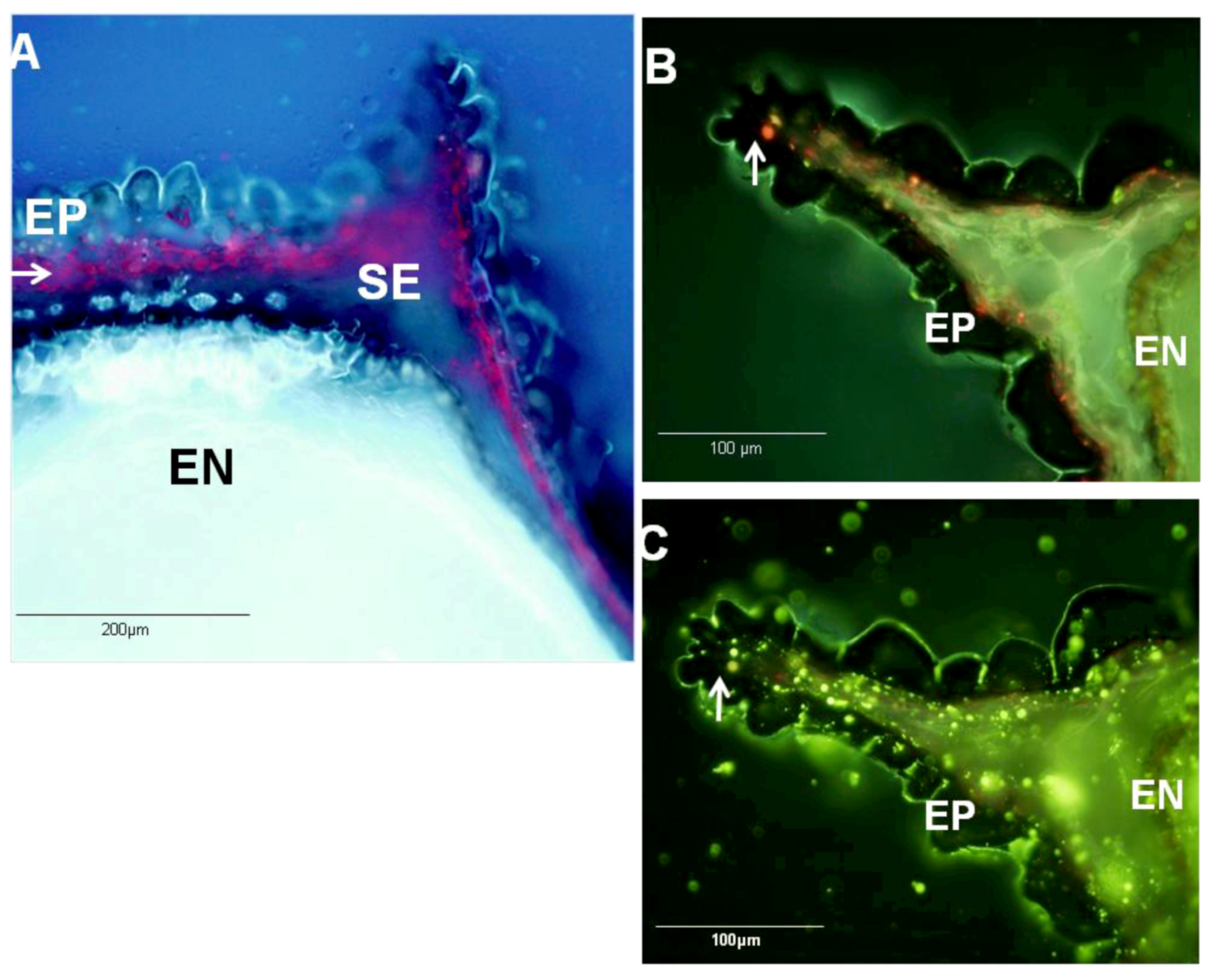


2.4. Ecological Relevance to the Distribution of Metabolites within Black Cumin Seed
3. Experimental
3.1. Chemicals
3.2. Plant Material
3.3. Separation of Seed Coat from Inner Seed Tissues
3.4. Extraction and Analysis of Volatile Compounds
3.5. Extraction and Analysis of Non-Volatile Compounds
3.6. Hystochemistry
4. Conclusions
Acknowledgments
References
- Padhye, S.; Banerjee, S.; Ahmad, A.; Mohammad, R.; Sarkar, F.H. From here to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008, 6, 495–510. [Google Scholar]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World, 3rd ed; Oxford University Press: New York, NY, USA, 2000; p. 206. [Google Scholar]
- Chevallier, A.M. The Encyclopedia of Medicinal Plants; Dorling Kindersley: New York, NY, USA, 1996; p. 237. [Google Scholar]
- Levey, M. The Medical Formulary; University of Wisconsin Press: Madison, WI, USA, 1966. [Google Scholar]
- Gunther, R.T. The Greek Herbal of Dioscorides; Hafner Pub. Co.: New York, NY, USA, 1959; p. 93. [Google Scholar]
- Muntner, S. Assaph (Harofe) the Physician (in Herbrew). Sefer Refuoth: Korot 4 . 1967–1969; 411. [Google Scholar]
- Muntner, S. Moshe Ben Maimon (Maimonides),Treatise on Poisons and Their Antidotes (in Hebrew); J.B. Lippincott: Philadelphia, PA, USA, 1966; pp. 115–123. [Google Scholar]
- Al-Akili, M.M. Natural Healing with the Medicine of the Prophet: From the Book of the Provisions of the Hereafter; Pearl Pub. House: Philadelphia, PA, USA, 1993; p. 195. [Google Scholar]
- Levey, M. Ibn Mäsawaih and His Treatise on Simple Aromatic Substances. J. Hist. Med. Allied. Sci. 1961, 6, 394–410. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Nigella sativa: Reduces the risk of various maladies. Crit. Rev. Food Sci. 2010, 50, 654–665. [Google Scholar]
- Zaid, H.; Silbermann, M.; Ben-Arye, E.; Saad, B. Greco-Arab and Islamic herbal-derived anticancer modalities: From tradition to molecular mechanisms. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Ibn, Sina. Kitab al-Qanun fī al-tibb. “The Canon of Medicine”; al-Muthanna Library: Baghdad, Iraq (in Arabic), 1877; p. 437, Ibn Sina (Avicenna). [Google Scholar]
- Ibn, al-Baytar. Kitab al-Jami li-Mufradat al-Adwiya wa-l-Aghdhiya. “The Comprehensive Book of Simple Drugs”; Bulaq: Cairo, Egypt (in Arabic), 1874; Volume II, p. 113. [Google Scholar]
- al, Qazwini. Abu Yahya Zakariya' ibn Muhammad. Kitab Aja’ib al-makhluqat wa Gharaib al-Mawjudat, “The Wonders of Creation”; Dar al-Sharq al-Arabi: Beyruth, Lebanon (in Arabic), 1981; p. 254. [Google Scholar]
- al-Antaki, Dawud. Tadhkirat uli al-albab wa-l-jami lil ’ajab al-‘ujab, “The Memorandum of the Intelligent, a Compendium of Wonders”; al-Ẓahir: Cairo, Egypt (in Arabic), 1935; pp. 219–220. [Google Scholar]
- Rosner, F.; Muntner, S. Moshe Ben Maimon (Maimonides), The Medical Aphorisms of Moses Maimonides; Yeshiva University Press: New York, NY, USA, 1970; pp. 15–58. [Google Scholar]
- Lev, E.; Amar, Z. Reconstruction of the inventory of materia medica used by members of the Jewish community of medieval Cairo according to prescriptions found in the Taylor-Schechter Genizah collection, Cambridge. J. Ethnopharmacol. 2006, 108, 428–444. [Google Scholar] [CrossRef]
- Ghanzafar, S.A. Handbook of Arabian Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1994; p. 180. [Google Scholar]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Tech. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Khan, M.A. Chemical composition and medical properties of Nigella sativa Linn. Inflammopharmacology 1999, 7, 15–35. [Google Scholar] [CrossRef]
- Aitzetmuller, K.; Werner, G.; Ivanov, S.A. Seeds oils of Nigella species and of closely related genera. OCL-Ol. Corps Gras Lip. 1997, 4, 385–388. [Google Scholar]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leucocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar]
- Benkaci-Ali, F.; Baaliouamer, A.; Meklati, B.Y.; Chemat, F. Chemical composition of seed essential oils from Algerian Nigella sativa extracted by microwave and hydrodistillation. Flavour Frag. J. 2007, 22, 148–153. [Google Scholar]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar]
- Chehl, N.; Chipitsyna, G.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB 2009, 11, 373–381. [Google Scholar] [CrossRef]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef]
- Ur-Rahman, A.; Malik, S.; Ahmed, S.; Chaudhry, I.; Ur-Rehman, H. Nigellimine-N-oxide—A new isoquinoline alkaloid from seeds of Nigella sativa. Heterocycles 1985, 23, 953–955. [Google Scholar] [CrossRef]
- Ur-Rahman, A.; Malik, S.; Cun-Hung, H.; Clardy, J. Isolation and structure determination of nigellicine, a novel alkaloid from seeds of Nigella sativa. Tetrahedron Lett. 1985, 26, 2759–2762. [Google Scholar]
- Ur-Rahman, A.; Malik, S.; Zaman, K. Nigellimine: A new isoquinoline alkaloid from the seeds of Nigella sativa. J. Nat. Prod. 1992, 55, 676–678. [Google Scholar]
- Ur-Rahman, A.; Malik, S.; Hassan, S.S.; Choudhary, M.I.; Ni, C.Z.; Clardy, J. Nigellidine—A new indazole alkaloid from seeds of Nigella sativa. Tetrahedron Lett. 1995, 36, 1993–1996. [Google Scholar]
- Morikawa, T.; Xu, F.; Kashima, Y.; Matsuda, H.; Ninomiya, K.; Yoshikawa, M. Novel dolabellane-type diterpene alkaloids with lipid metabolism promoting activities from the seeds of Nigella sativa. Org. Lett. 2004, 6, 869–872. [Google Scholar]
- Kumara, S.S.M.; Huat, B.T.K. Extraction, isolation and characterisation of antitumor principle, α-Hederin, from the seeds of Nigella sati. Planta Med. 2001, 67, 29–32. [Google Scholar] [CrossRef]
- Sieben, A.; Prenner, L.; Sorkalla, T.; Wolf, A.; Jakobs, D.; Runkel, F.; Häberlein, H. α-Hederin, but not hederacoside C and hederagenin from Hedera helix, affects the binding behavior, dynamics, and regulation of β2-adrenergic receptors. Biochemistry 2009, 48, 3477–3482. [Google Scholar]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C. Chemical constituents and antioxidant potentials of essential oil and acetone extract of Nigella sativa seeds. J. Sci. Food Agric. 2005, 85, 2297–2306. [Google Scholar]
- Wajs, A.; Bonikowski, R.; Kalemba, D. Composition of essential oil from seeds of Nigella sativa L. cultivated in Poland. Flavour Frag. J. 2008, 23, 126–132. [Google Scholar] [CrossRef]
- Skoula, M.; Gotsiou, P.; Naxakis, G.; Johnson, C.B. A chemosystematic investigation on the mono- andsesquiterpenoids in the genus Origanum (Labiatae). Phytochemistry 1999, 52, 649–657. [Google Scholar]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M.F. Enrichment of the thymoquinone content in volatile oil from Satureja montana using supercritical fluid extraction. J. Sep. Sci. 2009, 32, 328–334. [Google Scholar]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol. Biol. 2010, 73, 587–603. [Google Scholar]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.A.; Palavra, A.M.F. Composition and antioxidant activity of Thymus vulgaris volatiles: Comparison between supercritical fluid extraction and hydrodistillation. J. Sep. Sci. 2010, 33, 2211–2218. [Google Scholar]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Morreti, A.; Lovato, A.F.S. Seed yield, yield components, oil content and essential oil content and composition of Nigella sativa L. and Nigella damascena L. Ind. Crops Prod. 2002, 15, 59–69. [Google Scholar] [CrossRef]
- Hamrouni-Sellami, I.; Kchouk, M.E.; Marzouk, B. Lipid and aroma composition of black cumin (Nigella sativa L.) seeds from Tunisia. J. Food Biochem. 2008, 32, 335–352. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef]
- Camciuc, M.; Bessiere, J.M.; Vilarem, G.; Gaset, A. Volatile components in okra seed coat. Phytochemistry 1998, 48, 311–315. [Google Scholar]
- Caissard, J.C.; Joly, C.; Bergougnoux, V.; Hugueney, P.; Mauriat, M.; Baudino, S. Secretion mechanisms of volatile organic compounds in specialized cells of aromatic plants. Rec. Res. Dev. Cell Biol. 2004, 2, 1–15. [Google Scholar]
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Fahn, A. Structure and function of secretory cells. Adv. Bot. Res. 2000, 31, 37–75. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Dudai, N.; Tadmor, Y.; Katzir, I.; Ravid, U.; Putievsky, E.; Joel, D.M. Histochemical localization of citral accumulation in lemongrass leaves (Cymbopogon citratus (DC.) Stapf., Poaceae). Ann. Bot.-London 1998, 81, 35–39. [Google Scholar] [CrossRef]
- Gross, M.; Joel, D.M.; Cohen, Y.; Bar, E.; Friedman, J.; Lewinsohn, E. Ontogenesis of mericarps of bitter fennel (Foeniculum vulgare Mill. var. vulgare) as related to t-anethole accumulation. Isr. J. Plant Sci. 2006, 54, 309–316. [Google Scholar]
- Karcz, J.; Tomczok, J. Microstructural features of seeds surface in 6 species of the genus Nigella L. (Ranunculaceae). Acta Biol. 1987, 7, 111–125. [Google Scholar]
- Kanazawa, K.; Sakakibara, H. High content of dopamine, a strong antioxidant, in Cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis Seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef]
- Hafez, Y.M. Effectiveness of the antifungal black seed oil against powdery mildews of cucumber (Podosphaera xanthii) and barley (Blumeria graminis f.sp. hordei). Acta Biol. Szeged. 2008, 52, 17–25. [Google Scholar]
- Maraka, A.; Al-Sharo’a, N.F.; Farah, H.; Elbjeirami, W.M.; Shakya, A.K.; Sallal, A.K. Effect of Nigella sativa extract and oil on aflatoxin production by Aspergillus flavus. Turk. J. Biol. 2007, 31, 155–159. [Google Scholar]
- Chaubey, M.K. Insecticidal activity of Trachyspermum ammi (Umbelliferae), Anethum graveolens (Umbelliferae) and Nigella sativa (Ranunculaceae) essential oils against stored-product beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Afr. J. Agric. Res. 2007, 2, 596–600. [Google Scholar]
- Dudai, N.; Larkov, O.; Putievsky, E.; Lerner, H.R.; Ravid, U.; Lewinsohn, E.; Mayer, A.M. Biotransformation of constituents of essential oils by germinating wheat seed. Phytochemistry 2000, 55, 375–382. [Google Scholar]
- Reynolds, T. Comparative effect of alicyclic compounds and quinones on inhibition of lettuce fruit germination. Ann. Bot.-London 1987, 60, 215–223. [Google Scholar]
- Dudai, N.; Larkov, O.; Ravid, U.; Putievsky, E.; Lewinsohn, E. Developmental control of monoterpene content and composition in Micromeria fruticosa (L.) Druce. Ann. Bot.-London 2001, 88, 349–354. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Roessner, U.; Luedemann, A.; Brust, D.; Fiehn, O.; Linke, T.; Willmitzer, L.; Fernie, A.R. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 2001, 13, 11–29. [Google Scholar]
- Luedemann, A.; Strassburg, K.; Erban, A.; Kopka, J. TagFinder for the quantitative analysis of gas chromatography-Mass spectrometry (GC-MS)-based metabolite profiling experiments. Bioinformatics 2008, 24, 732–737. [Google Scholar] [CrossRef]
- Erban, A.; Schauer, N.; Fernie, A.R.; Kopka, J. Nonsupervised construction and application of mass spectral and retention time index libraries from time-of-flight gas chromatography-mass spectrometry metabolite profiles. Method. Mol. Cell. Biol. 2007, 358, 19–38. [Google Scholar]
- Hummel, J.; Selbig, J.; Walther, D.; Kopka, J. The Golm Metabolome Database: A Database for GC-MS Based Metabolite Profiling. In Metabolomics; Nielsen, J., Jewett, M., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany/New York, NY, USA, 2007; pp. 75–96. [Google Scholar]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with sudan red 7B or fluorol yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Caissard, J.C.; Bergougnoux, V.; Martin, M.; Mauriat, M.; Baudino, S. Chemical and histochemical analysis of ‘Quatre Saisons Blanc Mousseux’, a Moss Rose of the Rosa X damascena Group. Ann. Bot.-London 2006, 97, 231–238. [Google Scholar]
- Sample Availability: Samples of the compounds and N. sativa seed are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Botnick, I.; Xue, W.; Bar, E.; Ibdah, M.; Schwartz, A.; Joel, D.M.; Lev, E.; Fait, A.; Lewinsohn, E. Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses. Molecules 2012, 17, 10159-10177. https://doi.org/10.3390/molecules170910159
Botnick I, Xue W, Bar E, Ibdah M, Schwartz A, Joel DM, Lev E, Fait A, Lewinsohn E. Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses. Molecules. 2012; 17(9):10159-10177. https://doi.org/10.3390/molecules170910159
Chicago/Turabian StyleBotnick, Ilan, Wentao Xue, Einat Bar, Mwafaq Ibdah, Amnon Schwartz, Daniel M. Joel, Efraim Lev, Aaron Fait, and Efraim Lewinsohn. 2012. "Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses" Molecules 17, no. 9: 10159-10177. https://doi.org/10.3390/molecules170910159




