Dry Etching of Copper Phthalocyanine Thin Films: Effects on Morphology and Surface Stoichiometry
Abstract
:1. Introduction
2. Results and Discussion
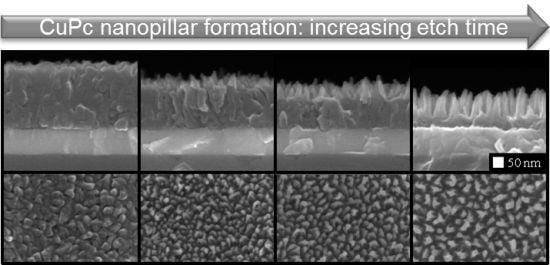
2.1. Columnar MPc Film Morphologies via Plasma Etching
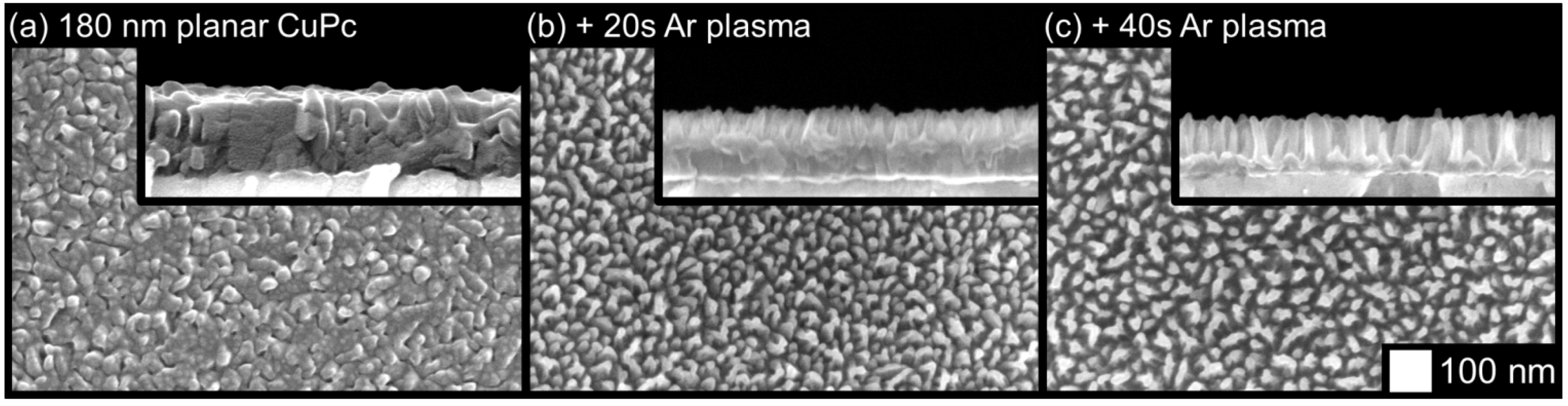

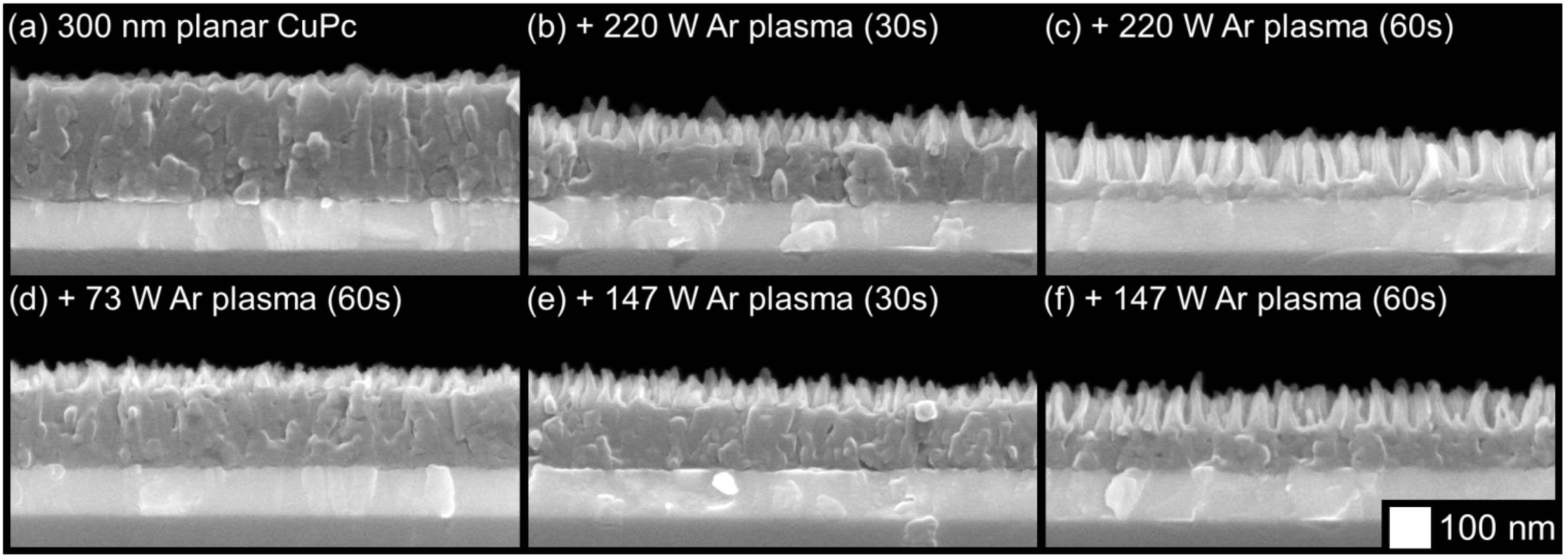

2.2. Surface Analysis and Electronic Properties


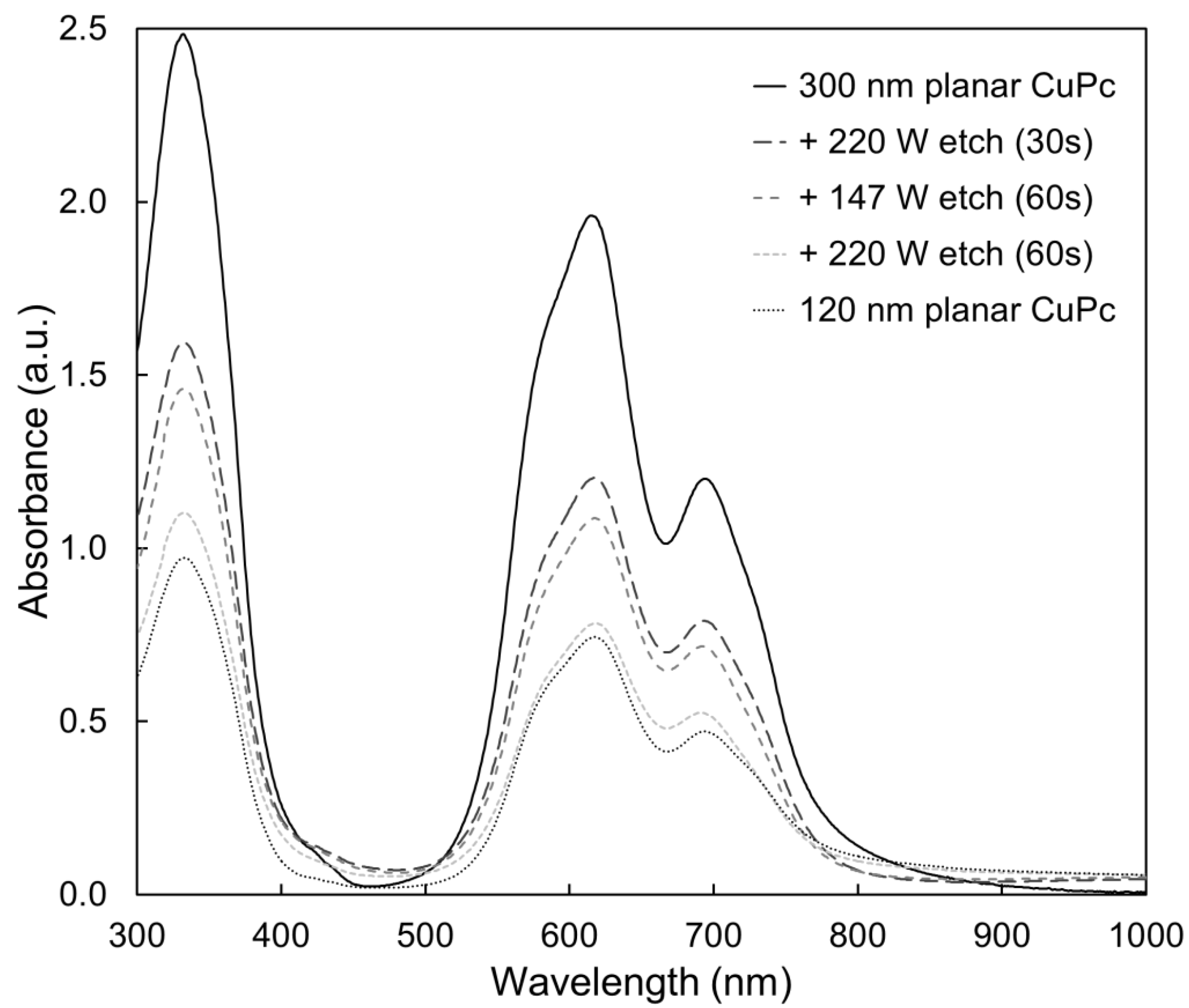
2.3. Bulk Film Analysis
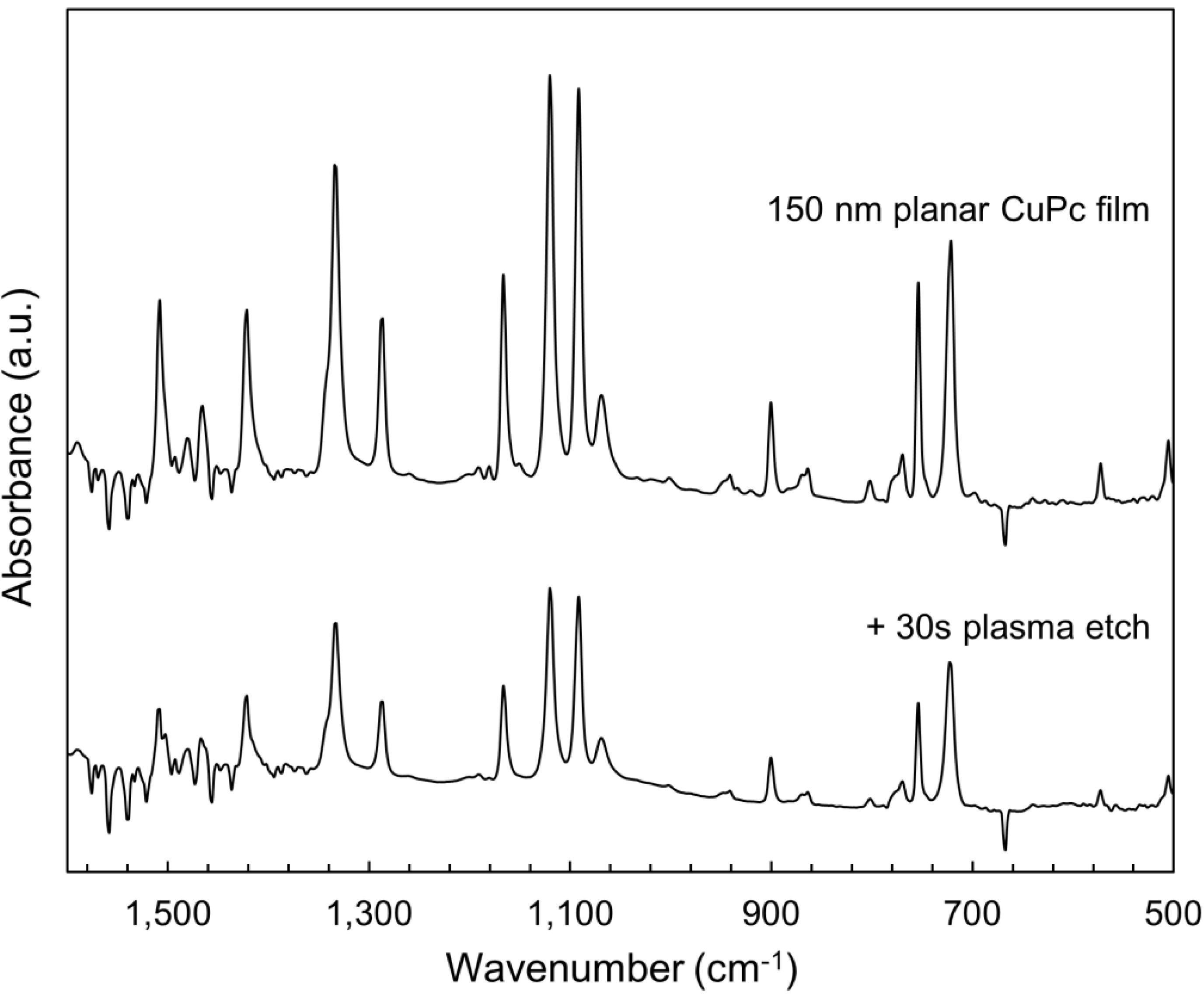
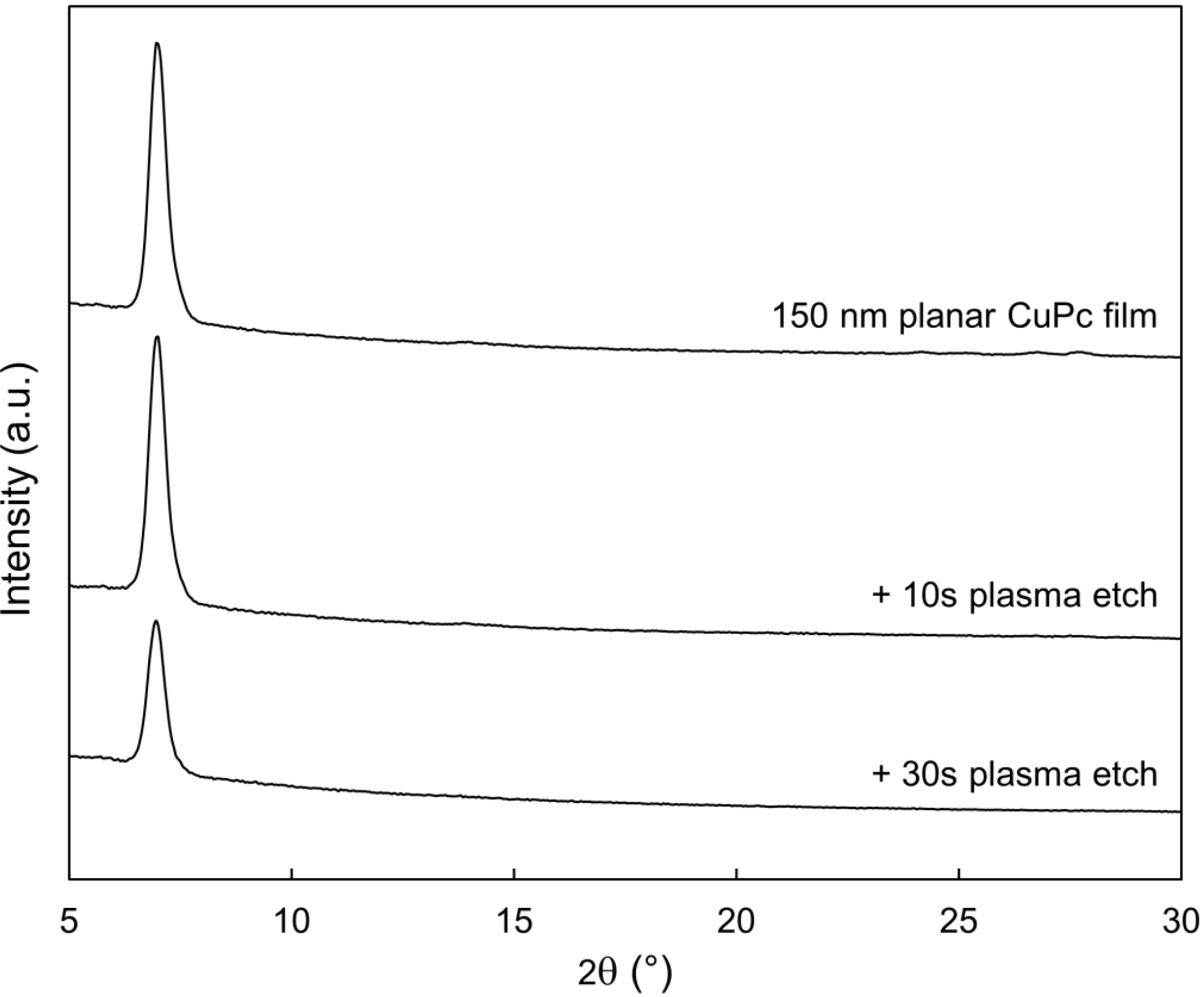
3. Experimental
4. Conclusions
Acknowledgments
References
- Nobes, M.J.; Colligon, J.S.; Carter, G. The equilibrium topography of sputtered amorphous solids. J. Mater. Sci. 1969, 4, 730–733. [Google Scholar] [CrossRef]
- Auciello, O.; Kelly, R. Ion Bombardment Modification of Surfaces; Elsevier Publishing Co.: Amsterdam, The Netherlands, 1984. [Google Scholar]
- King, B.V.; Tsong, I.S.T. The depth resolution of sputter profiling. Ultramicroscopy 1984, 14, 75–78. [Google Scholar] [CrossRef]
- Tanemura, M.; Okuyama, F. Topographical features of N2+-Sputtered Metal Sandwiches: Their Correlation with Auger Depth Resolution. Thin Solid Films 1988, 165, 193–209. [Google Scholar] [CrossRef]
- Cirlin, E.-H.; Vajo, J.J.; Doty, R.E.; Hasenberg, T.C. Ion-induced topography, depth resolution, and ion yield during sary ion mass spectrometry depth profiling of a GaAs/AlGaAs superlattice: Effects of sample rotation. J. Vac. Sci. Technol. A 1991, 9, 1395–1401. [Google Scholar] [CrossRef]
- Hofmann, S. Approaching the limits of high resolution depth profiling. Appl. Surf. Sci. 1993, 70–71, 9–19. [Google Scholar] [CrossRef]
- Carter, G. The effects of ion bombardment sputtering and atomic transport related roughening and smoothing on depth profiling resolution. Vacuum 1996, 47, 409–420. [Google Scholar] [CrossRef]
- Carter, G. The physics and applications of ion beam erosion. J. Phys. D Appl. Phys. 2001, 34, R1–R22. [Google Scholar] [CrossRef]
- Karmakar, P.; Mollick, S.A.; Ghose, D.; Chakrabarti, A. Role of initial surface roughness on ion induced surface morphology. Appl. Phys. Lett. 2008, 93, 103102. [Google Scholar] [CrossRef]
- Sulania, I.; Tripathi, A.; Kabiraj, D.; Lequeux, M.; Avasthi, D. Surface patterning on indium phosphide with low energy Ar atoms bombardment: an evolution from nanodots to nanoripples. Adv. Mat. Lett. 2010, 1, 118–122. [Google Scholar] [CrossRef]
- Zhang, K.; Brötzmann, M.; Hofsäss, H. Surfactant-driven self-organized surface patterns by ion beam erosion. New J. Phys. 2011, 13, 013033. [Google Scholar]
- Pamler, W.; Wangemann, K.; Kampermann, S.; Hosler, W. Depth Resolution in Auger Depth Profile Analysis of Aluminum Metallization in Microelectronics: The Effect of Crystalline Texture. Nucl. Instrum. Methods 1990, 51, 34–40. [Google Scholar] [CrossRef]
- Tanemura, M.; Fujimoto, S.; Okuyama, F. Dependence of Auger Depth Resolution and Surface Texturing on Primary Ion Species. Surf. Sci. 1990, 230, 283–289. [Google Scholar] [CrossRef]
- Cramer, H.-G.; Grehl, T.; Kollmer, F.; Moellers, R.; Niehuis, E.; Rading, D. Depth profiling of organic materials using improved ion beam conditions. Appl. Surf. Sci. 2008, 255, 966–969. [Google Scholar]
- Ninomiya, S.; Ichiki, K.; Yamada, H.; Nakata, Y.; Seki, T.; Aoki, T.; Matsuo, J. Molecular depth profiling of multilayer structures of organic semiconductor materials by sary ion mass spectrometry with large argon cluster ion beams. Rapid Commun. Mass Spectrom. 2009, 23, 3264–3268. [Google Scholar] [CrossRef]
- Weibel, D.E.; Lockyer, N.; Vickerman, J.C. C60 cluster ion bombardment of organic surfaces. Appl. Surf. Sci. 2004, 231–232, 153–158. [Google Scholar]
- Jones, E.A.; Fletcher, J.S.; Thompson, C.E.; Jackson, D.A.; Lockyer, N.P.; Vickerman, J.C. ToF-SIMS analysis of bio-systems: Are polyatomic primary ions the solution? Appl. Surf. Sci. 2006, 252, 6844–6854. [Google Scholar] [CrossRef]
- Winograd, N.; Postawa, Z.; Cheng, J.; Szakal, C.; Kozole, J.; Garrison, B.J. Improvements in SIMS continue—Is the end in sight? Appl. Surf. Sci. 2006, 252, 6836–6843. [Google Scholar]
- Shon, H.K.; Lee, T.G.; Kim, D.H.; Kang, H.J.; Lee, B.H.; Sung, M.M.; Moon, D.W. The effect of C60 cluster ion beam bombardment in sputter depth profiling of organic–inorganic hybrid multiple thin films. Appl. Surf. Sci. 2008, 255, 1055–1057. [Google Scholar]
- Miyayama, T.; Sanada, N.; Iida, S.-I.; Hammond, J.S.; Suzuki, M. The effect of angle of incidence to low damage sputtering of organic polymers using a C60 ion beam. Appl. Surf. Sci. 2008, 255, 951–953. [Google Scholar] [CrossRef]
- Yang, F.; Forrest, S.R. Photocurrent generation in nanostructured organic solar cells. ACS Nano 2008, 2, 1022–1032. [Google Scholar] [CrossRef]
- Placencia, D.; Wang, W.; Shallcross, R.C.; Nebesny, K.W.; Brumbach, M.; Armstrong, N.R. Organic Photovoltaic Cells Based On Solvent-Annealed, Textured Titanyl Phthalocyanine/C60 Heterojunctions. Adv. Funct. Mater. 2009, 19, 1913–1921. [Google Scholar] [CrossRef]
- Karak, S.; Ray, S.K.; Dhar, A. Improvement of efficiency in solar cells based on vertically grown copper phthalocyanine nanorods. J. Phys. D Appl. Phys. 2010, 43, 245101. [Google Scholar] [CrossRef]
- Yang, F.; Shtein, M.; Forrest, S.R. Controlled growth of a molecular bulk heterojunction photovoltaic cell. Nat. Mater. 2005, 4, 37–41. [Google Scholar] [CrossRef]
- Van Dijken, J.G.; Fleischauer, M.D.; Brett, M.J. Morphology control of CuPc thin films using Glancing Angle Deposition. In Proceedings of 33rd IEEE Photovoltaic Specialists Conference, San Diego, CA, USA, 11–16 May 2008; pp. 1222–1225.
- Zheng, Y.; Bekele, R.; Ouyang, J.; Xue, J. Organic photovoltaic cells with vertically aligned crystalline molecular nanorods. Org. Electron. 2009, 10, 1621–1625. [Google Scholar] [CrossRef]
- Li, N.; Forrest, S.R. Tilted bulk heterojunction organic photovoltaic cells grown by oblique angle deposition. Appl. Phys. Lett. 2009, 95, 123309. [Google Scholar]
- Van Dijken, J.G.; Fleischauer, M.D.; Brett, M.J. Controlled nanostructuring of CuPc thin films via glancing angle deposition for idealized organic photovoltaic architectures. J. Mater. Chem. 2011, 21, 1013–1019. [Google Scholar]
- Van Dijken, J.G.; Fleischauer, M.D.; Brett, M.J. Solvent effects on ZnPc thin films and their role in fabrication of nanostructured organic solar cells. Org. Electron. 2011, 12, 2111–2119. [Google Scholar]
- Hirade, M.; Nakanotani, H.; Yahiro, M.; Adachi, C. Formation of organic crystalline nanopillar arrays and their application to organic photovoltaic cells. ACS Appl. Mater. Interfaces 2011, 3, 80–83. [Google Scholar] [CrossRef]
- Van Dijken, J.G.; Wu, N.L.-Y.; Fleischauer, M.D.; Buriak, J.M.; Brett, M.J. Morphology control and nanoscale patterning of small molecule organic thin films. SPIE 2012, 8435, 8435. [Google Scholar]
- Pfuetzner, S.; Mickel, C.; Jankowski, J.; Hein, M.; Meiss, J.; Schuenemann, C.; Elschner, C.; Levin, A.A.; Rellinghaus, B.; Leo, K.; et al. The influence of substrate heating on morphology and layer growth in C60:ZnPc bulk heterojunction solar cells. Org. Electron. 2011, 12, 435–441. [Google Scholar] [CrossRef]
- Matsuo, Y.; Sato, Y.; Niinomi, T.; Soga, I.; Tanaka, H.; Nakamura, E. Columnar structure in bulk heterojunction in solution-processable three-layered p-i-n organic photovoltaic devices using tetrabenzoporphyrin precursor and silylmethyl[60]fullerene. J. Am. Chem. Soc. 2009, 131, 16048–16050. [Google Scholar]
- Auciello, O. Ion interaction with solids: Surface texturing, some bulk effects, and their possible applications. J. Vac. Sci. Technol. 1981, 19, 841–867. [Google Scholar] [CrossRef]
- Cuomo, J.J.; Rossnagel, S.M.; Kaufman, H.R. Handbook of Ion Beam Processing Technology—Principles, Deposition, Film Modification and Synthesis; Noyes Publications: Park Ridge, NJ, USA, 1989. [Google Scholar]
- Hawkeye, M.M.; Brett, M.J. Glancing angle deposition: Fabrication, properties, and applications of micro- and nanostructured thin films. J. Vac. Sci. Technol. A 2007, 25, 1317–1335. [Google Scholar] [CrossRef]
- Sorge, J.B.; Brett, M.J. Film morphology modification in ion-assisted glancing angle deposition. Thin Solid Films 2010, 519, 1356–1360. [Google Scholar] [CrossRef]
- Taschuk, M.T.; Sorge, J.B.; Steele, J.J.; Brett, M.J. Ion-Beam Assisted Glancing Angle Deposition for Relative Humidity Sensors. IEEE Sens. J. 2008, 8, 1521–1522. [Google Scholar] [CrossRef]
- Kupsta, M.R.; Taschuk, M.T.; Brett, M.J.; Sit, J.C. Reactive Ion Etching of Columnar Nanostructured TiO2 Thin Films for Modified Relative Humidity Sensor Response Time. IEEE Sens. J. 2009, 9, 1979–1986. [Google Scholar] [CrossRef]
- Kwan, J.K.; Sit, J.C. The use of ion-milling to control clustering of nanostructured, columnar thin films. Nanotechnology 2010, 21, 295301. [Google Scholar] [CrossRef]
- Jim, S.R.; Oko, A.J.; Taschuk, M.T.; Brett, M.J. Morphological modification of nanostructured ultrathin-layer chromatography stationary phases. J. Chromatogr. A 2011, 1218, 7203–7210. [Google Scholar] [CrossRef]
- Peters, C.H.; Sachs-Quintana, I.T.; Kastrop, J.P.; Beaupré, S.; Leclerc, M.; McGehee, M.D. High Efficiency Polymer Solar Cells with Long Operating Lifetimes. Adv. Energy Mater. 2011, 1, 491–494. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Naddaf, M.; Chakane, S.; Jain, S.; Bhoraskar, S.V.; Mandale, A.B. Modification of sensing properties of metallophthalocyanine by an ECR plasma. Nucl. Instrum. Meth. B 2002, 194, 54–60. [Google Scholar] [CrossRef]
- Pakhomov, G.L.; Drozdov, M.N.; Vostokov, N.V. Plasma irradiation effects in phthalocyanine films. Appl. Surf. Sci. 2004, 230, 241–248. [Google Scholar] [CrossRef]
- Kaplan, M.L.; Forrest, S.R.; Schmidt, P.H.; Venkatesan, T. Optical and electrical properties of ion-beam-irradiated films of organic molecular solids and polymers. J. Appl. Phys. 1984, 55, 732. [Google Scholar]
- Nath, A.; Prushan, M.J.; Gilbert, J.G. Can super-excited molecules survive fragmentation? J. Radioanal. Nucl. Chem. 2001, 247, 589–591. [Google Scholar] [CrossRef]
- Wei, H.X.; Li, J.; Xu, Z.Q.; Cai, Y.; Tang, J.X.; Li, Y.Q. Thermal annealing-induced vertical phase separation of copper phthalocyanine: Fullerene bulk heterojunction in organic photovoltaic cells. Appl. Phys. Lett. 2010, 97, 083302. [Google Scholar]
- Wei, H.X.; Li, J.; Cai, Y.; Xu, Z.Q.; Lee, S.T.; Li, Y.Q.; Tang, J.X. Electronic structures of planar and mixed C70/CuPc heterojunctions in organic photovoltaic devices. Org. Electron. 2011, 12, 1422–1428. [Google Scholar] [CrossRef]
- Grządziel, L.; Krzywiecki, M.; Peisert, H.; Chassé, T.; Szuber, J. Influence of ambient air exposure on surface chemistry and electronic properties of thin copper phthalocyanine sensing layers. Thin Solid Films 2011, 519, 2187–2192. [Google Scholar]
- Sample Availability: Samples of the films are not available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van Dijken, J.G.; Brett, M.J. Dry Etching of Copper Phthalocyanine Thin Films: Effects on Morphology and Surface Stoichiometry. Molecules 2012, 17, 10119-10130. https://doi.org/10.3390/molecules170910119
Van Dijken JG, Brett MJ. Dry Etching of Copper Phthalocyanine Thin Films: Effects on Morphology and Surface Stoichiometry. Molecules. 2012; 17(9):10119-10130. https://doi.org/10.3390/molecules170910119
Chicago/Turabian StyleVan Dijken, Jaron G., and Michael J. Brett. 2012. "Dry Etching of Copper Phthalocyanine Thin Films: Effects on Morphology and Surface Stoichiometry" Molecules 17, no. 9: 10119-10130. https://doi.org/10.3390/molecules170910119