Synthesis and Bioactivity Evaluation of New 6-Aryl-5-cyano Thiouracils as Potential Antimicrobial and Anticancer Agents
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry
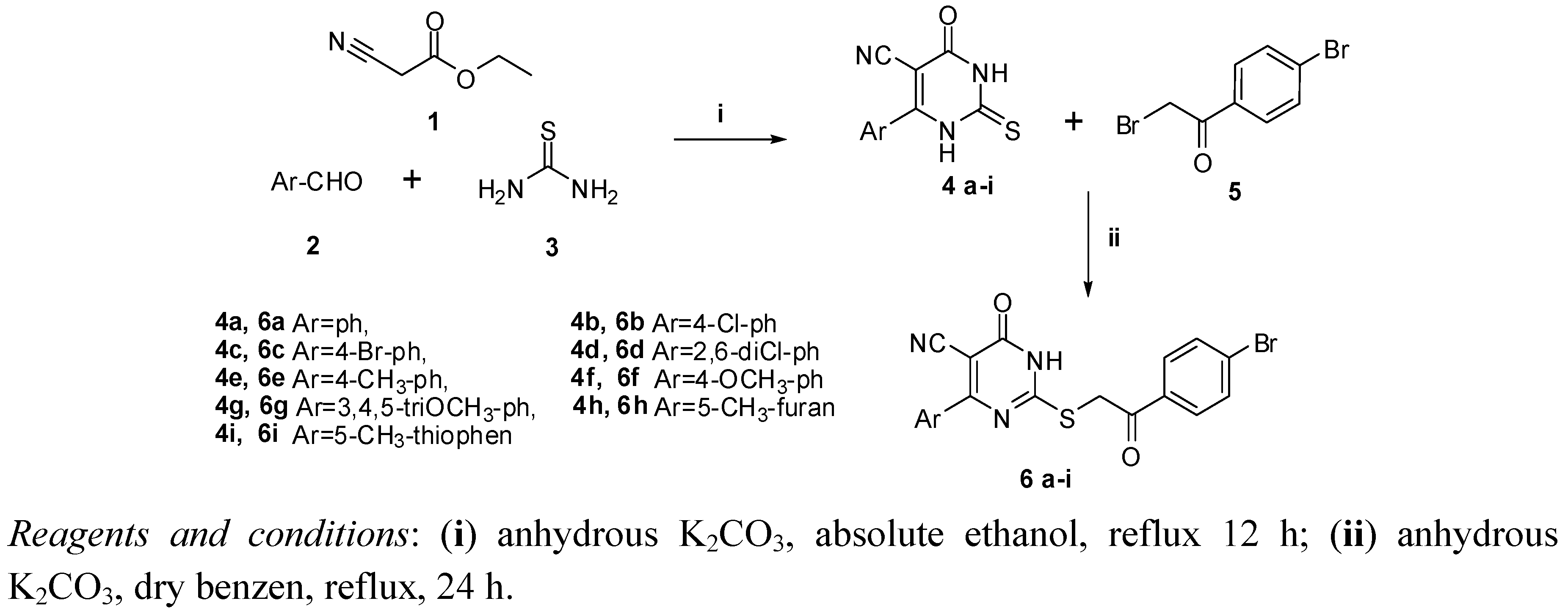
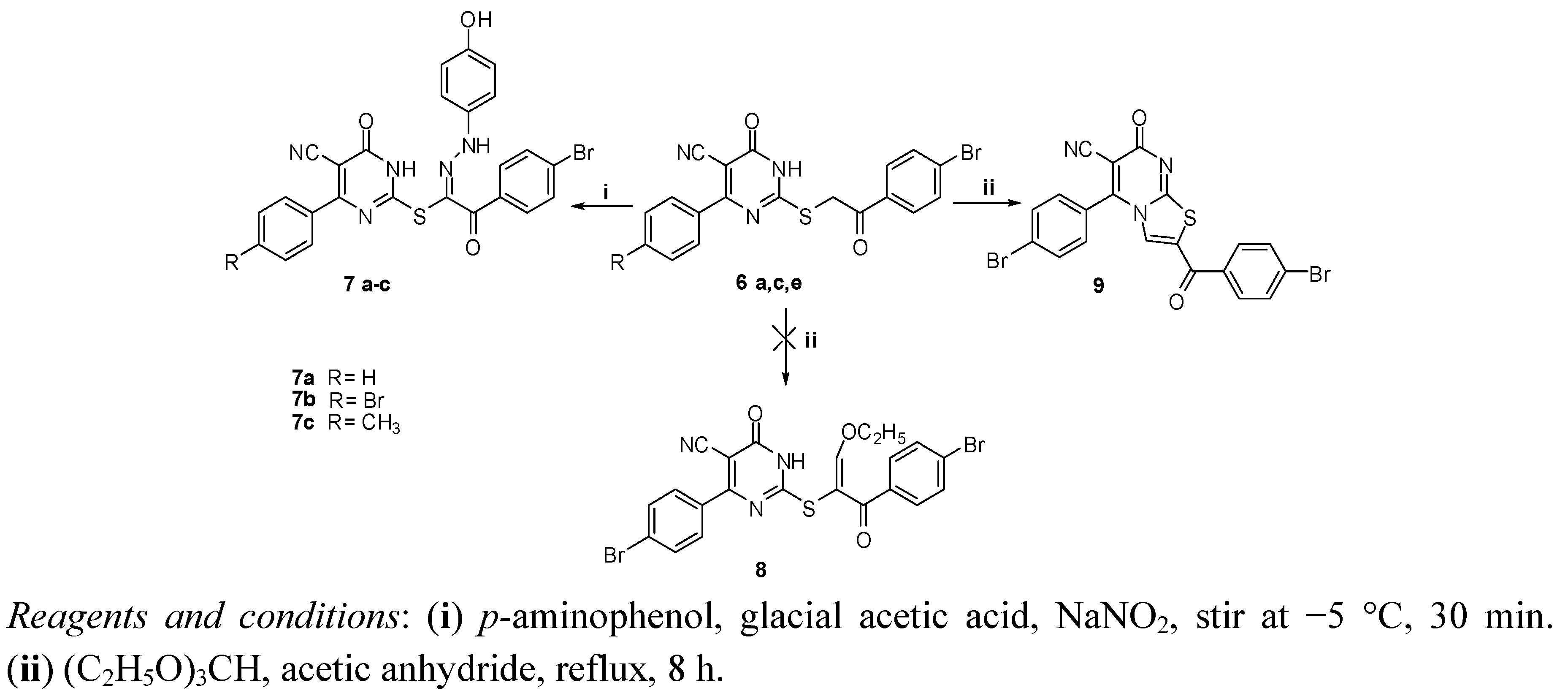

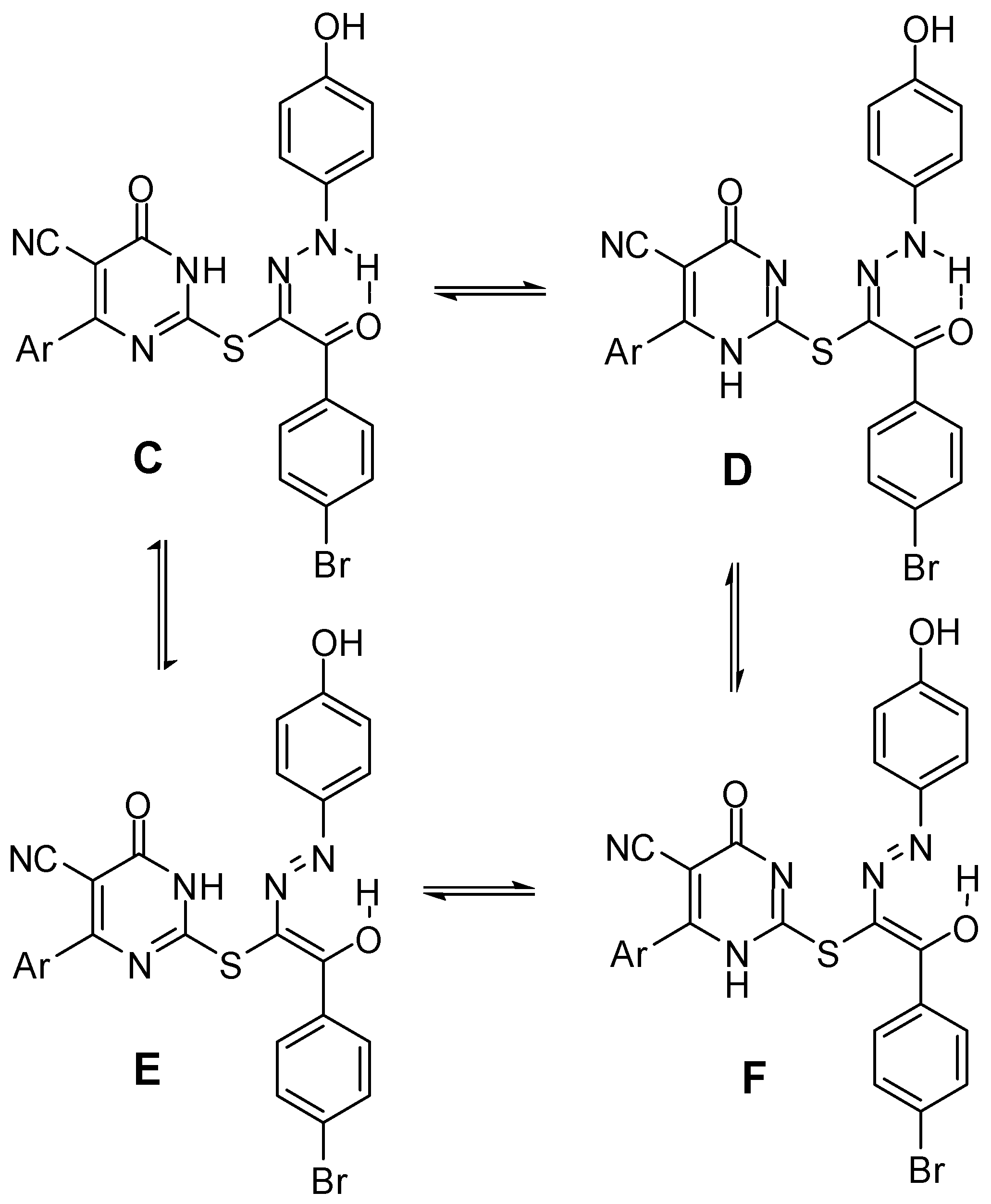
2.2. Biological Evaluation
2.2.1. Antimicrobial Activity
| Compound | Gram positive bacteria | Gram negative bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| S. aureus | B. subtlis | E. coli | P. aeruginosa | C. albicans | A. niger | ||
| 4d | MIC | 9.38 | 9.38 | >50 | >50 | >50 | >50 |
| MBC | 9.30 | 9.30 | >50 | >50 | >50 | >50 | |
| IC50 | 4.20 | 6.25 | >50 | >50 | >50 | >50 | |
| 4g | MIC | >50 | >50 | >50 | >50 | 9.38 | 18.75 |
| MBC | >50 | >50 | >50 | >50 | 9.30 | 18.75 | |
| IC50 | >50 | >50 | >50 | >50 | 6.25 | 12.50 | |
| 4h | MIC | 1.17 | 1.17 | >50 | >50 | >50 | >50 |
| MBC | 1.56 | 1.56 | >50 | >50 | >50 | >50 | |
| IC50 | 0.40 | 0.78 | >50 | >50 | >50 | >50 | |
| 4i | MIC | 2.34 | 9.38 | 9.38 | 9.38 | 2.34 | 4.69 |
| MBC | 2.30 | 9.30 | 9.30 | 9.30 | 2.30 | 6.25 | |
| IC50 | 1.17 | 6.25 | 3.13 | 3.13 | 1.17 | 3.13 | |
| 6a | MIC | 4.69 | 9.38 | 18.75 | 37.50 | >50 | >50 |
| MBC | 3.80 | 9.00 | 18.75 | 37.50 | >50 | >50 | |
| IC50 | 2.30 | 6.25 | 12.50 | 25.00 | >50 | >50 | |
| 6b | MIC | >50 | >50 | >50 | >50 | >50 | >50 |
| MBC | >50 | >50 | >50 | >50 | >50 | >50 | |
| IC50 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 6c | MIC | 37.50 | 37.50 | 37.50 | 37.50 | > 50 | > 50 |
| MBC | 37.50 | 37.50 | 37.50 | 37.50 | > 50 | > 50 | |
| IC50 | 12.50 | 25.00 | 25.00 | 25.00 | > 50 | > 50 | |
| 6d | MIC | >50 | >50 | >50 | >50 | >50 | >50 |
| MBC | >50 | >50 | >50 | >50 | >50 | >50 | |
| IC50 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 6e | MIC | >50 | >50 | >50 | >50 | >50 | >50 |
| MBC | >50 | >50 | >50 | >50 | >50 | >50 | |
| IC50 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 6f | MIC | 2.34 | 4.69 | >50 | >50 | >50 | >50 |
| MBC | 3.13 | 6.25 | >50 | >50 | >50 | >50 | |
| IC50 | 1.56 | 3.13 | >50 | >50 | >50 | >50 | |
| 6g | MIC | 4.69 | 4.69 | >50 | >50 | >50 | >50 |
| MBC | 4.70 | 4.70 | >50 | >50 | >50 | >50 | |
| IC50 | 1.56 | 1.56 | >50 | >50 | >50 | >50 | |
| 6h | MIC | >50 | >50 | >50 | >50 | >50 | >50 |
| MBC | >50 | >50 | >50 | >50 | >50 | >50 | |
| IC50 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 6i | MIC | >50 | >50 | >50 | >50 | >50 | >50 |
| MBC | >50 | >50 | >50 | >50 | >50 | >50 | |
| IC50 | >50 | >50 | >50 | >50 | >50 | >50 | |
| 7a | MIC | 18.75 | 18.75 | >50 | >50 | >50 | >50 |
| MBC | 18.75 | 18.75 | >50 | >50 | >50 | >50 | |
| IC50 | 12.50 | 12.50 | >50 | >50 | >50 | >50 | |
| 7b | MIC | 1.17 | 2.34 | >50 | >50 | >50 | >50 |
| MBC | 1.17 | 2.30 | >50 | >50 | >50 | >50 | |
| IC50 | 0.78 | 1.17 | >50 | >50 | >50 | >50 | |
| 7c | MIC | 0.19 | 1.17 | >50 | >50 | >50 | >50 |
| MBC | 0.20 | 1.17 | >50 | >50 | >50 | >50 | |
| IC50 | 0.15 | 0.40 | >50 | >50 | >50 | >50 | |
| 9 | MIC | 37.50 | 37.50 | >50 | >50 | >50 | 37.50 |
| MBC | 37.50 | 50.00 | >50 | >50 | >50 | 50.00 | |
| IC50 | 12.50 | 37.50 | >50 | >50 | >50 | 37.50 | |
| Amoxicillin | MIC | 1.25 | 150.00 | NA | NA | NA | NA |
| Gentamicin | MIC | NA | NA | 1.00 | 8.00 | NA | NA |
| Amphotericin B | MIC | NA | NA | NA | NA | 3.00 | 1.25 |

2.2.2. Anticancer Activity
| Compound | Mean growth, % | Range of growth, % | Panel | Most sensitive cell lines |
|---|---|---|---|---|
| growth, % | ||||
| 4d | 102.9 | 76.85–126.64 | Renal Cancer | UO-31 (76.85) |
| 4g | 102.53 | 81.19–121.32 | Renal Cancer | A498 (81.19) |
| 4h | 104.95 | 82.37–133.71 | Renal Cancer | UO-31 (82.37) |
| 4i | 105.24 | 76.99–127.08 | Melanoma | L OX IMVI (76.99) |
| Renal Cancer | 786-0 (83.54) , UO-31 (84.31) | |||
| 6a | 102.14 | 80.39–119.48 | Renal Cancer | A498 (85.49) , UO-31 (80.39) |
| 6b | 103.26 | 80.27–121.43 | Renal Cancer | A498 (80.27) |
| 6c | 102.26 | 80.07–117.90 | Renal Cancer | A498 (80.07) |
| 6d | 89.03 | 41.03–118.60 | Leukemia | HL-60(TB) (75.34), K-562 (73.85), MOLT-4 (73.33), |
| RPMI-8226 (72.35), SR (79.59). | ||||
| EKVX (79.94), HOP-92 (41.03), NCI-H522 (75.25). | ||||
| Non Small Cell Lung Cancer | HCT-116 (74.54), | |||
| HTC-15 (80.89). | ||||
| SF-295 (81.77). | ||||
| Colon Cancer | SK-MEL-2 (82.18), | |||
| CNS Cancer | UACC-62 (80.12) | |||
| Melanoma | 780-0 (73.64), A498 (75.93), CAKI-1 (71.34), UO-31 (80.25). | |||
| Renal Cancer | MCF-7 (83.81), T-47D (74.23), MDA-MB-468 (73.27) | |||
| Breast Cancer | ||||
| 6e | 101.92 | 82.81–119.49 | Renal Cancer | UO-31 (82.81) |
| 6f | 102.3 | 78.50–121.80 | Non-Small Cell Lung Cancer | HOP-92 (85.86) |
| Renal Cancer | ||||
| A498 (78.50), UO-31 (86.29) | ||||
| 6g | 102.98 | 83.81–124.78 | Renal Cancer | A498 (84.19), UO-31 (83.81) |
| 6h | 101.72 | 73.09–114.65 | Renal Cancer | UO-31 (73.09) |
| 6i | 102.41 | 42.38–119.33 | Leukemia | MOLT-4 (42.38) |
| Melanoma | L OX IMVI (75.06) | |||
| Renal Cancer | 786-0 (81.16), UO-31 (80.26) |
3. Experimental
3.1. Chemistry
3.2. Biological Evaluation
3.2.1. Determination of the Antimicrobial Activitiess
3.2.1.1. Determination of the Minimum Inhibitory Concentration (MIC)
3.2.1.2. Determination of the MBC and IC50
3.2.2. Anticancer Activity [37]
4. Conclusions
Acknowledgments
References
- Maga, G.; Radi, M.; Gerard, M.A.; Botta, M.; Ennifar, E. HIV-1 RT inhibitors with novel mechanism of action: NNRTIs that compete with the nucleotide substrate. Viruses 2010, 2, 880–899. [Google Scholar] [CrossRef]
- Callery, P.; Gannett, P. Cancer and cancer chemotherapy. In Foye’s Principles of Medicinal Chemistry, 5th; Williams, D.A., Lemke, T.L., Eds.; Lippincot Williams and Wilkins: Philadelphia, PA, USA, 2002; pp. 934–935. [Google Scholar]
- Deshmukh, M.B.; Salunkhe, S.M.; Patil, D.R.; Anbhule, P.V. A novel and efficient one step synthesis of 2-amino-5-cyano-6-hydroxy-4-aryl pyrimidines and their anti-bacterial activity. Eur. J. Med. Chem. 2009, 44, 2651–2654. [Google Scholar] [CrossRef]
- Masoud, M.S.; Ibrahim, A.A.; Khalil, E.A.; El-Marghany, A. Spectral properties of some metal complexes derived from uracil-thiouracil and citrazinic acid compounds. SpectrochimActa A Mol. Biomol. Spectrosc. 2007, 67, 662–668. [Google Scholar] [CrossRef]
- Fathalla, O.A.; Awad, S.M.; Mohamed, M.S. Synthesis of new 2-thiouracil-5-sulfonamide derivatives with antibacterial and antifungal activity. Arch. Pharm. Res. 2005, 28, 1205–1212. [Google Scholar] [CrossRef]
- Odani, A.; Kozlowski, H.; Swiatek-Kozlowska, J.; Brasun, J.; Operschall, B.P.; Sigel, H. Extent of metal ion-sulfur binding in complexes of thiouracil nucleosides and nucleotides in aqueous solution. J. Inorg. Biochem. 2007, 101, 727–735. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Sornsongkhram, N.; Pingaew, R.; Techatanachai, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and novel bioactivities of substituted 6-propylthiouracils. Eur. J. Sci. Res. 2009, 36, 236–245. [Google Scholar]
- Prachayasittikul, S.; Worachartcheewan, A.; Nantasenamat, C.; Chinworrungsee, M.; Sornsongkhram, N.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and structure-activity relationship of 2-thiopyrimidine-4-one analogues as antimicrobial and anticancer agents. Eur. J. Med. Chem. 2011, 46, 738–742. [Google Scholar] [CrossRef]
- He, Y.P.; Long, J.; Zhang, S.S.; Li, C.; Lai, C.C.; Zhang, C.S.; Li, D.X.; Zhang, D.H.; Wanga, H.; Cai, Q.Q.; Zheng, Y.T. Synthesis and biological evaluation of novel dihydro-aryl/alkylsulfanyl-cyclohexylmethyl-oxopyrimidines (S-DACOs) as high active anti-HIV agents. Bioorg. Med. Chem. Lett. 2011, 21, 694–697. [Google Scholar]
- He, Y.; Chen, F.; Sun, G.; Wang, Y.; Clercq, E.D.; Balzarini, J.; Pannecouque, C. Alkyl-2-[(aryl and alkyloxylcarbonylmethyl)thio]-6-(1-naphthylmethyl)pyrimidin-4(3H)-ones as an unique HIV reverse transcriptase inhibitors of S-DABO series. Bioorg. Med. Chem. Lett. 2004, 14, 3173–3176. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Ragab, F.AF.; Ramala, M.M.; El-Diwani, H.I. Novel benzimidazole-pyrimidine conjugates as potent antitumor agents. Eur. J. Med. Chem. 2010, 45, 2336–2344. [Google Scholar] [CrossRef]
- Galal, S.A.; Abdelsamie, A.S.; Tokuda, H.; Suzuki, N.; Lida, A.; El-Hefnawi, M.M.; Ramadan, R.A.; Atta, M.H.E.; El Diwani, H.I. Part I: Synthesis, cancer chemopreventive activity and molecular docking study of novel quinoxaline derivatives. Eur. J. Med. Chem. 2011, 46, 327–340. [Google Scholar] [CrossRef]
- Al-Abdullah, E.S.; Al-Obaid, A.-R.M.; Al-Deeb, O.A.; Habib, E.E.; El-Emam, A.A. Synthesis of novel 6-phenyl-2,4-disubstituted pyrimidine-5-carbonitriles as potential antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 4642–4647. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Awad, S.M.; Ahmed, N.M. Anticancer cancer activities of 6-aryl-5-cyano-2-thiouracil derivatives. Pharma Res. 2012, 6, 54–60. [Google Scholar]
- Mohamed, M.S.; Awad, S.M.; Ahmed, N.M. Synthesis and antimicrobial evaluation of some 6-aryl-5-cyano-2-thiouracil derivatives. Acta Pharm. 2011, 61, 171–185. [Google Scholar] [CrossRef]
- Fathalla, O.A.; Zeid, I.F.; Haiba, M.E.; Soliman, A.M. Synthesis, antibacterial and anticancer evaluation of some pyrimidine derivatives. World J. Chem. 2009, 4, 127–132. [Google Scholar]
- Chen, W.; Huang, Y.; Gundala, S.R.; Yan, H.; Li, M.; Tai, P.C.; Wang, B. The first low μM SecA inhibitors. Bioorg. Med. Chem. 2010, 18, 1617–1625. [Google Scholar]
- Ding, Y.; Girardet, J.L.; Smith, K.L.; Larson, G.; Prigaro, B.; Wu, J.Z.; Yao, N. Parallel synthesis of 5-cyano-6-aryl-2-thiouracil derivatives as inhibitors for hepatitis C viral NS5B RNA-dependent RNA polymerase. Bioorg. Chem. 2006, 34, 26–38. [Google Scholar] [CrossRef]
- Chen, H.; Tsalkova, T.; Mei, F.C.; Hu, Y.; Cheng, X.; Zhou, J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 2012, 22, 4038–4043. [Google Scholar]
- Rose, Y.; Ciblat, S.; Reddy, R.; Belley, A.; Dietrich, L.; McKay, G.; Rafai, A.; Delorme, D. Novel non-nucleobase inhibitors of Staphylococcus aureus DNA polymerase IIIC. Bioorg. Med. Chem. Lett. 2006, 16, 891–896. [Google Scholar]
- Onnis, V.; Cocco, M.T.; Fadda, R.; Congiu, C. Synthesis and evaluation of anticancer activity of 2-arylamino-6-trifluoromethyl-3-(hydrazonocarbonyl)pyridines. Bioorg. Med. Chem. 2009, 17, 6158–6165. [Google Scholar] [CrossRef]
- Edrees, M.M.; Farghaly, T.A.; El-Hag, F.A.A.; Abdalla, M.M. Antimicrobial, antitumor and 5α-reductase inhibitor activities of some hydrazonoyl substituted pyrimidinones. Eur. J. Med. Chem. 2010, 45, 5702–5707. [Google Scholar] [CrossRef]
- Shawali, A.S.; Elghandour, A.H.; Sayed, A.R. A Novel One-Pot Synthesis of 3-arylazo-[1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones. Synth. Commun. 2001, 31, 731–740. [Google Scholar] [CrossRef]
- Bedford, G.R.; Taylor, P.J.; Webb, G.A. 15N-NMR studies of guanidines. II-the fused-in guanidine unit of some oxoheterocycles: A combined 15N-NMR, 13C-NMR and IR study. Magn. Res. Chem. 1995, 33, 389–394. [Google Scholar] [CrossRef]
- Elguero, J.; Goya, P.; Martinez, A.; Rozas, I. On the tautomerism of 2-phenacyl-4-pyrimidinones and related compounds. Chem. Ber. 1989, 122, 919–924. [Google Scholar] [CrossRef]
- Greenhill, J.V.; Ismail, M.J.; Bedford, G.R.; Edwards, P.N.; Taylor, P.J.J. Conformational and tautomeric studies of acylguanidines. II: Vibrational and carbon-13 nuclear magnetic resonance spectroscopy. Chem. Soc. Perkin Trans. 1985, 2, 1265–1274. [Google Scholar]
- Reiter, J.; Bongo, L.; Dyortsok, P. Synthesis and tautomeric structure of 2-[N-aryl-2-oxo-2-arylethanehydrazonoyl]-6-methyl-4(3H)-pyrimidinones. Tetrahedron 1987, 43, 2497–2504. [Google Scholar] [CrossRef]
- Jones, R.; Rayan, A.J.; Sternhell, S.; Wright, S.E. The structures of some 5-pyrazolones and derived 4-arylazo-5-pyrazolones. Tetrahedron 1963, 19, 1497–1507. [Google Scholar] [CrossRef]
- Shawali, A.S.; Farghaly, T.A. Synthesis and tautomeric structure of 2-[N-aryl-2-oxo-2-arylethanehydrazonyl]-6-methyl-4(3H)-pyrimidinone. Tetrahedron 2004, 60, 3051–3057. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing, 19th Informational Supplement; CLSI: Wayne, PA, USA, 2009.
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, Carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Prindle, R.F.; Wright, E.S. Phenolic compounds. In Disinfection, Sterilization and Preservation; Block, S.S., Ed.; Lea and Febiger: Philadelfia, PA, USA, 1997; pp. 115–118. [Google Scholar]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolfe, A.; et al. Feasibility of a high-flux anticancer drug screen utilizing a diverse panel of human tumor cell lines in culture. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar]
- Boyd, M.R. Cancer Drug Discovery and Development. In Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials and Approval; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA, 1997; Volume 2, pp. 23–43. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.; Bokesch, H.; Kenney, S.; Boyd, M. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Parmar, J.M.; Modha, J.J.; Parikh, A.R. Synthesis of azetidinones and thiazolidinones from hydrazinopyrimidine as potential antimicrobial agents. Indian J. Chem. 1999, 38, 440–444. [Google Scholar]
- Khodair, A.I.; Ibrahim, E.E.; Ashry, E.S.H. Glycosylation of 2-thiouracil derivatives. A synthetic approach to 3-glycosyl-2,4-dioxypyrimidines. Nucleosides Nucleotides 1997, 16, 433–444. [Google Scholar]
- Abdou, I.B.; Strekowski, L. A facile synthesis of 6-aryl-5-cyano-1-(β-d-pyranosyl or β-d-furanosyl)-2-thiocytosines. Tetrahedron 2000, 56, 8631–8636. [Google Scholar] [CrossRef]
- Azéma, J.; Guidetti, B.; Korolyov, A.; Kiss, R.; Roques, C.; Constant, P.; Daffé, M.; Malet-Martino, M. Synthesis of lipophilic dimeric C-7/C-7-linked ciprofloxacin and C-6/C-6-linked levofloxacin derivatives. Versatile in vitro biological evaluations of monomeric and dimeric fluoroquinolone derivatives as potential antitumor, antibacterial or antimycobacterial agents. Eur. J. Med. Chem. 2011, 46, 6025–6038. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 6a–i, 7a–c are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Taher, A.T.; Abou-Seri, S.M. Synthesis and Bioactivity Evaluation of New 6-Aryl-5-cyano Thiouracils as Potential Antimicrobial and Anticancer Agents. Molecules 2012, 17, 9868-9886. https://doi.org/10.3390/molecules17089868
Taher AT, Abou-Seri SM. Synthesis and Bioactivity Evaluation of New 6-Aryl-5-cyano Thiouracils as Potential Antimicrobial and Anticancer Agents. Molecules. 2012; 17(8):9868-9886. https://doi.org/10.3390/molecules17089868
Chicago/Turabian StyleTaher, Azza Taher, and Sahar Mahmoud Abou-Seri. 2012. "Synthesis and Bioactivity Evaluation of New 6-Aryl-5-cyano Thiouracils as Potential Antimicrobial and Anticancer Agents" Molecules 17, no. 8: 9868-9886. https://doi.org/10.3390/molecules17089868




