The Active Ingredients of Jiang-Zhi-Ning: Study of the Nelumbo nucifera Alkaloids and Their Main Bioactive Metabolites
Abstract
:Abbreviations
| DSC | differential scanning calorimetry |
| HL | hyperlipidemia |
| HPLC | high performance liquid chromatography |
| JZN | Jiang-Zhi-Ning |
| LC/MS | liquid chromatography–mass spectrometry |
| 2H1M | 2-hydroxy-1-methoxyaporphin |
| UV | ultra-violet spectrophotometry |
1. Introduction
2. Results and Discussion
2.1. Identification and Attribution of the Effective Components of JZN
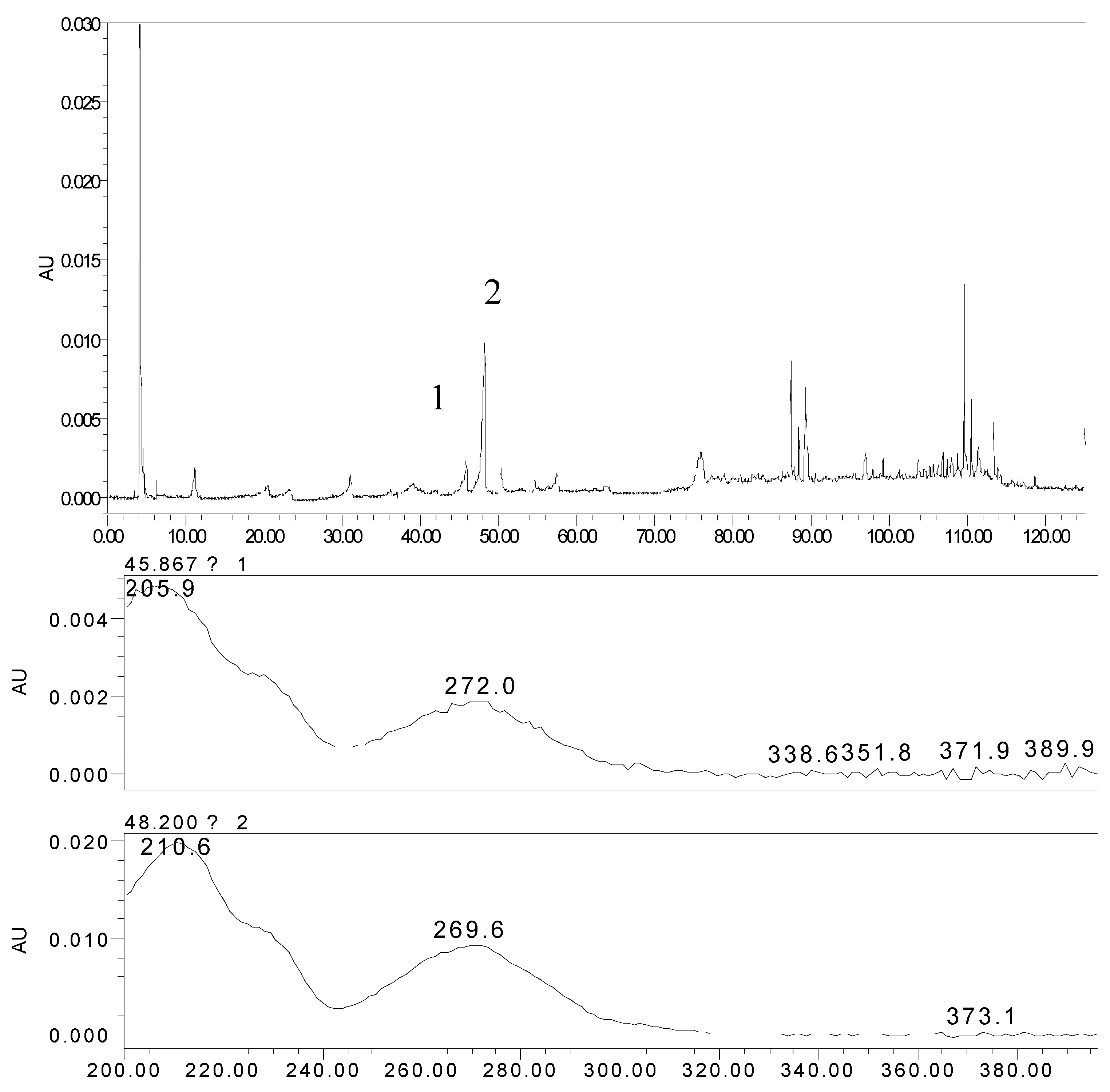
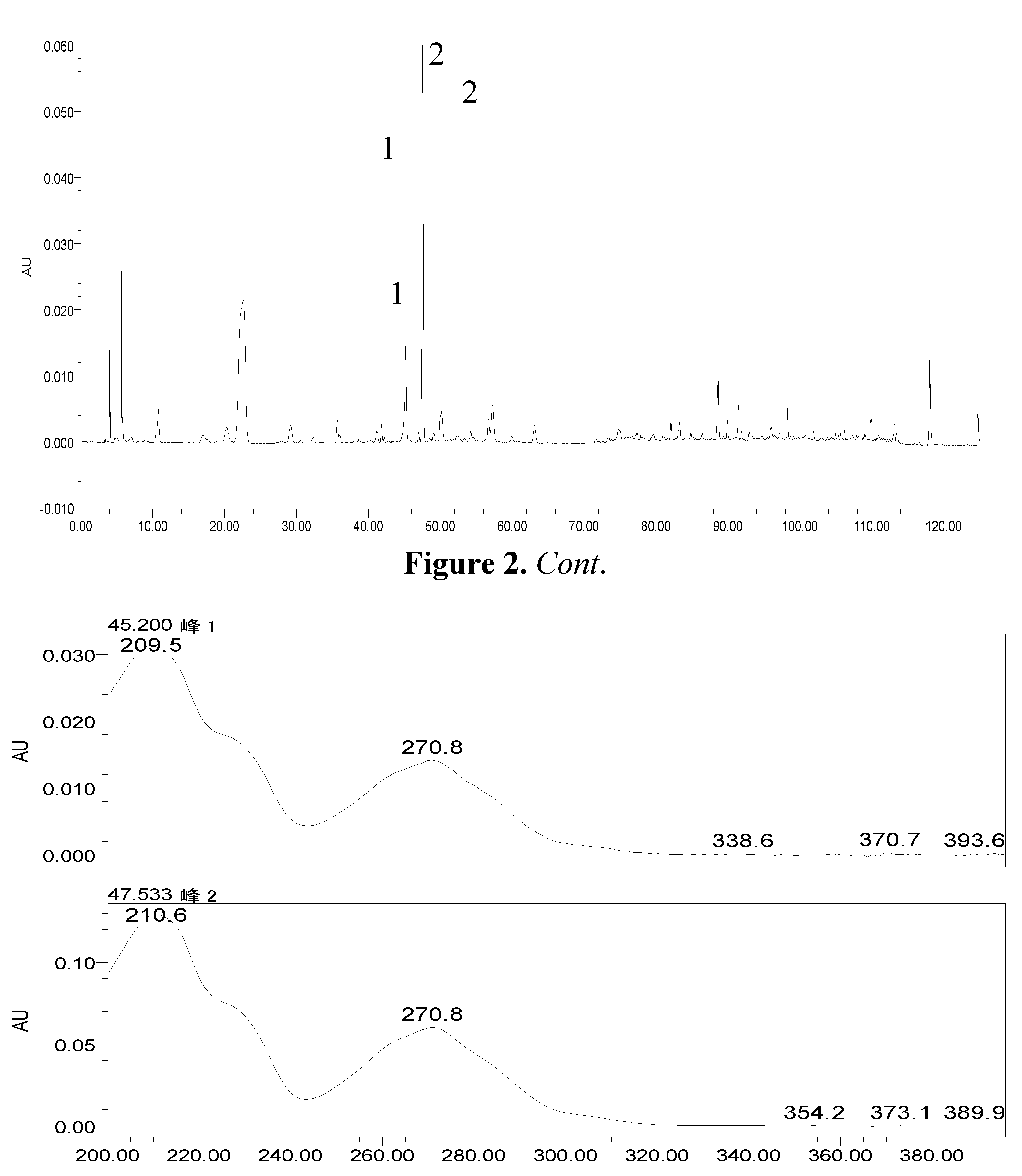
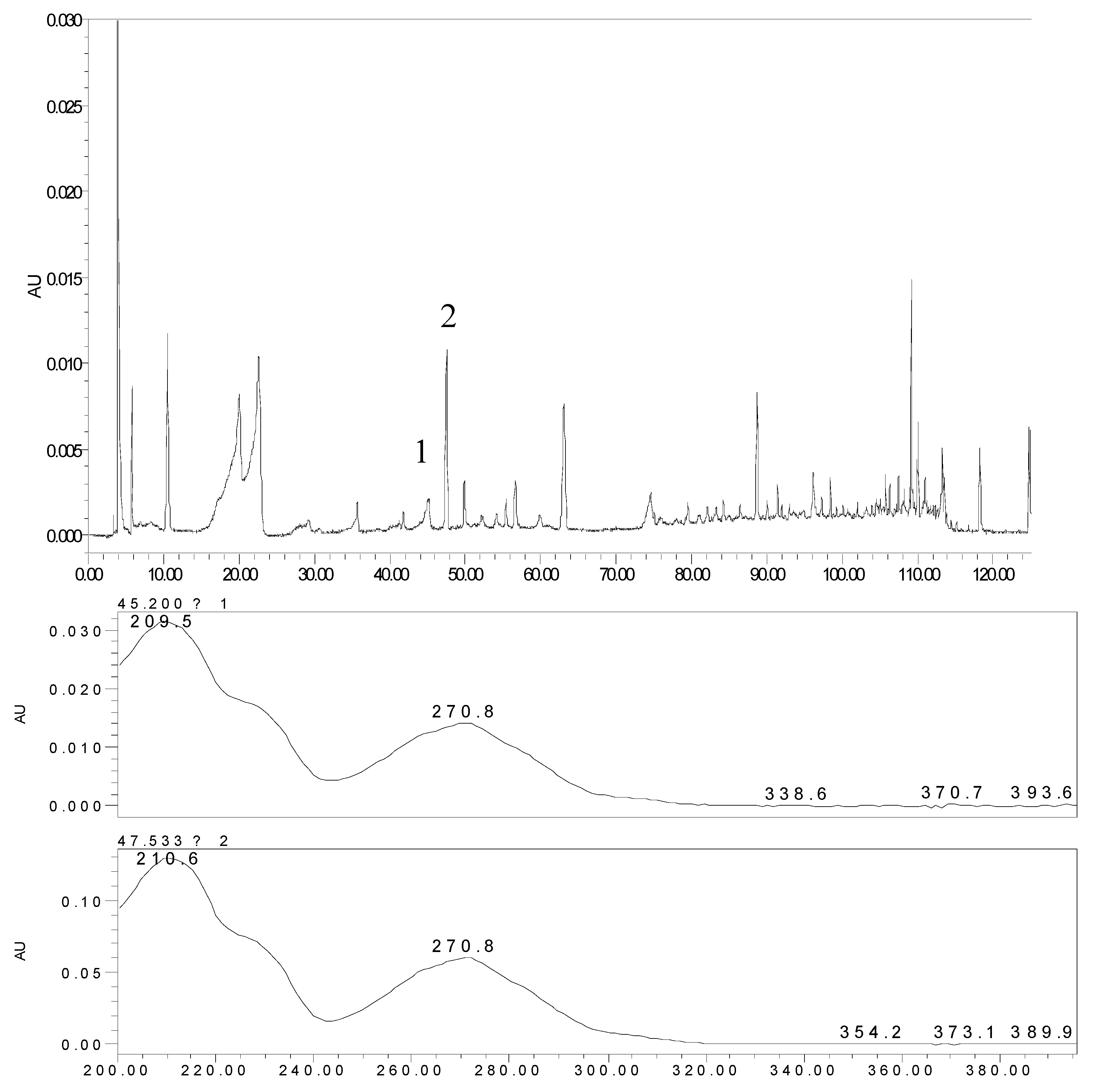
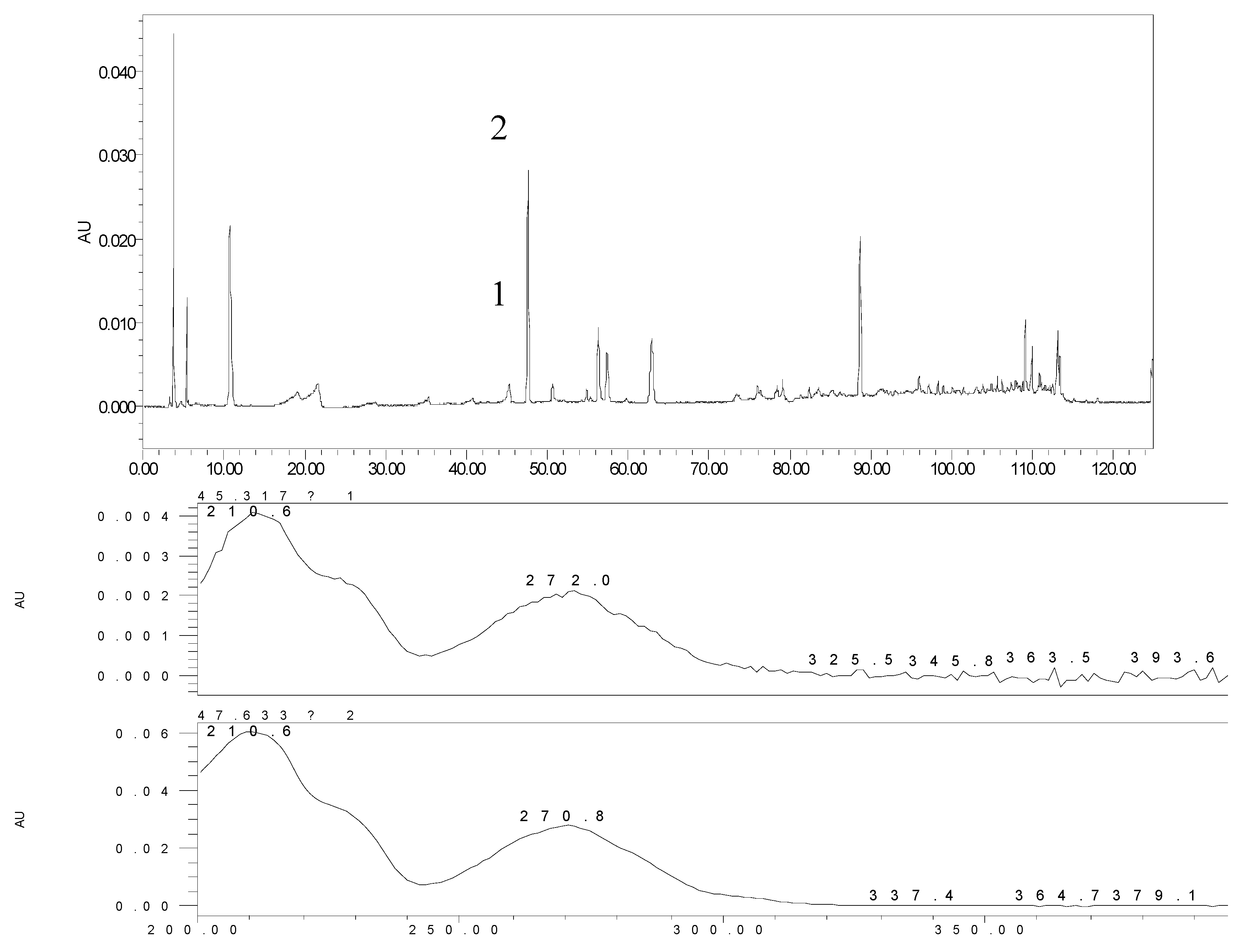


2.2. The Metabolites of 2H1M
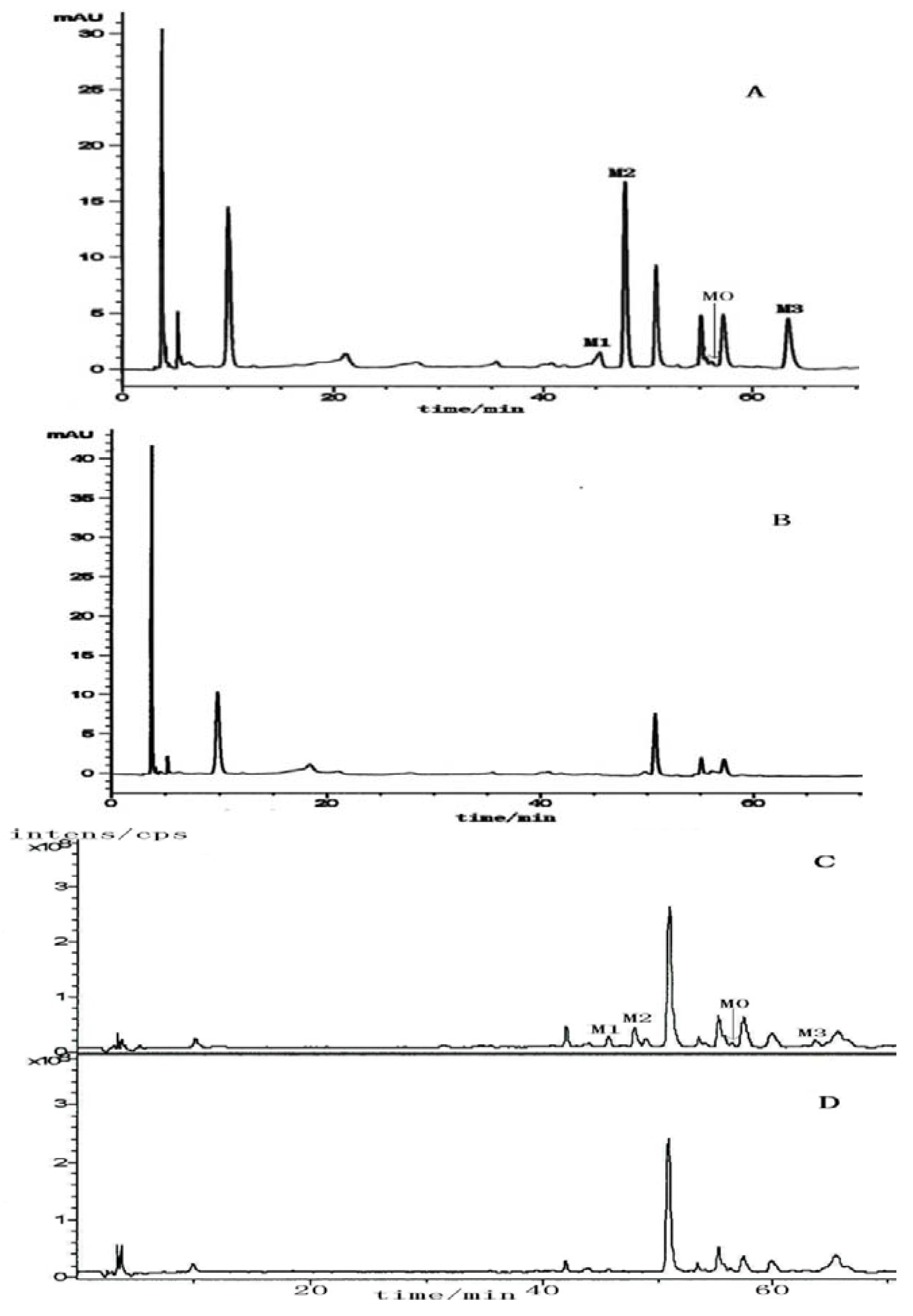
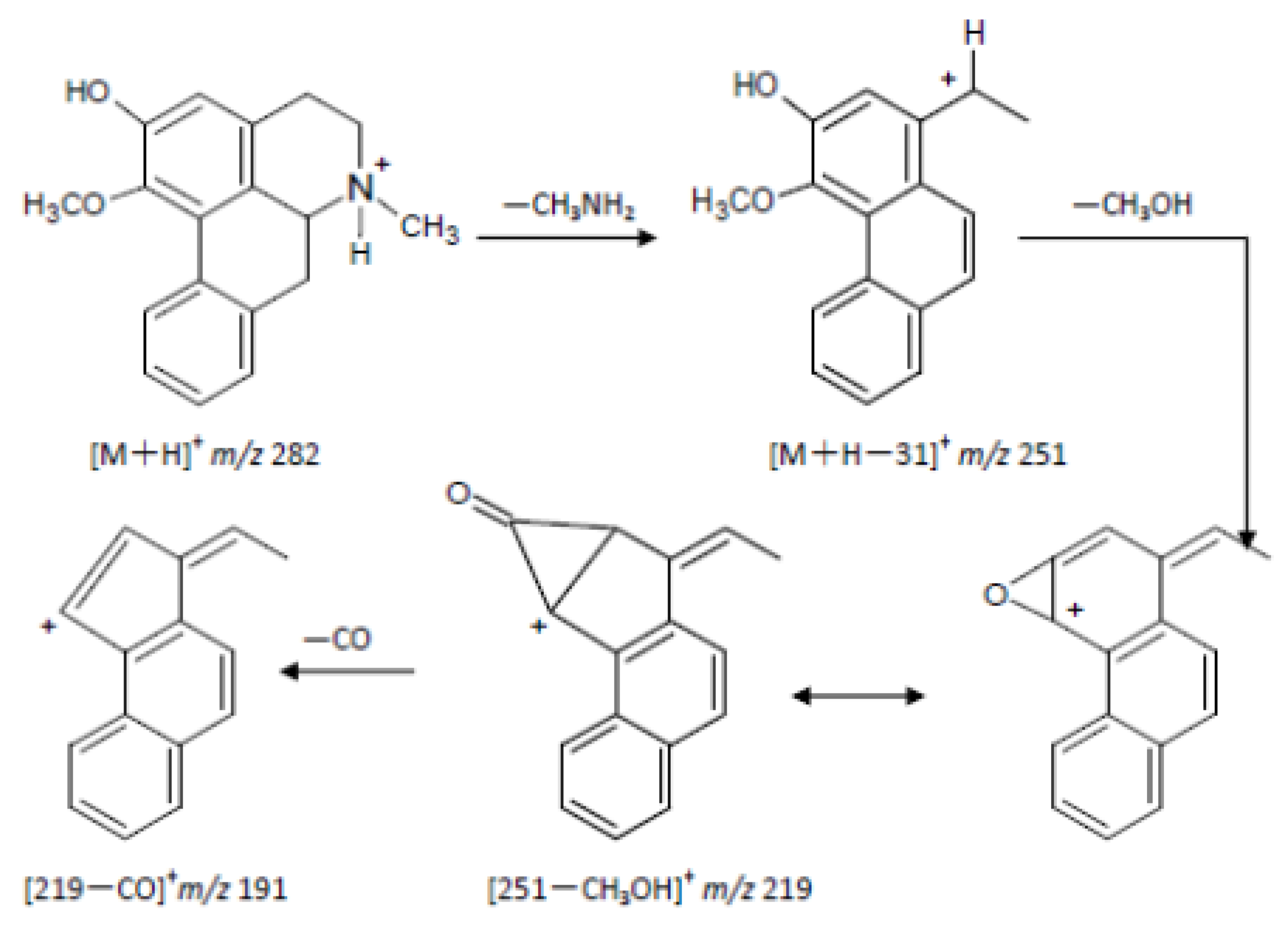
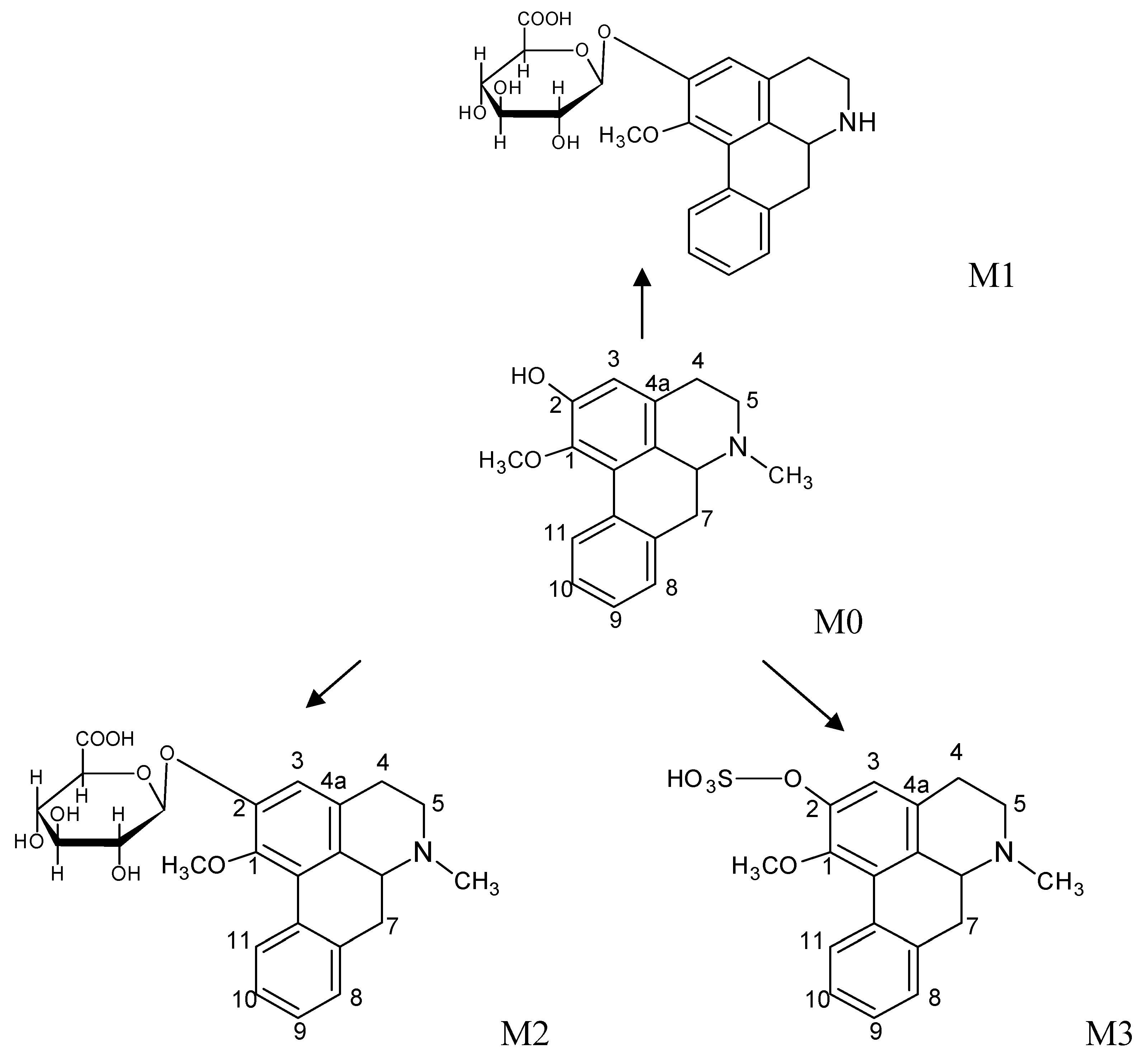

3. Experimental
3.1. General
3.2. Identification and Attribution of Effective Ingredients of JZN
3.2.1. Experimental Animals and Sample Preparation
3.2.2. Chromatographic Conditions
| Time (min) | Acetonitrile (%) | Water (0.01% Formic acid) (%) |
|---|---|---|
| 0 | 2 | 98 |
| 20 | 2 | 98 |
| 50 | 16 | 84 |
| 65 | 16 | 84 |
| 90 | 40 | 60 |
| 100 | 60 | 40 |
| 110 | 100 | 0 |
3.2.3. Source of Precursor and Identification
3.2.4. Attribution of Precursors in Different Alkaloid Fractions of Nelumbo nucifera
3.2.5. Structure Identification of 2H1M
3.3. Experiments Investigating the Metabolites of 2H1M
3.3.1. Isolation of 2H1M
3.3.2. Plasma Sample Collection and Preparation
3.3.3. LC/MSn Conditions
4. Conclusions
Acknowledgements
References
- Chen, J.; Zhao, H.; Yang, Y.; Liu, B.; Ni, J.; Wang, W. Lipid-lowering and antioxidant activities of Jiang-Zhi-Ning in Traditional Chinese Medicine. J. Ethnopharmacol. 2011, 134, 919–930. [Google Scholar] [CrossRef]
- Wang, F.G.; Liu, B.; Wang, W.; Shi, R.B. Study on adscription of drug constituents in Beagle dog plasma after intragastric administration with Jiangzhining extract. Zhongguo Zhong Yao Za Zhi 2008, 33, 912–914. [Google Scholar]
- Guan, J.H.; Liang, A.H.; Feng, Q.J.; Hu, S.S. Influence of the watery extract of jiangzhining decoction on the genetic expression of hepatocyte LDLR of hyperlipidemic rats. Zhongguo Zhong Yao Za Zhi 2002, 27, 289–291. [Google Scholar]
- Xu, D.Y.; Liu, B.; Wang, W. Study on Quality Evaluation of Jiangzhining Based on Anti—oxidative Activity. Chin. J. Exp. Tradit. Med. Formulae 2010, 16, 44–47. [Google Scholar]
- Zeng, M.; Wang, J.; Zhao, G.; Bian, J. Study on Lipid-Regulating Function of Shouming Jiangzhining. Chin. Pharm. 2009, 18, 17–18. [Google Scholar]
- Yang, Z.D.; Zhang, X.; Du, J.; Ma, Z.J.; Guo, F.; Li, S.; Yao, X.J. An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure-activity correlations. Nat. Prod. Res. 2012, 26, 387–392. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Y.; Hu, R.; Chen, J.; Zhang, Z.; Pan, Y. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta 2010, 80, 1292–1297. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, B.; Shi, R.B. HPLC determination of 2-hydroxy-1-methoxyaporphine, pronuciferine, nuciferine and roemerine in Nelumbo nucifera and its alkaloid fraction. Zhongguo Zhong Yao Za Zhi 2008, 33, 1713–1716. [Google Scholar]
- Kunitomo, J. Alkaloids of Nelumbo nucifera. Phytochemistry 1973, 12, 699–701. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshio, Y.; Chen, Y.-P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbonucifera, and structure-activity correlation with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar]
- Boustie, J.; Stigliani, J.L.; Montanha, J.; Amoros, M.; Payard, M.; Girre, L. Antipoliovirus structure-activity relationship of some aporphine alkaloid. J. Nat. Prod. 1998, 61, 480–484. [Google Scholar] [CrossRef]
- Stevigny, C.; Jiwan, J.-L.H.; Rozenberg, R.; de Hoffmann, E.; Quetin-Leclercq, J. Key fragmentation patterns of aporphine alkaloids by electrospray ionization with multistage mass spectr ometry. Rapid Commun. Mass Spectrom. 2004, 18, 523–528. [Google Scholar] [CrossRef]
- Lv, C.Y.; Zhang, L.T. Application of multiple technique of LC/MS in the study of metabolism of natural product and traditional Chinese medicine. Chin. Pharm. J. 2006, 41, 241–244. [Google Scholar]
- Lin, M.B.; Zhang, W.; Zhao, X.W.; Lu, J.X.; Wang, M.; Chen, L.G. Biotransformation of tetrandrine in rats and in men. Acta Pharmacol. Sin. 1982, 17, 728–735. [Google Scholar]
- Sample Availability: Samples of the compounds 2-hydroxy-1-methoxyaporphin (2H1M) are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Ma, X.; Gao, K.; Wang, Y.; Zhao, H.; Wu, H.; Wang, J.; Xie, H.; OuYang, Y.; Luo, L.; et al. The Active Ingredients of Jiang-Zhi-Ning: Study of the Nelumbo nucifera Alkaloids and Their Main Bioactive Metabolites. Molecules 2012, 17, 9855-9867. https://doi.org/10.3390/molecules17089855
Chen J, Ma X, Gao K, Wang Y, Zhao H, Wu H, Wang J, Xie H, OuYang Y, Luo L, et al. The Active Ingredients of Jiang-Zhi-Ning: Study of the Nelumbo nucifera Alkaloids and Their Main Bioactive Metabolites. Molecules. 2012; 17(8):9855-9867. https://doi.org/10.3390/molecules17089855
Chicago/Turabian StyleChen, Jianxin, Xueling Ma, Kuo Gao, Yong Wang, Huihui Zhao, Hao Wu, Juan Wang, Hua Xie, Yulin OuYang, Liangtao Luo, and et al. 2012. "The Active Ingredients of Jiang-Zhi-Ning: Study of the Nelumbo nucifera Alkaloids and Their Main Bioactive Metabolites" Molecules 17, no. 8: 9855-9867. https://doi.org/10.3390/molecules17089855




