3. Experimental
3.1. General Methods
NMR spectra were recorded on a Bruker Avance 300 spectrometer (300 MHz for 1H-NMR, 75 MHz for 13C) or AMX 500 spectrometer (500 MHz for 1H-NMR). Optical rotation was measured at a concentration of g/100 mL, with a Perkin-Elmer polarimeter (accuracy 0.002°). GC-MS analyses were performed on a Perkin-Elmer Turbomass-Autosystem XL. Analytical thin-layer chromatography was performed on precoated silica gel plates, with spot visualization via ultraviolet light and/or by staining with ninhydrin solution in ethanol. Chromatographic separations were performed on a silica gel column by flash chromatography (Kiesel gel 40, 0.040–0.063 mm; Merck). Yields are given after purification, unless explicitly stated. Reactions requiring anhydrous conditions were performed under nitrogen or argon gas.
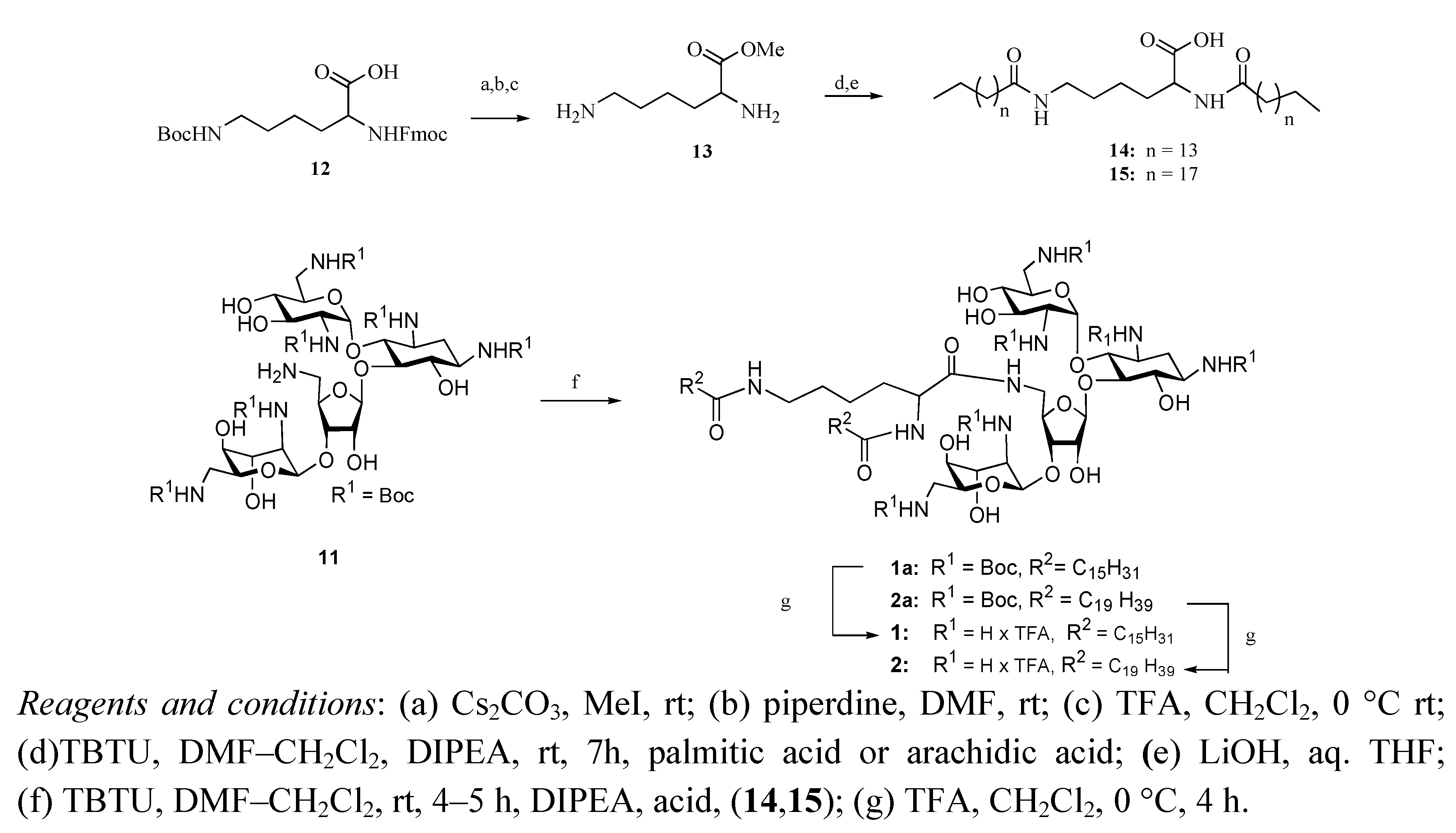
3.2. Synthesis of Lysine-Dilipid Conjugates 14 and 15
Commercially available side chain protected Fmoc-Lys(NHBoc)-COOH was converted to methyl ester using TBTU in DMF followed by removal of Fmoc- and Boc-protecting groups followed by condensation to palmitic acid/arachidic. Ester hydrolysis was performed with LiOH in a mixture of THF/H2O (2:1).
N,N-di-Hexadecanoyl-Lys-acid (14). Yield = 65%; 1H-NMR (300 MHz, CD3OD and CDCl3): δ 6.22 (d, 1H, J = 7.9 Hz), 5.76 (t, 1H, J = 5.0 Hz), 4.55 (m, 1H), 3.22 (t, 2H, J = 6.3 Hz), 2.19 (m, 4H), 1.84 (m, 2H), 1.61 (m, 4H), 1.41 (m, 4H), 1.24 (s, 48H), 0.87 (t, 6H, J = 6.6 Hz); EIMS: calcd for C38H74NaN2O4+ 645.56 Found: 645.88 [M+Na]+.
N,N-di-Nonadecanoyl-Lys-acid (15). Yield = 61%; 1H-NMR (300 MHz, CD3OD and CDCl3): δ 6.22 (d, 1H, J = 7.9 Hz), 5.76 (t, 1H, J = 5.0 Hz), 4.55 (m, 1H), 3.32 (t, 2H, J = 6.3 Hz), 2.19 (m, 4H), 1.84 (m, 2H), 1.73–1.41 (m, 8H), 1.24 (s, 64H), 0.87 (t, 6H, J = 6.6 Hz); EIMS: calcd for C46H90N2NaO4+ 775.69 Found: 775.97 [M+Na]+.
3.3. General Procedure for Coupling Reaction of Neomycin B-based Amine 11
To a solution of neomycin amine
11 (1 equiv.) [
12] in dry DMF, TBTU (2 equiv.), the lipid of choice (1 equiv.) and DIPEA (3 equiv.) were added. The mixture was stirred at room temperature for 2 h, then extracted with water and ethyl acetate. The ethyl acetate layer was washed with water, brine, dried over sodium sulfate and concentrated. The crude residue was purified using flash silica gel (CH
2Cl
2/MeOH). Spectroscopic data is provided below.
3.4. General Procedure for Final Deprotection
All Boc-protected lipids were treated with 95:5 TFA–H2O at 0 °C for 3 min, following which the solvent was removed at reduced pressure. To the residue 2% methanol in ether was added and the solvent was decanted to get the solid neomycin-lipid conjugate as salt. The spectroscopic data is given below.
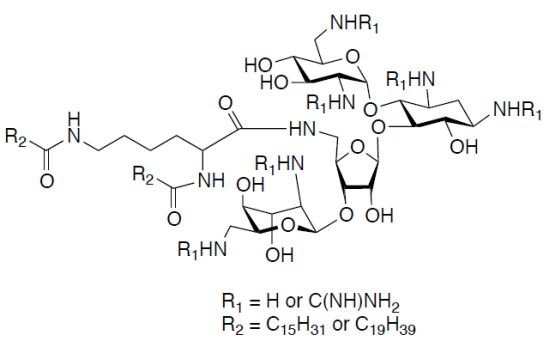
5"-N-(N,N-di-Hexadecanoyl-Lys)-1,3,2',6',2''',6'''-hexa-N-(tert-butoxycarbonyl)-5''-deoxy-neomycin (1a). Yield = 78%; Rf 0.35 (MeOH/CH2Cl2 1:9); 1H-NMR (300 MHz, CD3OD): δ 5.48 (br s, 1H), 5.09 (br s, 1H), 4.88 (s, 1H), 4.31 (d, 1H, J = 3.4 Hz), 4.07 (t, 1H, J = 4.4 Hz), 3.96–3.80 (m, 5H), 3.76 (m, 2H), 3.56 (m, 4H), 3.48 (br t, 3H, J = 3.9 Hz), 3.39 (m, 4H), 3.27 (d, 2H, J = 7.6 Hz), 3.20 (m, 2H), 3.12 (m, 1H), 2.34 (m, 3H), 1.97 (dt, 1H, J = 4.3, 11.1 Hz), 1.64 (m, 4H), 1.51–144 (m, 56H), 1.43 (s, 9H), 1.29 (s, 45H), 0.90 (t, 6H, J = 6.1 Hz); 13C-NMR (75 MHz, CD3OD): δ 176.6 (amide C=O), 176.4 (×2) (peptide amide C=O), 158.9–158.7 (Boc-CO), 112.1, 100.4, 98.8 (anomeric carbons), 88.4, 80.8–80.3, 79.4, 76.2, 75.6, 74.5, 74.1, 73.4, 71.7, 69.0, 69.3, 56.8, 53.6, 51.3, 52.5, 43.9, 42.7, 42.5, 38.9, 37.0–23.7 (aliphatic CH2), 14.5 (aliphatic CH3); [α]D25 = +32.0 (c 0.53, MeOH); EIMS: calcd for C91H167N9NaO27+ 1841.22 Found: 1841.45 [M+Na]+; Anal. Calcd. for C91H167N9O27 C, 60.08; H, 9.25; N, 6.93; Found: C, 60.33; H, 9.45; N, 7.03.
1,3,2',6',2''',6'''-Hexaammonium-5"-deoxy-5"-N-(N,N-di-hexadecanoyl-Lys)-neomycin hexakis-(trifluoroacetate) (1). Yield = 89%; Rf 0.10 (NH4OH/MeOH/CH2Cl2 2:9:4); 1H-NMR (300 MHz, CD3OD): δ 5.86 (d, 1H, J = 3.1 Hz), 5.40 (d, 1H, J = 4.4 Hz), 5.30 (s, 1H), 4.41 (t, 1H, J = 5.4 Hz), 4.30 (m, 2H), 4.22 (m, 1H), 4.16 (m, 2H), 4.08 (t, 2H, J = 5.9 Hz), 3.99 (t, 2H, J = 3.6 Hz), 3.89 (t, 2H, J = 8.8 Hz), 3.68 (m, 4H), 3.52 (dd, 2H, J = 2.6, 11.1 Hz), 3.45 (m, 3H), 3.41 (t, 1H, J = 3.6 Hz), 3.37 (d, 1H, J = 1.6 Hz), 3.24 (m, 1H), 3.20 (m, 1H), 2.48 (d, 2H, J = 11.8 Hz), 2.26 (d, 3H, J = 7.8 Hz), 2.08 (d, 2H, J = 11.8 Hz), 1.61 (m, 3H), 1.29 (s, 52H), 0.90 (t, 6H, J = 6.1 Hz); 13C-NMR (75 MHz, CD3OD): δ 177.4 (amide C=O), 177.3 (×2) (peptide amide C=O), 164.0–162.7 (TFA, q with J 2CF ~ 34.8 Hz), 124.1–112.4 (q with J 1CF ~ 292.0 Hz), 109.5, 97.2, 96.7 (anomeric carbons), 86.6, 83.4, 78.1, 76.6, 76.4, 75.6, 74.1, 74.0, 72.9, 72.5, 72.0, 71.9, 71.8, 69.4, 67.6, 64.9, 55.4, 52.9, 51.1, 50.3, 41.9, 41.7, 37.2–23.7 (aliphatic CH2), 14.7 (CH3); [α]D25 = +24.0 (c 1.58, MeOH); EIMS: calcd for C61H120N9O15+1218.88 Found: 1219.09 [M+H]+; Anal. Calcd. for C73H125F18N9O27 C, 46.08; H, 6.62; F, 17.97; N, 6.63; Found: C, 46.23; H, 6.76; F, 18.01; N, 6.46.
5"N-(N,N-di-Nonadecane-Lys)-1,3,2',6',2''',6'''-hexa-N-(tert-butoxycarbonyl)-5"-deoxy-neomycin (2a). Yield = 70%; Rf 0.33 (MeOH/CH2Cl2 1:9); 1H-NMR (300 MHz, 2:1 mixture of CD3OD and CDCl3): δ 5.08 (m, 2H), 4.74 (m, 1H), 4.36 (m, 2H), 4.22 (m, 2H), 4.03 (t, 1H, J = 7.3 Hz), 3.99 (m, 4H), 3.79 (m, 2H), 3.70 (m, 2H), 3.55 (m, 3H), 3.46 (m, 4H), 3.43 (m, 2H), 3.25 (m, 2H), 3.13 (m, 1H), 2.34 (m, 2H), 2.24 (t, 2H, J = 7.9 Hz), 1.99 (m, 2H), 1.69 (m, 2H), 1.55–1.40 (m, 72H), 1.28 (s, 54H), 0.89 (t, 6H, J = 7.1 Hz); 13C-NMR (75 MHz, CD3OD): δ 176.2 (amide C=O), 175.7 (×2) (peptide amide C=O), 158.3–157.2 (Boc-CO), 101.6, 100.3, 97.6 (anomeric carbons), 87.5, 81.4–80.6, 78.4, 76.4, 75.5, 75.3, 74.5, 73.5, 73.3, 72.4, 71.4, 68.9, 57.1, 56.1, 53.4, 52.3, 44.2, 42.8, 40.0, 38.0, 37.7–24.0 (aliphatic CH2), 14.4(CH3); [α]D25 = +55.0 (c 0.73, MeOH); EIMS: C99H183N9NaO27+ 1953.32 Found: 1953.67 [M+Na]+; Anal. Calcd. for C99H183N9O27 C, 61.56; H, 9.55; N, 6.53; Found: C, 62.22; H, 10.09; N, 6.53.
1,3,2',6',2"',6"'-Hexaammonium-5"-deoxy-5"-N-(N,N-di-nonadecanoyl-Lys)-neomycin hexakis-(trifluoroacetate) (2). Yield = 87%; Rf 0.10 (NH4OH/MeOH/CH2Cl2 2:9:4); 1H-NMR (300 MHz, 2:1 mixture of CD3OD and CDCl3): δ 5.40 (d, 1H, J = 3.1 Hz), 5.25 (d, 1H, J = 4.4 Hz), 5.20 (s, 1H), 4.79 (m, 1H), 4.62 (m, 1H), 4.40–4.00 (m, 9H), 3.89 (t, 1H, J = 5.9 Hz), 3.68 (m, 2H), 3.60 (m, 1H), 3.44 (m, 6H), 3.22 (m, 4H), 2.45 (m, 1H), 2.21 (d, 2H, J = 8.1 Hz), 2.19 (d, 2H, J = 7.1 Hz), 2.00 (d, 2H, J = 6.4 Hz), 1.60 (m, 5H), 1.22 (s, 70H), 0.90 (t, 6H, J = 7.2 Hz); 13C-NMR (75 MHz, CD3OD): δ 177.2 (amide C=O), 176.1 (×2) (peptide amide C=O), 164.0–162.7 (TFA, q with J 2CF ~ 34.8 Hz), 124.1–112.4 (q with J 1CF ~ 292.0 Hz), 109.5, 97.2, 96.3 (anomeric carbons), 86.3, 83.0, 79.2, 78.2, 75.9, 75.5, 73.8, 72.9, 72.4, 72.1, 71.9, 71.7, 69.4, 69.0, 61.9, 55.9, 52.9, 51.8, 50.3, 41.9, 41.7, 40.6, 40.5, 37.5–23.7 (aliphatic CH2) 14.7 (CH3); [α]D25 = +55.0 (c 0.71, MeOH); EIMS: calcd for C69H136N9O15+ 1331.01 Found: 1331.45 [M+H]+; Anal. Calcd for C81H141F18N9O27 C, 48.28; H, 7.05; F, 16.97; N, 6.26 Found: C, 48.28; H, 7.05; F, 16.97; N, 6.26.
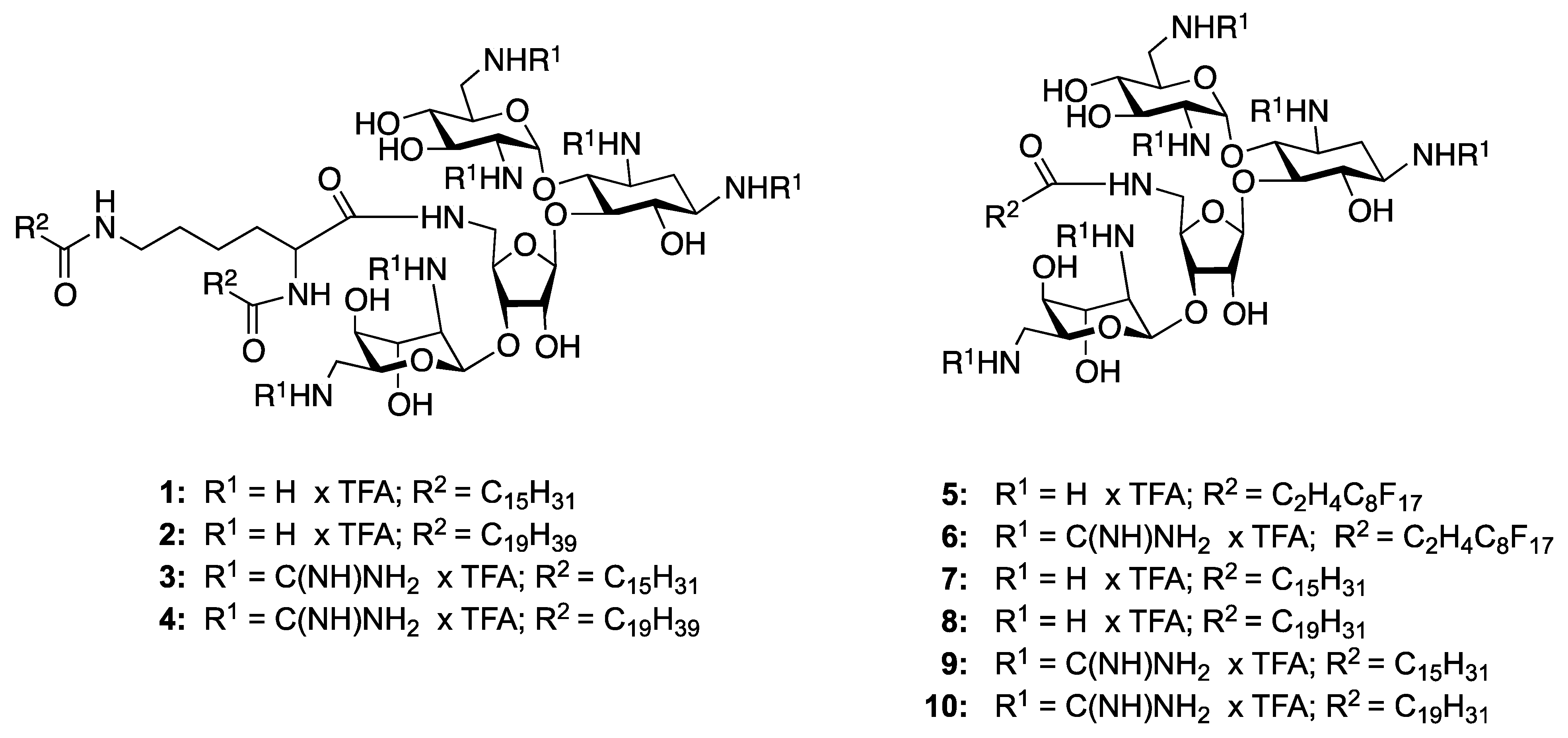
5"-N-(Heptadecafluoro-decanoyl)-1,3,2',6',2"',6"'-hexa-N-(tert-butoxycarbonyl)-5"-deoxy-neomycin (5a). Yield = 88%; Rf 0.56 (MeOH/CH2Cl2 1:9); 1H-NMR (300 MHz, CD3OD): δ 5.54 (br s, 1H), 5.10 (br s, 1H), 4.89 (br s, 1H), 4.34 (d, J = 4.38, 1H), 4.11–4.04 (m, 2H), 3.97–3.85 (m, 4H), 3.79–3.72 (m, 2H), 3.59–3.47 (m, 6H), 3.44–3.36 (m, 3H), 3.28–3.18 (m, 2H), 3.08 (dd, J = 8.6, 13.6 Hz, 1H), 2.82–2.47 (m, 4H), 1.97–192 (d, J =12.6 Hz, 1H), 1.67–1.60 (m, 2H), 1.51–1.43 (m, 54H), 1.39–1.22 (m, 2H); 13C-NMR (75 MHz, CD3OD): δ 173.0 (amide-CO) 159.1, 158.9, 158.7, 158.6, 158.3, 157.9 (Boc-CO), 112.3, 100.3, 98.7 (anomeric carbons), 88.6, 81.0, 80.8, 80.7, 80.4, 80.3, 76.3, 75.5, 74.5, 73.4, 72.7, 72.5, 71.7, 69.1, 56.8, 53.6, 52.7, 51.3, 44.2, 42.7, 42.0, 35.7, 28.9–27.5 (Boc CH3 and aliphatic CH2); EIMS: calcd. for C64H99F17N7O25+ 1688.64. Found: 1688.45 [M+H]+.
1,3,2',6',2''',6"'-Hexaammonium-5"-deoxy-5"-N-(heptadecafluoro-decanoyl)-neomycin hexakis-(trifluoroacetate) (5). Yield = 89%; Rf 0.14 (NH4OH/MeOH/CH2Cl2 1:5:5); 1H-NMR (300 MHz, CD3OD): δ 5.85 (d, J = 3.7 Hz, 1H), 5.40 (d, J = 4.8 Hz, 1H), 5.29 (br s, 1H), 4.41–4.33 (m, 1H), 4.25 (m, 4H), 4.14 (m, 1H), 4.08 (m, 1H), 4.00 (m, 2H), 3.88 (dd, 1H, J = 9.0, 7.8 Hz), 3.73–3.66 (m, 2H), 3.64 (m, 1H), 3.55–3.34 (m, 8H), 3.26–3.21 (m, 1H), 3.16 (dd, 1H, J = 13.4, 8.1 Hz), 2.58 (m, 3H), 2.46 (m, 1H), 2.07 (m, 1H); 13C-NMR (75 MHz, CD3OD): δ 173.70 (NH-CO-), 163.3 (TFA), 123.93, 120.38, 120.09, 120.07, 119.86, 119.82 (F attached), 117.2 (TFA), 109.61, 97.33, 96.71 (anomeric carbons), 86.55, 83.19, 78.18, 76.50, 75.58, 73.99, 73.05, 72.04, 71.94, 69.48, 69.33, 69.18, 66.87, 55.03, 52.88, 51.14, 50.31, 42.21, 41.96, 41.68, 29.43, 27.51; EIMS: calcd. for C34H51F17N7O13+ 1088.32. Found: 1088.45 [M+H]+.
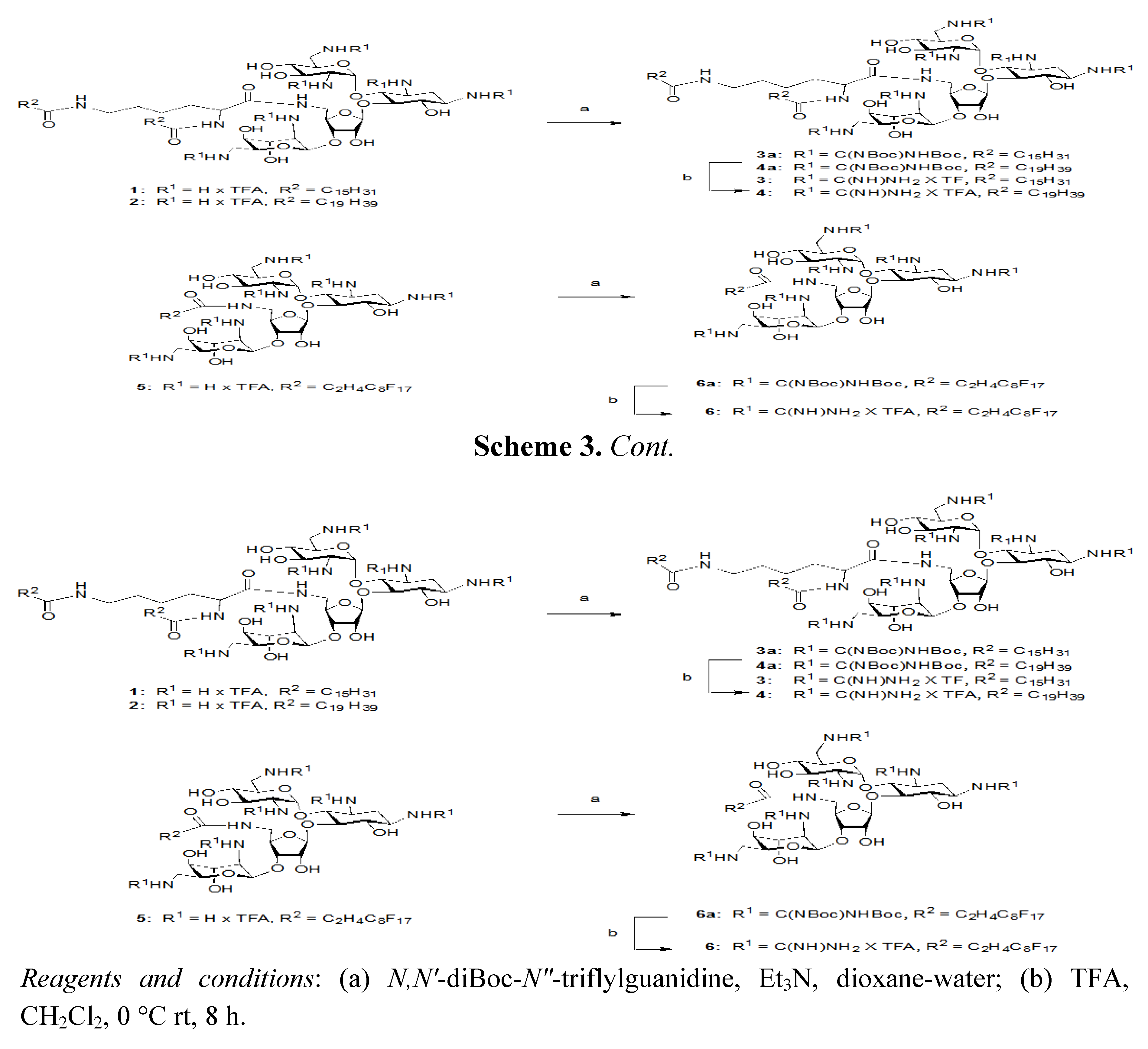
3.5. General Procedure for the Guanidinylation of Aminoglycosides
N,N'-DiBoc-N"-triflylguanidine was purchased from Fluka and used without further purification. To a solution of aminoglycoside (5 amines, 0.054 mmol) in H2O (0.5 mL) was added 1,4-dioxane (2.5 mL) and N,N'-diBoc-N"-triflylguanidine (0.82 mmol) in alternating portions so the solution remained relatively clear. After 5 min, NEt3 (0.82 mmol) was added at room temperature. After 3–4 days, the 1,4-dioxane was removed under reduced pressure. The remaining residue and H2O was extracted with CH2Cl2 (3 × 10 mL), washed with H2O and brine, and dried (MgSO4). The fully guanidinylated product can be isolated by flash column chromatography (FCC) on silica gel (CH2Cl2/MeOH).
3.6. General Procedure for the Deprotection of Guanidinoglycosides
A solution of TFA: CH2Cl2 (1:1, 1 mL) was added to the protected guanidinoglycoside (0.041 mmol) at room temperature. After approximately 4 h, the solution was diluted with toluene, concentrated in vacuo, and dissolved in H2O. Subsequent lyophilization of H2O provided the deprotected guanidinoglycoside as a fluffy white powder.
5"-Deoxy-5"-N-(N,N-di-hexadecanoyl-Lys)-1,3,2',6',2"',6'''-hexa-N-(tert-butoxycarbonyl-guanidino)-5"-deoxy-neomycin (3a). Yield = 75%; Rf 0.33 (MeOH/CH2Cl2 1:9); 1H-NMR (300 MHz, CD3OD): δ 5.62 (s, 1H), 5.22 (s, 2H), 5.08 (s, 1H), 4.55 (m, 1H), 4.38 (m, 2H), 4.23 (m, 3H), 4.10 (m, 4H), 3.99 (m, 2H), 3.91–3.77 (m, 2H), 3.72 (m, 2H), 3.63–3.50 (m, 6H), 3.40 (t, 1H, J = 5.3 Hz), 3.20 (m, 1H), 2.26 (m, 4H), 1.72 (m, 2H), 1.50 (s, 128H), 1.30 (s, 36H), 0.89 (t, 6H, J = 7.1 Hz); 13C-NMR (75 MHz, CD3OD, HSQC): δ 176.5 (amide C=O), 176.2 (×2) (peptide amide C=O), 164.4–164.1 (Boc C=O), 158.0–157.4 (guanidine C=NH), 154.4–153.7 (Boc C=O), 111.3, 97.7, 95.8 (anomeric carbons), 88.3, 84.8–84.1, 82.6, 80.7–80.4, 79.6, 77.3, 76.5, 75.3, 74.2, 73.1, 72.6, 71.7, 71.2, 68.4, 56.4, 54.2, 52.9, 50.8, 51.7, 44.4, 43.6, 43.3, 41.8, 39.7–22.6 (aliphatic CH3) 13.4 (CH3); [α]D25 = +56.0 (c 0.56, MeOH); EIMS: calcd for C127H227N21NaO39+ 2693.64 Found: 2693.89 [M+Na]+; Anal. Calcd. for C127H227N21O39 C, 57.08; H, 8.56; N, 11.01; Found: C, 57.67; H, 9.11; N, 11.41.
1,3,2',6',2''',6'''-Hexaammonium-guanidinyl-5"-deoxy-5"-N-(N,N-di-hexadecanoyl-Lys)-neomycin hexakis(trifluoroacetate) (3). Yield = 81%; Rf 0.10 (NH4OH/MeOH/CH2Cl2 2:9:4); 1H-NMR (300 MHz, CD3OD): δ 5.64 (d, 1H, J = 2.7 Hz), 5.18 (m, 2H), 5.06 (m, 1H), 5.03 (d, 1H, J = 5.3 Hz), 4.77 (m, 1H), 4.23 (m, 1H), 4.15–4.08 (m, 3H), 4.01 (m, 2H), 3.80–3.77 (m, 3H), 3.67 (m, 3H), 3.57–3.48 (m, 6H), 3.42 (m, 2H), 3.37 (m, 1H), 3.19 (t, 1 H, J = 7.3 Hz), 2.24 (m, 3H), 2.19 (m, 2H), 1.72 (m, 2H), 1.61 (m, 4H), 1.46 (m, 2H), 1.29 (s, 49H), 0.91 (t, 6H, J = 6.5 Hz); 13C-NMR (75 MHz, CD3OD): δ 177.6 (amide C=O), 177.4 (×2) (peptide amide C=O), 159.6, 159.2 (×4), 158.6 (guanidine C=NH), 100.6, 97.9, 97.6 (anomeric carbons), 88.4, 86.6, 83.9, 82.4, 79.6, 79.4, 76.4, 75.2, 74.0, 72.7, 72.0, 71.4, 70.9, 69.8, 57.1, 55.4, 52.9, 51.7, 50.3, 43.6, 41.9, 41.7, 38.9–23.7 (aliphatic CH2), 14.7 (aliphatic CH3); [α]D25 = +44.0 (c 0.67, MeOH); EIMS: calcd for C67H131N21O15+ 1471.01 Found: 1471.22 [M+H]+; Anal. Calcd. for C79H137F18N21O27 C, 44.03; H, 6.41; F, 15.87; N, 13.65; Found: C, 44.22; H, 6.82; F, 16.11; N, 14.15.
5''-Deoxy-5"-N-((N,N-di-nonadecanoyl-Lys)-1,3,2',6',2''',6'''-hexa-N-(tert-butoxycarbonylguanidino)-5"-deoxyneomycin (4a). Yield = 71%; Rf 0.32 (MeOH/CH2Cl2 1:9); 1H-NMR (300 MHz, CD3OD and CDCl3): δ 5.74 (s, 1H), 5.05 (m, 2H), 4.74 (m, 1H), 4.55 (m, 1H), 4.38 (m, 2H), 4.18 (m, 3H), 4.03 (m, 1H), 3.99 (m, 2H), 3.83 (m, 3H), 3.72–3.35 (m, 6H), 3.23 (t, 2H, J = 9.3 Hz), 3.17 (d, 1H, J = 6.7 Hz), 3.43 (d, 3H, J = 13.4 Hz), 2.25 (t, 2H, J = 6.9 Hz), 2.17 (t, 2H, J = 7.3 Hz), 2.03 (m, 2H), 1.72 (m, 2H), 1.50 (s, 108H), 1.30 (s, 74H), 0.89 (t, 6H, J = 7.1 Hz); 13C-NMR (75 MHz, CD3OD, HSQC): δ 176.2 (amide C=O), 176.1 (×2) (peptide amide C=O), 164.6–163.9 (Boc C=O), 158.2–157.3 (guanidine C=NH), 154.4–153.7 (Boc C=O), 111.3, 97.7, 95.8 (anomeric carbons), 86.8, 85.1–84.0, 82.0, 80.9–80.3, 78.7, 78.5, 75.3, 74.2, 73.5, 72.6, 71.7, 71.2, 70.6, 69.4, 67.6, 67.3, 66.2, 63.3, 53.9, 50.1, 51.4, 48.1, 47.3, 42.6, 41.3, 40.0, 39.7–22.6 (aliphatic CH2) 13.4 (aliphatic CH3); [α]D25 = +48.0 (c 0.49, MeOH); EIMS: calcd for C135H243N21NaO39+ 2815.77 Found: 2816.45 [M+Na]+; Anal. Calcd for C135H243N21O39 C, 58.23; H, 8.80; N, 10.56; Found: C, 58.44; H, 9.10; N, 10.56.
1,3,2',6',2''',6'''-Hexaammonium-guanidinyl-5''-deoxy-5''-N-(N,N-di-nonadecanoyl-Lys)-neomycin hexakis(trifluoroacetate) (4). Yield = 84%; Rf 0.10 (NH4OH/MeOH/CH2Cl2 2:9:4); 1H-NMR (300 MHz, CD3OD and CDCl3): δ 5.06 (m, 2H), 4.74 (m, 2H), 4.08 (m, 2H), 3.81 (m, 3H), 3.60 (m, 1H), 3.50 (m, 5H), 3.43 (m, 3H), 3.42–3.26 (m, 10H), 2.91 (m, 1H), 1.95 (dt, 4H, J = 6.3 Hz), 1.33 (m, 3H), 1.03 (s, 74H), 0.89 (t, 6H, J = 7.1 Hz); 13C-NMR (75 MHz, CD3OD): δ 177.2 (amide C=O), 176.4 (×2) (peptide amide C=O), 164.0–162.7 (TFA, q with J 2CF ~ 34.8 Hz), 159. 6 (×2), 159.4 (×3), 159.2 (guanidine C=NH), 124.1–112.4 (q with J 1CF ~ 292.0 Hz), 112.1, 99.2, 97.3 (anomeric carbons), 83.0, 79.2, 78.2, 75.9, 74.0, 73.8, 72.9, 72.4, 72.1, 71.9, 71.7, 70.8, 68.8, 61.9, 56.9, 55.1, 53.2, 51.8, 43.4, 43.0, 40.8, 40.0, 37.5–23.7 (aliphatic CH3) 14.7 (aliphatic CH3); [α]D25 = +42.0 (c 0.65, MeOH); EIMS: calcd for C75H148N21O15+ 1583.14 Found: 1583.14 [M+H]+; Anal. Calcd for C87H153F18N21O27 C, 46.09; H, 6.80; F, 15.08; N, 12.97; Found: C, 46.54; H, 7.22; F, 15.28; N, 13.19.
5''-N-(Hexadecafluorodecanoyl)-1,3,2',6',2''',6'''-hexa-N-(tert-butoxycarbonyl-guanidino)-5''-deoxy-neomycin (6a). Yield = 86%; Rf 0.36 (MeOH/CH2Cl2 1:9); 1H-NMR (300 MHz, CD3OD): δ 5.88 (d, 1H, J = 3.68 Hz), 5.05 (dd, 2H, J =5.56, 1.70 Hz), 4.63–4.55 (m, 2H), 4.38 (m, 1H), 4.32–4.23 (m, 3H), 4.16 (m, 1H), 4.05–3.68 (m, 10H), 3.59–3.44 (m, 5H), 3.27 (m, 1H), 2.75–2.45 (m, 4H), 2.2 (m, 1H), 1.59–1.45 (m, 108H); 13C-NMR (75 MHz, CD3OD): δ 172.4,164.6.164.5, 164.3, 164.2, 164.1, 164.0, 158.8, 158.0, 157.9, 157.8, 157.6, 157.5, 154.7, 154.4, 154.2, 154.1, 154.0, 153.3, 153.2, 151.4, 140.5, 129.9, 127.7, 123.4, 120.4, 119.2, 114.9, 113.3, 99.3, 97.1, 89.1, 84.8, 84.7, 84.6, 84.5, 84.1, 84.0, 83.0, 80.7, 80.6, 80.5, 80.4, 80.3, 77.1, 76.7, 75.6, 74.3, 73.3, 72.0, 71.1, 68.1, 55.5, 53.0, 51.8, 50.2, 44.3, 44.0, 41.7, 35.2, 28.8–27.6 (Boc CH3 and aliphatic CH2).
1,3,2',6',2''',6'''-Hexaammonium-guanidinyl-5''-deoxy-5''-N-(hexadecafluorodecanoyl-neomycin hexakis(trifluoroacetate) (6). Yield = 91%; Rf 0.19 (NH4OH/MeOH/CH2Cl2 1:5:5); 1H-NMRR (300 MHz, CD3OD): δ 5.91 (d, 1H, J = 2.9 Hz), 5.11 (d, 1H, J = 3.7 Hz), 5.01 (br s, 1H), 4.25–4.20 (m, 1H), 4.14 (dd, 1H, J = 6.2, 3.8 Hz), 4.08 (dd, 1H, J = 10.2, 6.0 Hz), 4.02 (dd, 1H, J = 5.6, 2.5 Hz), 3.98 (dd, 1H, J = 8.6, 4.2 Hz), 3.81 (m, 5H), 3.66–3.49 (m, 9H), 3.38 (m, 3H), 2.73–2.40 (m, 4H), 2.13 (m, 1H), 1.74 (m, 1H); 13C-NMR (75 MHz, CD3OD): δ 173.77 (NH-CO-), 163.3 (TFA), 159.62, 159.51, 159.30, 159.25, 159.18, 158.56 (–NHC(NH)NH2), 129.85, 123.85, 123.44, 120.45, 120.09, 119.21, 116.22 (F- attached carbons), 111.63, 100.14, 97.19 (anomeric carbons), 87.67, 82.18, 80.07, 77.50, 76.65, 75.60, 74.22, 74.04, 71.97, 71.50, 70.89, 68.81, 57.14, 55.36, 53.63, 51.75, 43.46, 43.14, 42.34, 33.79, 28.10, 27.57; EIMS: calcd. for C40H63F17N19O13+ 1340.45. Found: 1340.9 [M+H]+.
3.7. Determination of the MIC Values for Aminoglycoside-Lipid Conjugates 1–6
Bacterial isolates were obtained from the American Type Culture Collection (ATCC). Isolates were kept frozen in skim milk at −80 °C until minimum inhibitory concentration (MIC) testing was carried out. Following two subcultures from frozen stock, the
in vitro activities of peptides were determined by macrobroth dilution in accordance with the Clinical and Laboratory Standards Institute (CLSI) 2006 guidelines [
19]. Stock solutions of peptides were prepared and dilutions made as described by CLSI. Test tubes contained doubling antimicrobial dilutions of cation adjusted Mueller-Hinton broth and inoculated to achieve a final concentration of approximately 5 × 10
5 CFU/mL then incubated in ambient air for 24 h prior to reading. Colony counts were performed periodically to confirm inocula. Quality control was performed using ATCC QC organisms.
3.8. Determination of the Hemolytic Activity for Aminoglycoside-Lipid Conjugates 5–6
Freshly isolated human erythrocytes isolated as recently described [
20] were incubated for 1 h at 37 °C with 50, 100 µg/mL solution of peptide dissolved in PBS. The samples were centrifuged (4,000 rpm, 5 min) before the absorbance at 540 nm of the supernatant was measured by a microtiter negative and positive controls, respectively. Peptide concentrations corresponding to 50% hemolysis (EC50) were determined from the dose-response curves.