2.1. Myographic Recording
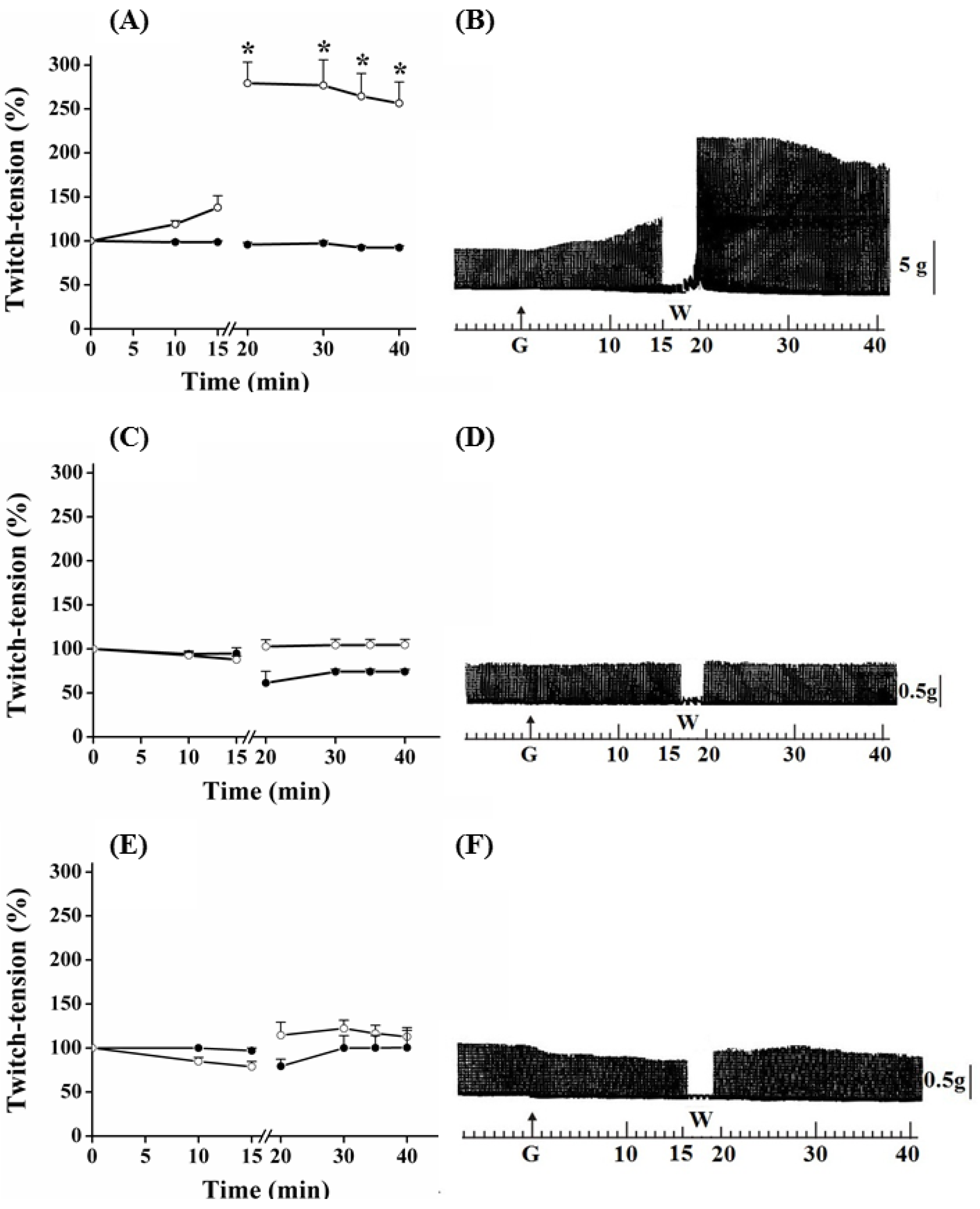
Myographic recordings of PND, EDL and SOL preparations incubated with guanidine for 15, 30 and 60 min, respectively are shown in
Figure 1,
Figure 2 and
Figure 3. In PND preparation, guanidine (10 mM) determined a gradual neurotransmission facilitation which achieved a 35% increase after 15 min (
P ≤ 0.05). The removal of guanidine from bath led to a second phase of facilitation of the contractile response, which was immediate rather than gradual, and almost four times (~170%) the amplitude of the initial record. Twenty minutes after guanidine removal by washing the preparation, the increase in the contractile response still remained 150% above that observed before guanidine administration and in relation to that presented by preparations incubated with Tyrode only (
Figure 1A,B).
Figure 1.
Effect of guanidine (10 mM) (white circles) for 15 min on the twitch response of PND (A), EDL (C) and SOL (E) (n = 8/each) in comparison with matched controls (black circles) (n = 3, 8, 8, respectively). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Panels B (PND), D (EDL) and F (SOL) display the myographic isometric twitch record of preparations incubated with guanidine (G). The vertical bars (5, 0.5 g) represent muscle tension.
Figure 1.
Effect of guanidine (10 mM) (white circles) for 15 min on the twitch response of PND (A), EDL (C) and SOL (E) (n = 8/each) in comparison with matched controls (black circles) (n = 3, 8, 8, respectively). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Panels B (PND), D (EDL) and F (SOL) display the myographic isometric twitch record of preparations incubated with guanidine (G). The vertical bars (5, 0.5 g) represent muscle tension.
Guanidine (10 mM) did not promote facilitation of neurotransmission in EDL and SOL preparations after 15 min of incubation (
Figure 1C,D and 1E,F, respectively). On the contrary, both tended to have reduced responses, which was more evident in SOL. Guanidine washing caused a nonsignificant tendency to increase the contractile response up to the remaining 20 min of observation. Likewise, neither 1 mM nor 5 mM guanidine promoted facilitation in EDL preparation even after its removal by washing (not shown).
PND contractile response, after the addition of guanidine (10 mM) in the bath for 30 min, is shown in
Figure 2. In these preparations, guanidine induced a gradual increase in the neuromuscular activity, which peak reached 65% of increase at 20 min; thereafter, there was facilitation reduction, although it has remained approximately 20% above baseline at the end of 30 min. Removal of guanidine and replacement for Tyrode caused immediate facilitation in a proportion of 95% (almost twice the initial, primary, facilitation).
Figure 2.
Effect of guanidine (10 mM) (white circles) for 30 min on the twitch response of the PND (A), EDL (C) and SOL (E) (n = 8/each) in comparison with matched controls (black circles) (n = 3, 8, 8, respectively). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Myographic isometric twitch records of PND (B), EDL (D) and SOL (F) incubated with guanidine (G). The vertical bars (5, 0.5 g) correspond to muscle tension.
Figure 2.
Effect of guanidine (10 mM) (white circles) for 30 min on the twitch response of the PND (A), EDL (C) and SOL (E) (n = 8/each) in comparison with matched controls (black circles) (n = 3, 8, 8, respectively). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Myographic isometric twitch records of PND (B), EDL (D) and SOL (F) incubated with guanidine (G). The vertical bars (5, 0.5 g) correspond to muscle tension.
The amplitude of the contractile response increased during the next 15 min, after which it remained stable; however, it was approximately 150% superior when comparing with baseline values (
Figure 2A,B) (
P ≤ 0.05). Guanidine (30 min) to the EDL preparation (
Figure 2C,D) depressed not significantly the muscle contractile activity, whereas it caused no change to SOL (
Figure 2E,F); the posterior tendency towards a secondary facilitation after guanidine removal from bath was not statistically significant. Neither 1 mM nor 5 mM altered the EDL contractile amplitude (not shown).
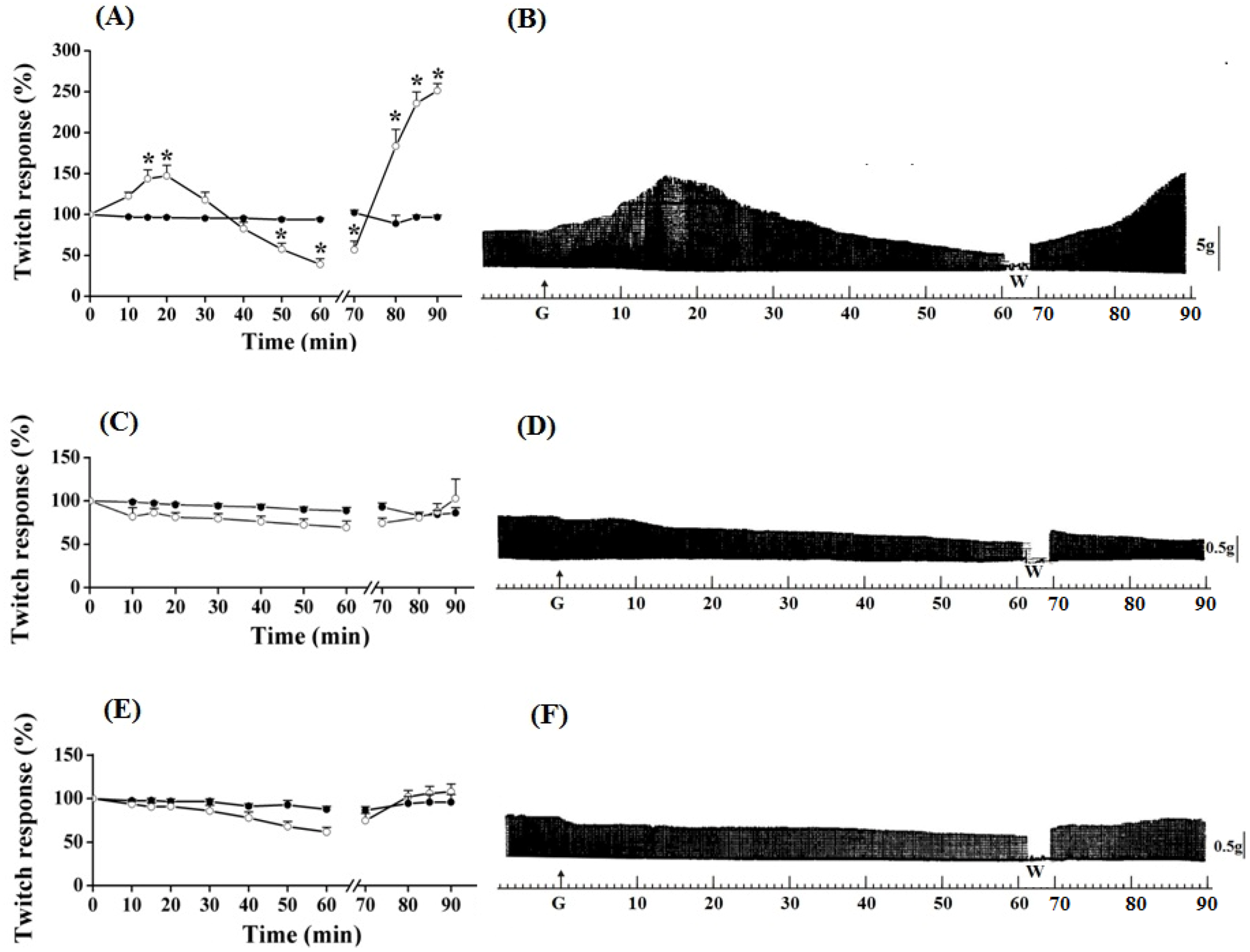
The triphasic effect (primary facilitation/blockade/secondary facilitation of neurotransmission) of guanidine on motor response was selective for PND preparation (
Figure 3). A gradual increase in the contraction amplitude was carried out, which peak (45–50% increase) occurred between 15 and 20 min. In sequence, it gradually decreased leading to total blockade within approximately 40 min and reaching negative values, approximately 70% under the baseline value, at 60 min (
P ≤ 0.05).
Figure 3.
Effect of guanidine (10 mM) (white circles) for 60 min on twitch responses of PND (A), EDL (C) and SOL (E) (n = 8/each) in comparison with matched controls (black circles) (n = 3, 8, 8, respectively). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Myographic isometric twitch records of PND (B), EDL (D) and SOL (F) incubated with guanidine (G). The vertical bars (5, 0.5 g) correspond to muscle tension.
Figure 3.
Effect of guanidine (10 mM) (white circles) for 60 min on twitch responses of PND (A), EDL (C) and SOL (E) (n = 8/each) in comparison with matched controls (black circles) (n = 3, 8, 8, respectively). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Myographic isometric twitch records of PND (B), EDL (D) and SOL (F) incubated with guanidine (G). The vertical bars (5, 0.5 g) correspond to muscle tension.
In the diaphragm, the guanidine washout from bathing medium promoted a gradual reversal of neuromuscular blockade, reaching ~150% of facilitation (secondary) above baseline at 20 min (
Figure 3A,B) (
P ≤ 0.05). The secondary facilitation can be explained by the ability of guanidine to cross the Na
+ channel [
30,
31], and this reduced the miniature endplate potentials (MEPPs) after washing preparations prior incubation with TTX [
21]. Neither the primary nor the secondary facilitation was observed in EDL (
Figure 3C,D) and SOL (
Figure 3E,F) preparations incubated with guanidine for 60 min. The absence of primary and second facilitations in EDL (10 mM, 5 mM or 1 mM, see
Figure 1,
Figure 2,
Figure 3 and
Figure 4) and SOL (10 mM,
Figure 1,
Figure 2 and
Figure 3) indicates that the triphasic effect of guanidine on neurotransmission occurs by a complex mechanism of action, depending on intrinsic muscle phenotypic and molecular characteristics.
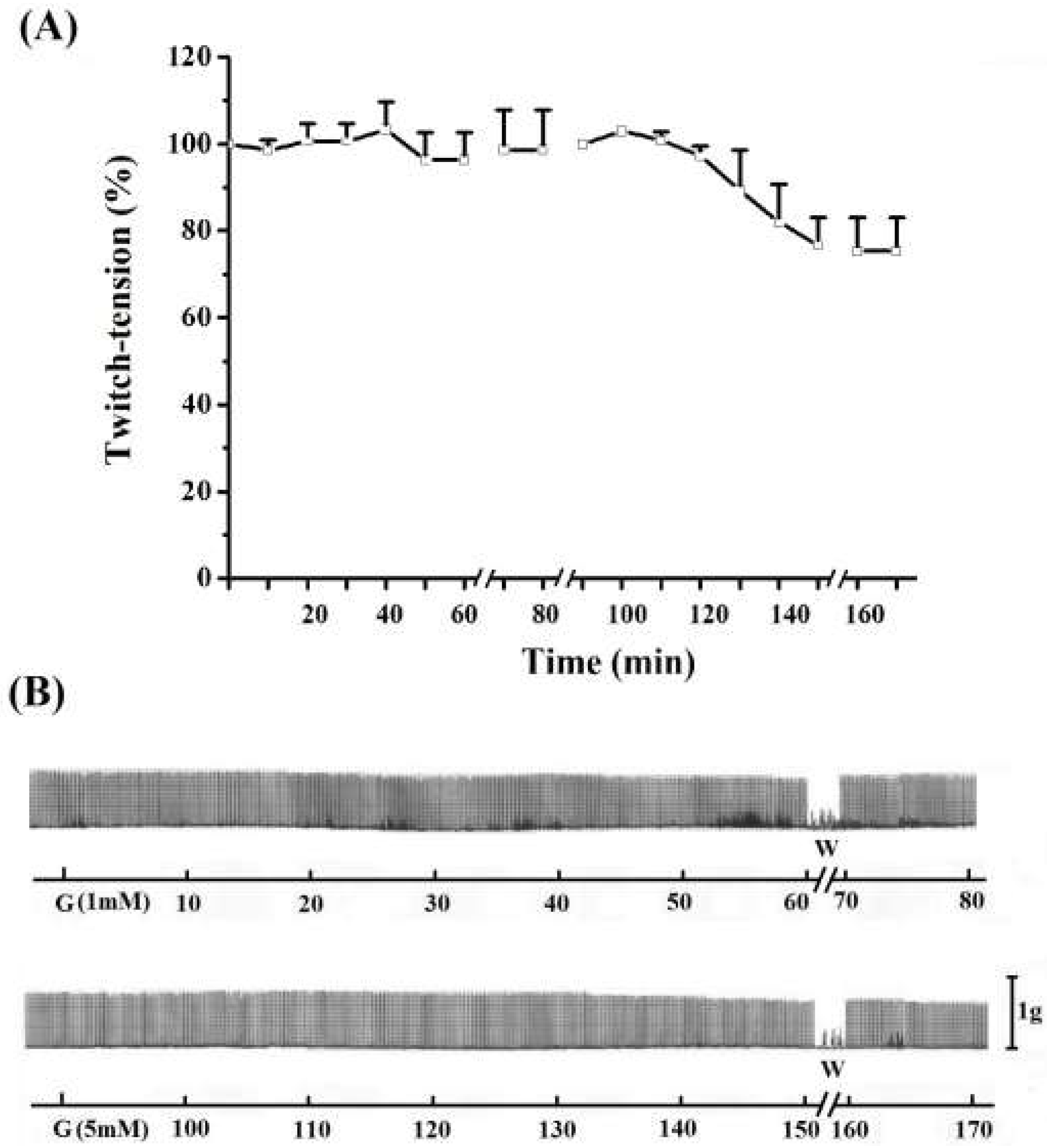
Figure 4.
Effect of 1 mM (A) and 5 mM (B)guanidine (G) for 60 min on the twitch response of EDL (n = 3/each). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Panel B displays the myographic isometric twitch record of these preparations. The vertical bar (1 g) represents muscle tension.
Figure 4.
Effect of 1 mM (A) and 5 mM (B)guanidine (G) for 60 min on the twitch response of EDL (n = 3/each). The line breaking corresponds to the preparation washing (W). Each point represents the mean ± S.E.M; one-way ANOVA followed by Tukey test, * P ≤ 0.05. Panel B displays the myographic isometric twitch record of these preparations. The vertical bar (1 g) represents muscle tension.
In addition, the effect of 5 mM guanidine was investigated by pre- or post-incubation with 4-aminopyridine (4-AP) in order to check either PND response before or after the blockade of potassium channels. Incubation of PND with 4-AP (10 µg/mL) produced as expected, a rise in the twitch response. The removal of 4-AP from bath by washing returned the twitch amplitude to baseline level. The subsequent addition of 5 mM guanidine produced facilitation of neurotransmission. After 80 min incubation with this low concentration of guanidine there was no blockade or inhibition of the amplitude of twitch response as was usual when 10 mM was used. Instead, the facilitation was maintained. After another washing for guanidine removal from bath the secondary facilitation did occur, almost achieving the level promoted by 4-AP (figure not shown). It was of note that there is a delay (which was variable in time) for starting the neurotransmission facilitation induced by guanidine after the 4-AP incubation and first washing.
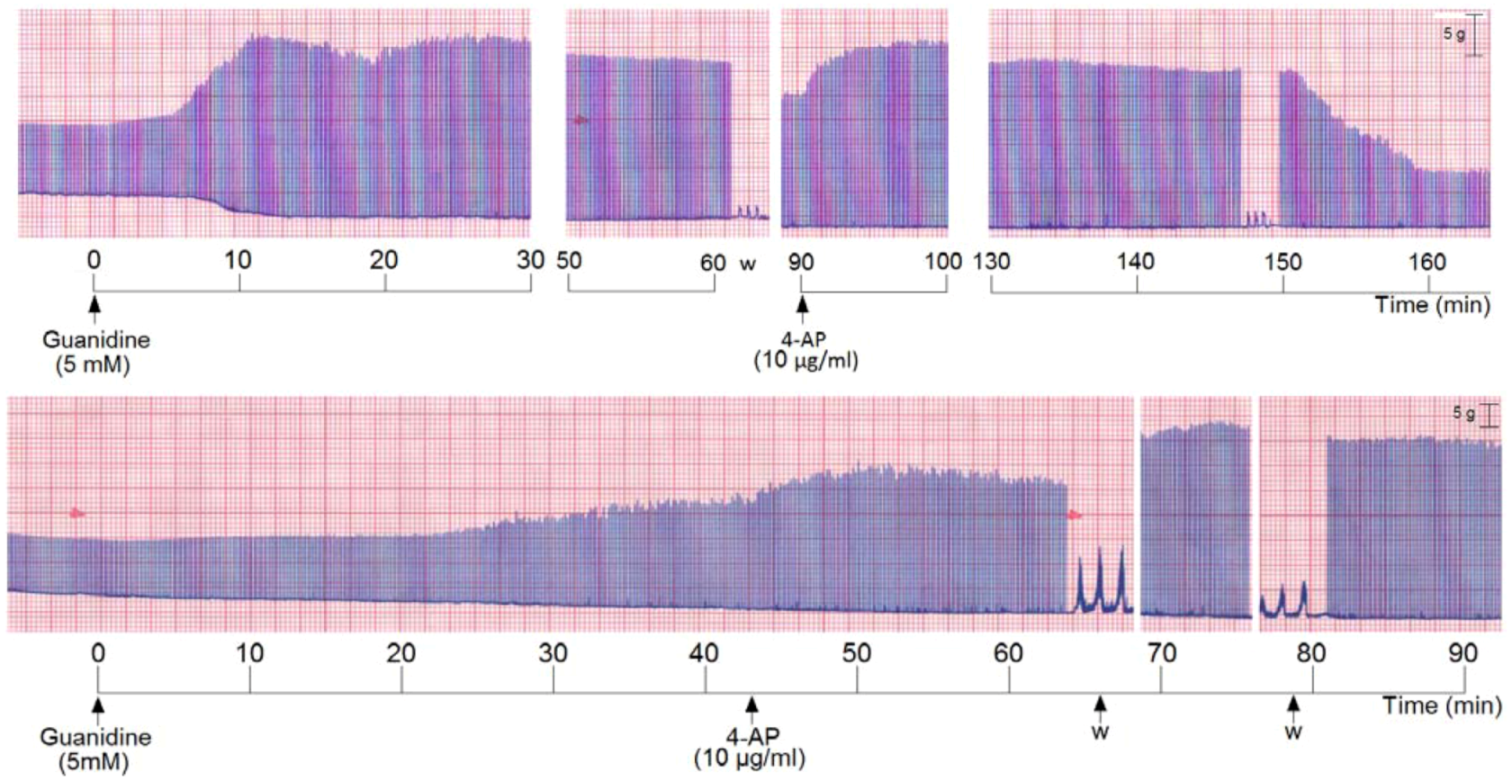
We also incubated PND with 5 mM guanidine, then the preparation was washed and 4-AP was added. The addition of guanidine promoted a marked primary facilitation whose amplitude was reduced after 70–80 min. The subsequent washing and addition of 4-AP promoted another facilitation of neurotransmission which was maintained for the subsequent 60 min. The removal of 4-AP from bath by washing the preparation (three times) promoted an almost immediate decrease of the amplitude of the muscle contraction (
Figure 5, upper register). A variation of these experiments was done which consisted in incubation of PND with 5 mM guanidine and after 45 min addition of 4-AP (10 µg/mL) without pre-washing. The 4-AP promoted an immediate rise in the twitch amplitude (~20%) which persisted until preparation was washed. The washing promoted a secondary facilitation and a third facilitation after the second washing of the preparation. The findings indicate that since guanidine was internalized into the fiber (by traversing sodium channels), the 4-AP pre-treatment did not interfere with the guanidine typical effect in diaphragm preparation (
Figure 5, lower register).
Figure 5.
Representative recordings (of four experiments each) from indirectly stimulated PND incubated with 5 mM guanidine, followed by washing (w) and subsequent addition of 4-aminopiridinte (4-AP) and another washing after 60 min additional incubation (upper recording); in the lower recording there was no guanidine removal by washing before 4-AP addition.
Figure 5.
Representative recordings (of four experiments each) from indirectly stimulated PND incubated with 5 mM guanidine, followed by washing (w) and subsequent addition of 4-aminopiridinte (4-AP) and another washing after 60 min additional incubation (upper recording); in the lower recording there was no guanidine removal by washing before 4-AP addition.
Taken together, the findings show that the blockade of K+ channels by 4-AP did not prevent the triphasisc effect characteristic of guanidine. In other words, 4-AP does not interfere with the primary and secondary increase of ACh release by guanidine (even with half of the concentration previously used). The findings suggest that even though both the 4-AP and guanidine are potassium channel blockers, guanidine exhibits some peculiar mechanisms not found in 4-AP. Interestingly when 4-AP is added without previous removal of the guanidine from preparation, there occurs two other facilitatory effects, after 4-AP removal by washing, in addition to the primary one.
Although several authors have suggested that guanidine effect relies on its action on voltage-gated Na
+, K
+ and Ca
2+ channels, present either in the nerve terminal or skeletal muscle, its mechanism of action remains still not totally elucidated [
11,
21,
22,
30,
32,
33,
34,
35,
36,
37]. The time-dependent facilitatory effect of guanidine seen here in the diaphragm could be probably related to presynaptic action of the drug, increasing Ca
2+ influx and enhancing release of ACh by nerve impulse [
21,
32], consequent to the blockade of K
+ conductance in the nerve terminal, as well as increasing the duration of the presynaptic action potential [
32,
33,
34,
35,
36].
The type of stimulation (indirect or direct field stimulation) and/or the guanidine concentration were/was responsible for differences in contractile responses between PND and EDL/SOL preparations seeming unlikely. PND even with direct stimulation responded with the typical triphasic effect (data not shown), and incubation with low or high doses of guanidine (5 mM, 20 or 25 mM) did not cause the triphasic effect on EDL and SOL preparations even after 60 min (data not shown).
2.2. Morphology and Morphometry
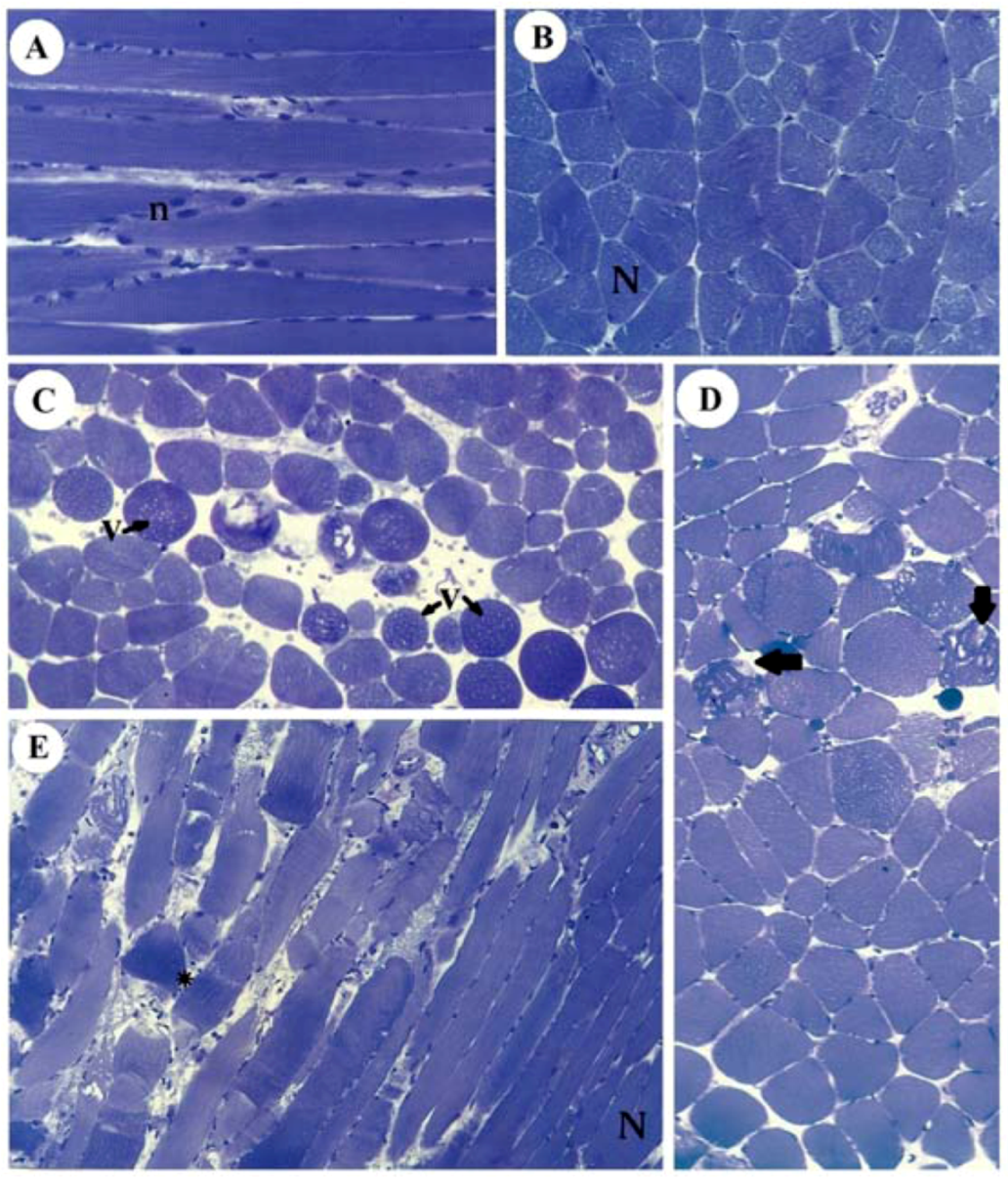
Figure 6 illustrates the normal aspect of muscle fibers of diaphragm incubated with Tyrode (panels A,B) and their different pathological stages in SOL (panel C), EDL (panel D) and diaphragm (panel E) after incubation with 10 mM guanidine for 60 min. Guanidine major effect was the appearance of vacuolated cells in SOL and EDL, which could be due to SR cisternae swelling, as reported for diaphragm [
22].
Figure 6.
Light micrographs of PND incubated with Tyrode show normal fibers (N) with their well-positioned nuclei (n) (A: longitudinal section, B: transversal section) and of SOL (C), EDL (D) and PND (E) showing different stages of cellular disorganization after incubation with 10 mM guanidine for 60 min, under indirect electrical stimulation; altered cells show vacuolation (V), worm-like threads of myofibrils interspersed among empty- looking regions of sarcoplasm due to sarcolemma disruption (arrows) or hypercontraction caused by densely packed myofibrils (*). See also edematous (round in shape) and non-homogeneously stained myofibers (toluidine blue). Bar: 35 µm.
Figure 6.
Light micrographs of PND incubated with Tyrode show normal fibers (N) with their well-positioned nuclei (n) (A: longitudinal section, B: transversal section) and of SOL (C), EDL (D) and PND (E) showing different stages of cellular disorganization after incubation with 10 mM guanidine for 60 min, under indirect electrical stimulation; altered cells show vacuolation (V), worm-like threads of myofibrils interspersed among empty- looking regions of sarcoplasm due to sarcolemma disruption (arrows) or hypercontraction caused by densely packed myofibrils (*). See also edematous (round in shape) and non-homogeneously stained myofibers (toluidine blue). Bar: 35 µm.
Moreover, instead of a typical polygonal profile in transverse sections, a number of fibers in all three preparations incubated with the organic cation guanidine showed spherical outline characteristic of swollen fibers; only a relative minor number of myofibers presented advanced pathological stages with color change due to condensed tortuous bands of myofibrils, or with clear spaces indicative of myofilament loss and sarcolemma rupture. The proportion of affected fibers caused by guanidine and Tyrode incubation is illustrated in
Figure 7.
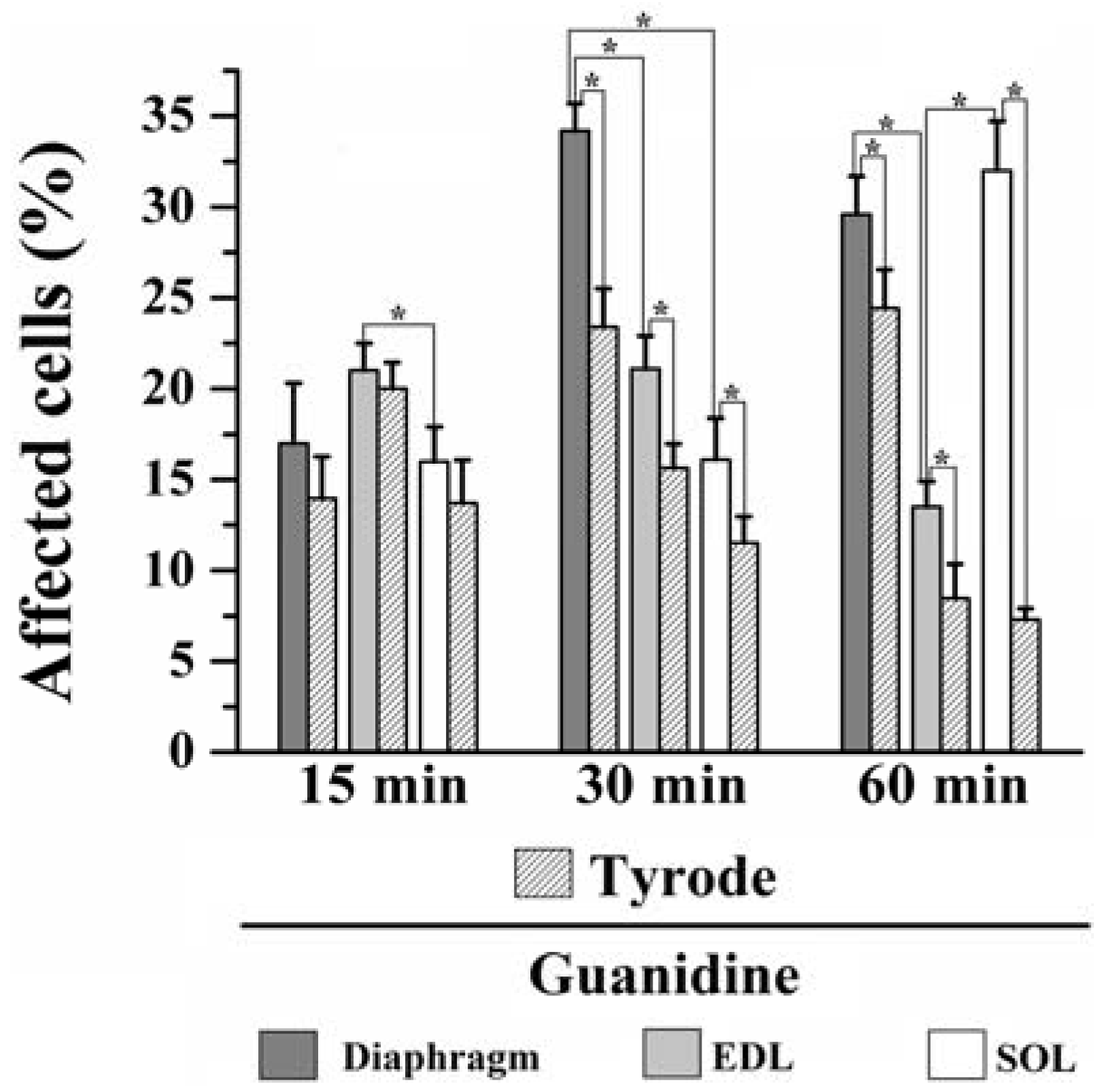
Figure 7.
Proportion of affected cells in PND, EDL and SOL muscles after 15, 30 and 60 min of 10 mM guanidine incubation or Tyrode solution (control). One-way analysis of variance (ANOVA) followed by Tukey test, * P ≤ 0.05.
Figure 7.
Proportion of affected cells in PND, EDL and SOL muscles after 15, 30 and 60 min of 10 mM guanidine incubation or Tyrode solution (control). One-way analysis of variance (ANOVA) followed by Tukey test, * P ≤ 0.05.
15 minutes incubation: relative to Tyrode-incubated preparations, guanidine did not induce morphological alterations in PND, EDL or SOL (13% vs. 17% for PND; 20% vs. 21% for EDL and 13% vs. 16% for SOL of affected fibers). However, guanidine affected significantly more the EDL than SOL when compared to each other (P ≤ 0.05).
30 minutes incubation: relative to control, guanidine promoted a significant increase in the number of affected fibers in all three muscles compared to paired-controls (23% vs. 33% for PND; 15% vs. 21% for EDL and 11% vs. 16% for SOL), (P ≤ 0.05). In addition, there was significant difference when comparing the impact of guanidine effect in all three muscles which was highest for PND and lowest for SOL (P ≤ 0.05).
60 min incubation: compared to control, guanidine promoted a significant increase in the proportion of fibers damaged in all three muscles (24% vs. 28% for PND, 8% vs. 13% for EDL and 7.5% vs. 31% for SOL), (P ≤ 0.05). After 60 min, PND and SOL showed higher proportion of damage than EDL (P ≤ 0.05).
The findings give evidences that in vitro incubation with Tyrode solution also promoted fiber morphological alterations. Interestingly, for PND the higher the period of incubation (15, 30 and 60 min) the higher the proportions of fibers affected (13%, 23% and 24%, respectively), whilst for EDL and SOL the higher the time of incubation the lower proportion of fibers affected (20%, 15% and 8% for EDL and 13%, 11% and 7.5% for SOL). Taken the findings together, it seems that the interaction of in vitro condition and guanidine effect may give the relative injury caused by guanidine itself. The changes in the proportion of fiber damage may result, at least partially, from a direct action of guanidine on muscle fibers, but also from artifact changes in morphology which can be produced even under satisfactory gassing incubation conditions resulting in cell injury. Curiously, the inverse relation of fibers affected versus time duration of Tyrode incubation seen in EDL and SOL suggests that changes were transitory and likely did not involve severe structural alterations in fibers. Based on the findings, the effect of guanidine in EDL may be related with high number of sodium channels in this muscle which interference by the drug provokes alteration of ionic conductance and osmotic disturbances in muscle fibers. This interpretation is in line with the regression in the proportion of fibers affected which passed from 21% after 15/30 min to 13% at the end of 60 mi. On the other hand, in SOL guanidine effect may involve mitochondrial effect (see below).
The antagonistic effect of guanidine on dantrolene effect [
38], a substance able to block the entry of Ca
2+ into SR, as well as the secondary facilitation (evoked even under direct stimulation), supports the idea that guanidine has a muscular action, what was currently corroborated. The increase of cytosolic Ca
2+ concentration (after K
+ channel blockade) inside muscle fibers in response to membrane depolarization triggers neurotransmitter release and muscle contraction; the increase in intracellular Na
+ results in the interruption of the change between extracellular sodium and intracellular calcium. As a result, the high intracellular Ca
2+ concentration activates intracellular calcium-dependent proteases, which hydrolyze the components of muscle cells [
22,
39]. Therefore, the data indicate that, in addition to presynaptic pharmacological effects, guanidine also produces a muscle pharmacological action on the diaphragm [
22,
40], EDL and SOL.
Molecular characteristics of skeletal muscle types are herein considered as an attempt to explain the differences in the contractile response of PND, EDL and SOL. As premise, it is common sense that the preservation of the membrane electrochemical gradients for Na+, K+, and Ca2+ is vital to the muscle processes of EC coupling, regardless the muscle phenotype. Herein, we found that evoked end-plate potentials produced a membrane depolarization of diaphragm which was greater in the post-washing secondary facilitation than in the former. This peculiar effect did not find similarity to fast- and slow-twitch muscles. Why EDL and SOL do not respond that way; what are the possible causes of this?
Studies have shown that density and types of ion channels involved in neurotransmission may differ in different muscle fiber types. For instance, NMJ of fast-contracting fibers (glycolytic) have high density of Na
+ channels, and this density is determined by the type of motor innervation [
41,
42,
43]. Based on this, it was expected that PND and EDL muscles would present a higher density of Na
+ channels than SOL, since they have a higher proportion of glycolytic fibers with fast contraction. As a result, PND and EDL are expected to present a larger proportion of damaged fibers than SOL muscles, which actually occurred in preparations incubated for 15 and 30 min, but not in those incubated for 60 min. Guanidine is able to cross Na
+ channels [
37,
38], being prevented from returning to the extracellular compartment by the selectivity of the Na
+ pump, which does not recognize it [
44]. The accumulation of Na
+ and Ca
2+ into the nerve terminal, along with the guanidinium cation influx, is expected to promote osmotic fluid entry and activation of calcium-dependent endoproteases.
Likewise, the existence of different types/proportion of K
+ channels in all three muscles could have a role in their response to guanidine. K
+ channels are membrane integral proteins with different types of pore-forming α-subunits; they are present ubiquitously in several types of cells where they play key roles in cell function and homeostasis; in skeletal muscle, they maintain the resting membrane potential, regulate the action potential duration and neurotransmitter release [
45,
46]. Recent studies provided evidences that ATP-regulated potassium (K
+ATP) channel densities and channel subunit composition vary in different muscle fiber types, contributing to their differential physiological performance and determining differential pharmacological actions of drugs modulating the channel activity [
47,
48]. K
ATP channels were shown to have muscle-specific roles and are physiologically and pharmacologically regulated. They determine muscle phenotype-specific pharmacological control of electrical activity, being its density higher in fast-twitch than in slow-twitch muscles; in so doing, K
ATP channels play important roles in the control of contractility, protecting tissue against calcium overload (see Flagg
et al. [
48] for review). Similarly, functional and pharmacological properties of calcium-activated K
+ channels in skeletal muscle tissue were shown to be also dependent on muscle phenotypic characteristics [
49].
The contractile and relaxing activity (EC coupling) of muscle fibers is crucially dependent on the calcium signaling and handling apparatus; as part of the process, there are myriads of regulatory proteins, such as the main proteins (ryanodine receptor, the SR Ca
2+ release channel; troponin, which mediates contraction through the action on Ca
2+ interaction with myofibrils; Ca
2+ pump, responsible for reuptaking Ca
2+ into the SR, and calsequestrin, the Ca
2+ storage protein in the SR) and accessory proteins (annexins, beta-actinin, calcineurin, calmodulin, calpain, myosin light chains, parvalbumin, S100 and sorcin), all of them involved in muscle response following a nerve impulse and action potential propagation. Since a range of heavy myosin isoforms may constitute muscle fiber types, the contractile properties of them vary according to their constitutive regulatory and accessory protein modulation [
50,
51]; thereby a series of Ca
2+-dependent physiologic processes will be differentially modulated, including transmitter release in motor nerve endings and contraction in metabolic/physiologic distinct muscle types [
27]. For instance, Zubrzycka-Gaarn
et al. [
51,
52] found that the protein amount of Ca
2+-ATPase and calsequestrin, both responsible for transporting Ca
2+ back into the sarcoplasmic reticulum (SR), was several times lower in the slow-twitch muscle SR vesicles, and that the active Ca
2+ transport and formation of the phosphorylated intermediate were several times lower than that observed in fast-twitch muscles. The authors suggested that the slow rate of calcium transport, found in slow-twitch muscle SR vesicles, may be related to a low content of Ca
2+-transporting ATPase in the membrane. In addition, the impact of aging on the Ca
2+ pump function of skeletal muscle SR, differing in soleus and gastrocnemius of rats, indicates that muscle contractile properties and SR function are muscle specific [
52].
It is now established that the regulation of the EC coupling mechanism differs between fast-twitch and slow-twitch fibers and that there is a direct involvement of calsequestrin in the Ca
2+ release process, given that protein represents an endogenous modulator of ryanodine receptors (SR Ca
2+ release channels) [
53,
54]. Evidences have been provided that key membrane proteins involved in Ca
2+ homeostasis detected fiber-type-specific differences in auxiliary subunits of dihydropyridine receptor, ryanodine receptor and Ca
2+-ATPase, as well as in triad markers and various Ca
2+-binding and ion-regulatory proteins [
55]. The authors concluded that the type of motor innervation has a profound impact on the levels and isoform expression pattern of Ca
2+-regulatory membrane proteins and reflects differences in the regulation of Ca
2+ homeostasis and contractile response in different types of muscle.
Feldmeyer
et al. [
56] suggested that the inhibitory action of guanidinium ion on excitation-contraction coupling is due to a depression of calcium release from the SR. It is possible that the differential contractile response in all three studied muscles is also related to the population of mitochondria present in each type of muscle fiber. Since Ca
2+ is one of the main protagonists in the genesis of the triphasic effect of guanidine, and relating it to the fact that mitochondria and SR are the main organelles involved in intracellular Ca
2+ homeostasis, it is expected that muscle fibers with a larger number of mitochondria are the most affected by guanidine [
57]. In fact, SOL muscle, which increased the number of affected cells only after 60 min of incubation (by more than twice compared to that observed at 15 and 30 min) is predominantly constituted of oxidative fibers with a larger proportion of mitochondria. It is known that mitochondria are the main organelles involved in alterations provoked by guanidine, in addition to the participation of the SR in pathophysiological processes of the drug [
22,
57].
Whether the amount of cells altered by the guanidine action was responsible for such differences is unclear. What is known is that the physiological characteristics of diaphragm [
34,
35,
36,
37] distinguish this muscle from the peripheral ones, responding differentially to endogenous or exogenous pharmacological stimuli.
The primary and secondary facilitations caused by guanidine specifically for PND suggest that the stimulation threshold for PND to produce a maximum depolarization of the end-plate is not the same stimulation threshold for EDL and SOL muscles,
i.e., the threshold to cause an end-plate potential greater for PND is likely smaller than the stimulation threshold for EDL and SOL muscles. What mechanisms can account for such differences? Since diaphragm is a vital muscle, it has the ability to support adverse conditions that peripheral muscles do not have. The term “safety factor” (SF) at the NMJ is used to indicate a property of the diaphragm that maintains neuromuscular transmission operative in conditions not supported by non-vital muscles. The SF relies on both the amount of presynaptic transmitter released per nerve impulse, being greater than that required to trigger an action potential in the muscle fiber, and on elaborate postsynaptic specializations to enhance the transmitter response [
58,
59]. It also relies on the diaphragm, possessing a higher density of nAChR and ACh quantal contents than peripheral muscles [
23]. This means that the SF makes the diaphragm more capable of amplifying the responses to depolarization, promoting the transduction process that leads to contraction than EDL and SOL muscles. Interestingly, Ermilov
et al. [
60] found that SF varies for neuromuscular transmission across muscle fiber types (even within a single muscle), being larger for type IIX or IIB fibers (fast-twitch types) than for type I or IIA fibers (slow-twitch types). As known, SOL muscle has high predominance of fiber I and IIA types, whereas diaphragm and EDL have high predominance of fiber IIX and IIB types.
A growing interest has been directed towards the mechanism of action of guanidine and its alkyl analogs on potassium channels [
20,
61]. The increase of ACh release into the synaptic cleft and stimulation of neuromuscular transmission resulting from this action has profound implication in the prospect of drugs targeting neuromuscular diseases. A recent study has shown that guanidine and derivatives bind within the intracellular pore of potassium channel and disturb the hydrophobic subunit interface to stabilize a closed state of the channel [
61].