Ginsenoside Rb1 Attenuates Intestinal Ischemia Reperfusion Induced Renal Injury by Activating Nrf2/ARE Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Ginsenoside Rb1 on Renal Functional Injury Induced by IIR
| Sham | IIR | NS | Rb1-30 | Rb1-60 | |
|---|---|---|---|---|---|
| BUN | 9.37 ± 1.19 | 21.55 ± 1.50 * | 18.87 ± 2.16 * | 15.52 ± 1.23 # | 12.91 ± 1.41 # |
| Cr | 10.26 ± 1.25 | 20.65 ± 1.53 * | 19.53 ± 1.19 * | 16.00 ± 1.50 # | 13.14 ± 1.22 # |
| NAGL | 71.65 ± 4.65 | 155.93 ± 8.07 * | 150.11 ± 6.09 * | 124.44 ± 6.52 # | 101.76 ± 12.20 # |
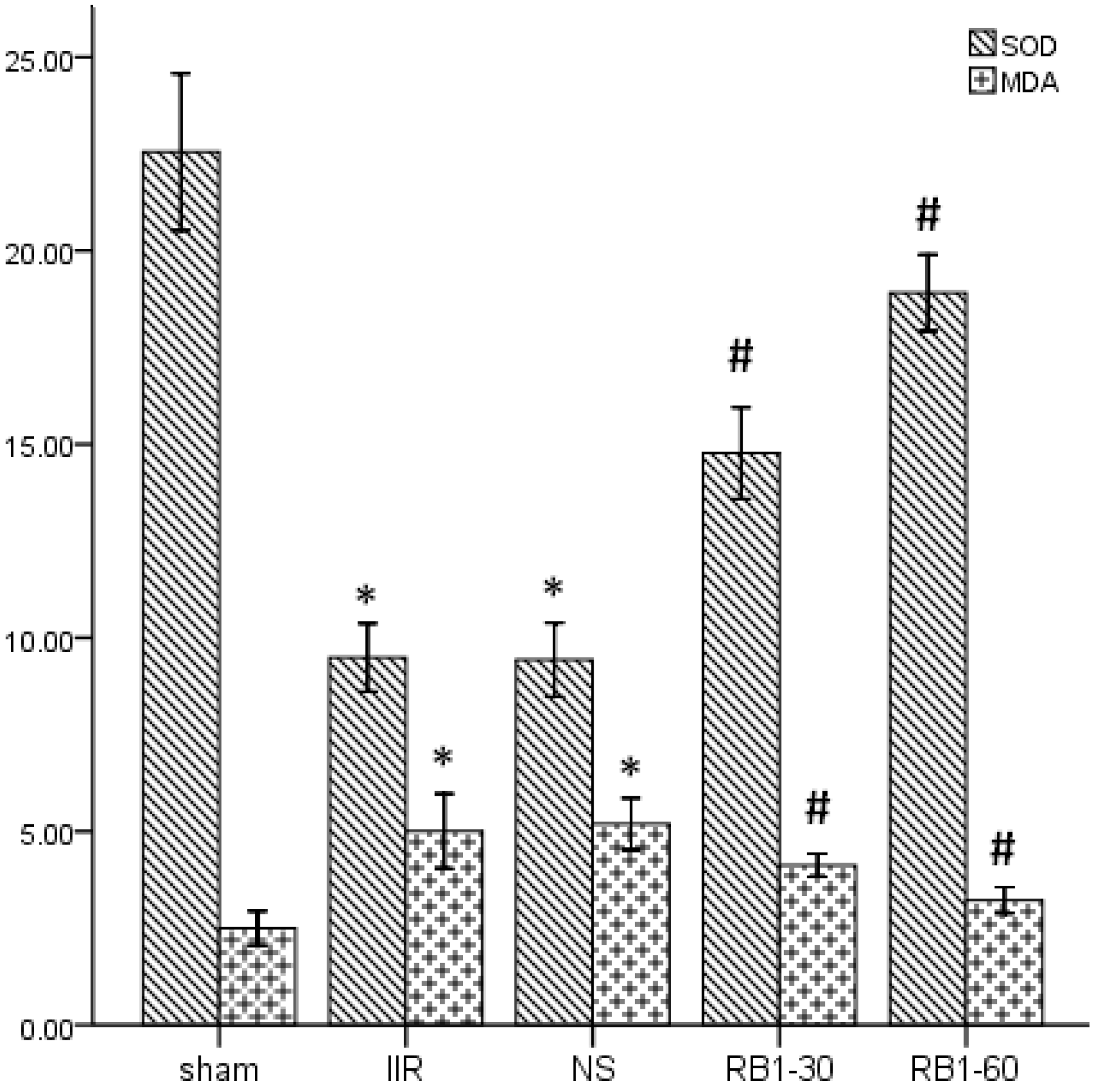
2.2. Effects of Ginsenoside Rb1 on SOD and MDA Levels in Renal Tissues
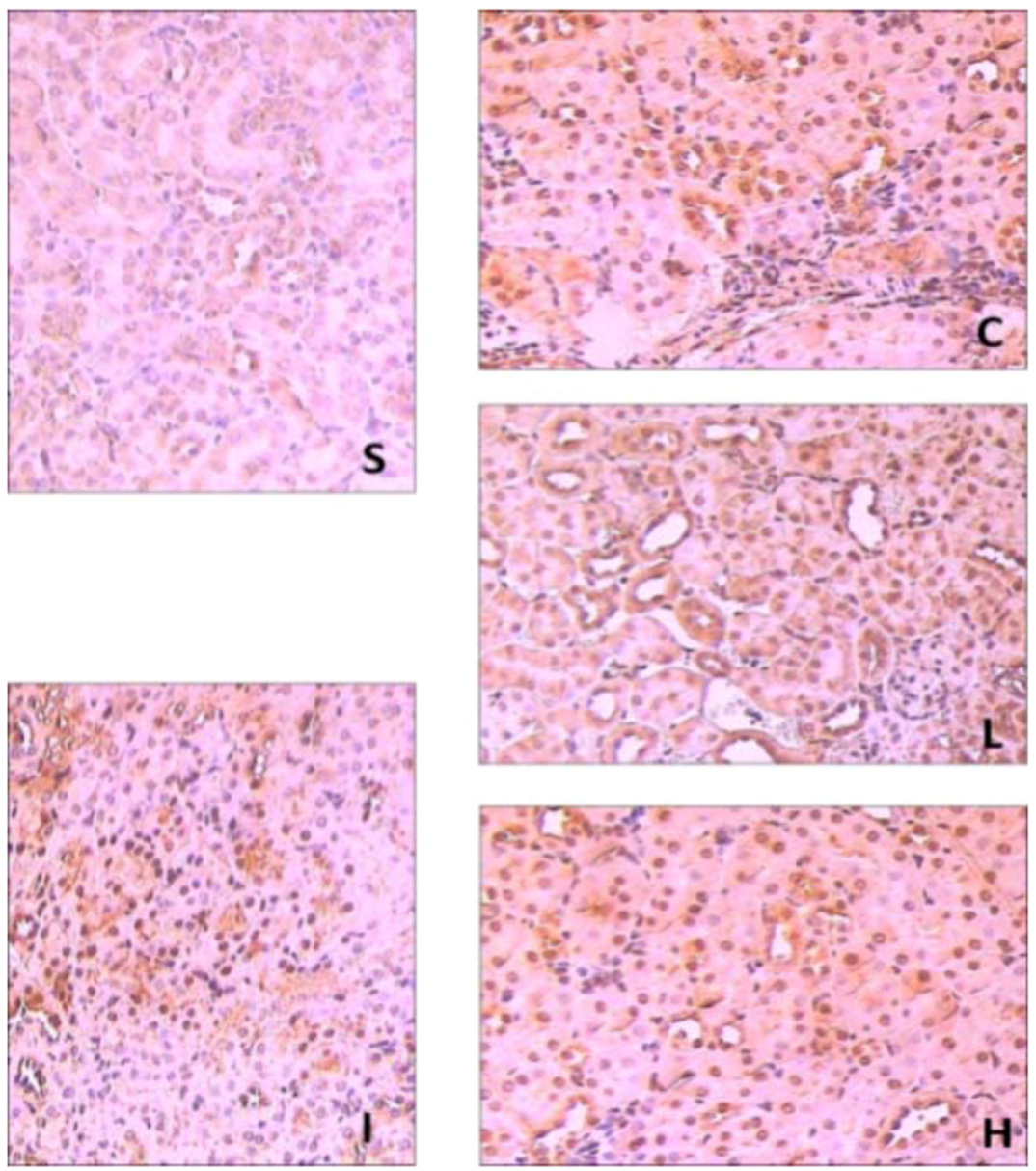
2.3. Effects of Ginsenoside Rb1 on HO-1 and Nrf2 Expressions in Renal Tissues by Immunohisto- Chemical Analysis


2.4. Effects of Ginsenoside Rb1 on HO-1 and Nrf2 Expressions in Renal Tissues by Western Blot Analysis

2.5. Renal Histopathological Assessment

3. Experimental
3.1. Materials
3.2. Experimental Protocol
3.3. Measurement of Serum BUN, Cr and NGAL
3.4. Renal Tissues SOD and MDA Assay
3.5. Renal HO-1 and Nrf2 Immunohistochemical Assays
3.6. Renal Tissues HO-1 and Nrf2 Western Blot Analysis
3.7. Renal Histopathological Assessment
3.8. Statistical Analysis
4. Conclusions
Acknowledgements
Conflict of Interest
- Sample Availability: Not available.
Reference and Notes
- Zhang, F.; Tong, L.; Qiao, H.; Dong, X.; Qiao, G.; Jiang, H.; Sun, X. Taurine attenuates multiple organ injury induced by intestinal ischemia reperfusion in rats. J. Surg. Res. 2008, 149, 101–109. [Google Scholar] [CrossRef]
- de Lima, F.M.; Villaverde, A.B.; Albertini, R.; Corrêa, J.C.; Carvalho, R.L.; Munin, E.; Araújo, T.; Silva, J.A.; Aimbire, F. Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: Action on anti- and pro-inflammatory cytokines. Laser. Surg. Med. 2011, 43, 410–420. [Google Scholar] [CrossRef]
- Zhao, H.D.; Zhang, F.; Shen, G.; Li, Y.B.; Li, Y.H.; Jing, H.R.; Ma, L.F.; Yao, J.H.; Tian, X.F. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J. Gastroenterol. 2010, 16, 3002–3010. [Google Scholar]
- Stefanutti, G.; Vejchapipat, P.; Williams, S.R.; Pierro, A.; Eaton, S. Heart energy metabolism after intestinal ischaemia and reperfusion. J. Pediatr. Surg. 2004, 39, 179–183. [Google Scholar] [CrossRef]
- Kazantzidou, D.; Tsalis, K.; Vasiliadis, K.; Kaldrymidou, H.; Papageorgiou, G.; Koliakou, K.; Tsali, E.; Lazaridis, C. Alanine-glutamine dipeptide pretreatment protects rat renal function from small intestine ischemia-reperfusion injury. Minerva Chir. 2010, 65, 515–525. [Google Scholar]
- Lai, I.R.; Ma, M.C.; Chen, C.F.; Chang, K.J. The effect of an intestinal ischemia-reperfusion injury on renal nerve activity among rats. Shock 2003, 19, 480–485. [Google Scholar] [CrossRef]
- Kubiak, B.D.; Albert, S.P.; Gatto, L.A.; Snyder, K.P.; Maier, K.G.; Vieau, C.J.; Roy, S.; Nieman, G.F. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock 2010, 34, 525–534. [Google Scholar] [CrossRef]
- Kostopanagiotou, G.; Avgerinos, E.; Costopanagiotou, C.; Arkadopoulos, N; Andreadou, I.; Diamantopoulou, K.; Lekka, M.; Smyrniotis, V.; Nakos, G. Acute lung injury in a rat model of intestinal ischemia-reperfusion: The potential time depended role of phospholipases A (2). J. Surg. Res. 2008, 147, 108–116. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2 -mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, L.H.; Zhang, J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef]
- Zeng, H.S.; Liu, Z.X.; Liu, X.C. Effect of ginsenoside-Rb1 and Re against cardiomyocyte apoptosis and expression of the related gene proteins in the experimental cardiac ischemiareperfusion in rats. Chin. J. Phys. Med. Rehabil. 2003, 25, 402–405. [Google Scholar]
- Geng, Q.; Wu, D.; Xie, Y.C.; Zhang, B.G.; Wu, H.; Yin, W.H. Effects of ginsenoside Rb1 on apoptosis and Bcl-2, Bax gene expression in rabbits with lung ischemia/reperfusion injury. Chin. J. TCM. WM. Crit. Care 2005, 12, 159–161. [Google Scholar]
- Wang, J.; Qiao, L.; Li, Y.; Yang, G. Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion- induced liver injury by inhibiting NF-κB activation. Exp. Mol. Med. 2008, 40, 686–698. [Google Scholar] [CrossRef]
- Li, F.C.; Tao, Z.Y.; Liu, A.M.; Li, J.L.; Wu, Z.H.; Lin, J.H. Effect of ginsenoside Rb1 on the expression of GLUT1 in penumbra area after cerebral ischemia/reperfusion injury in rats. Chin. J. Exp. Surg. 2005, 22, 196–197. [Google Scholar]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Mori, K.; Lee, H.T.; Rapoport, D.; Drexler, I.R.; Foster, K.; Yang, J.; Schmidt-Ott, K.M.; Chen, X.; Li, J.Y.; Weiss, S.; et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Invest. 2005, 115, 610–621. [Google Scholar]
- Arumugam, T.V.; Shiels, A.A.; Woodruff, T.M.; Granger, D.N.; Taylor, A.M. The role of the complement system in ischemia-reperfusion injury. Shock 2004, 21, 401–409. [Google Scholar] [CrossRef]
- Teke, Z.; Kabay, B.; Aytekin, F.O.; Yenisey, C.; Demirkan, N.C.; Sacar, M.; Erdem, E.; Ozden, A. Pyrrolidine dithiocarbamate prevents 60 minutes of warm mesenteric ischemia/reperfusion injury in rats. Am. J. Surg. 2007, 194, 255–262. [Google Scholar] [CrossRef]
- Röth, E.; Hejjel, L.; Jaberansari, M.; Jancso, G. The role of free radicals in endogenous adaptation and intracellular signals. Exp. Clin. Cardiol. 2004, 9, 13–16. [Google Scholar]
- Leng, Y.F.; Zhang, Y.; Zhang, Y.; Xue, X.; Wang, T.; Kang, Y.Q. Ischemic post-conditioning attenuates the intestinal injury induced by limb ischemia/reperfusion in rats. Braz. J. Med. Biol. Res. 2011, 44, 411–417. [Google Scholar]
- Gutteridge, J.M.C.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. TIBS 1990, 15, 129–135. [Google Scholar]
- Sykiotis, G.P.; Habeos, I.G.; Samuelson, A.V.; Bohmann, D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 41–48. [Google Scholar] [CrossRef]
- Chan, J.Y.; Kwong, M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta 2000, 1517, 19–26. [Google Scholar]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar]
- Shah, Z.A.; Li, R.C.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef]
- Vijayan, V.; Mueller, S.; Baumgart-Vogt, E.; Immenschuh, S. Heme oxygenase-1 as a therapeutic target in inflammatory disorders of the gastrointestinal tract. World J. Gastroenterol. 2010, 16, 3112–3119. [Google Scholar]
- Nakao, A.; Kaczorowski, D.J.; Sugimoto, R.; Billiar, T.R.; McCurry, K.R. Application of heme oxygenase-1,carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2008, 42, 78–88. [Google Scholar] [CrossRef]
- Tomohisa, T.; Yuji, N.; Toshikazu, Y. The Expression of Heme Oxygenase-1 Induced by Lansoprazole. J. Clin. Biochem. Nutr. 2009, 45, 9–13. [Google Scholar] [CrossRef]
- Mutlu, G.; Abbasoğlu, L.; Doğru-Abbasoğlu, S.; Solakoğlu, S.; Bulut, M. Morphologic changes and lipid peroxidation in renal tissues of young rats following intestinal ischemia-reperfusion. Pediatr. Surg. Int. 2002, 18, 337–340. [Google Scholar] [CrossRef]
- McWhinnie, D.L.; Thompson, J.F.; Taylor, H.M.; Chapman, J.R.; Bolton, E.M.; Carter, N.P.; Wood, R.F.; Morris, P.J. Morphometric analysis of cellular infiltration assessed by monoclonal antibody labelling in sequential human renal allograft biopsies. Transplantation 1986, 42, 352–358. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, Q.; Meng, Q.-T.; Jiang, Y.; Xia, Z.-Y. Ginsenoside Rb1 Attenuates Intestinal Ischemia Reperfusion Induced Renal Injury by Activating Nrf2/ARE Pathway. Molecules 2012, 17, 7195-7205. https://doi.org/10.3390/molecules17067195
Sun Q, Meng Q-T, Jiang Y, Xia Z-Y. Ginsenoside Rb1 Attenuates Intestinal Ischemia Reperfusion Induced Renal Injury by Activating Nrf2/ARE Pathway. Molecules. 2012; 17(6):7195-7205. https://doi.org/10.3390/molecules17067195
Chicago/Turabian StyleSun, Qian, Qing-Tao Meng, Ying Jiang, and Zhong-Yuan Xia. 2012. "Ginsenoside Rb1 Attenuates Intestinal Ischemia Reperfusion Induced Renal Injury by Activating Nrf2/ARE Pathway" Molecules 17, no. 6: 7195-7205. https://doi.org/10.3390/molecules17067195