Fluorescent Nanoprobes Dedicated to in Vivo Imaging: From Preclinical Validations to Clinical Translation
Abstract
:1. Introduction
2. Clinical Applications of in Vivo Fluorescence Imaging Probes and Associated Constraints
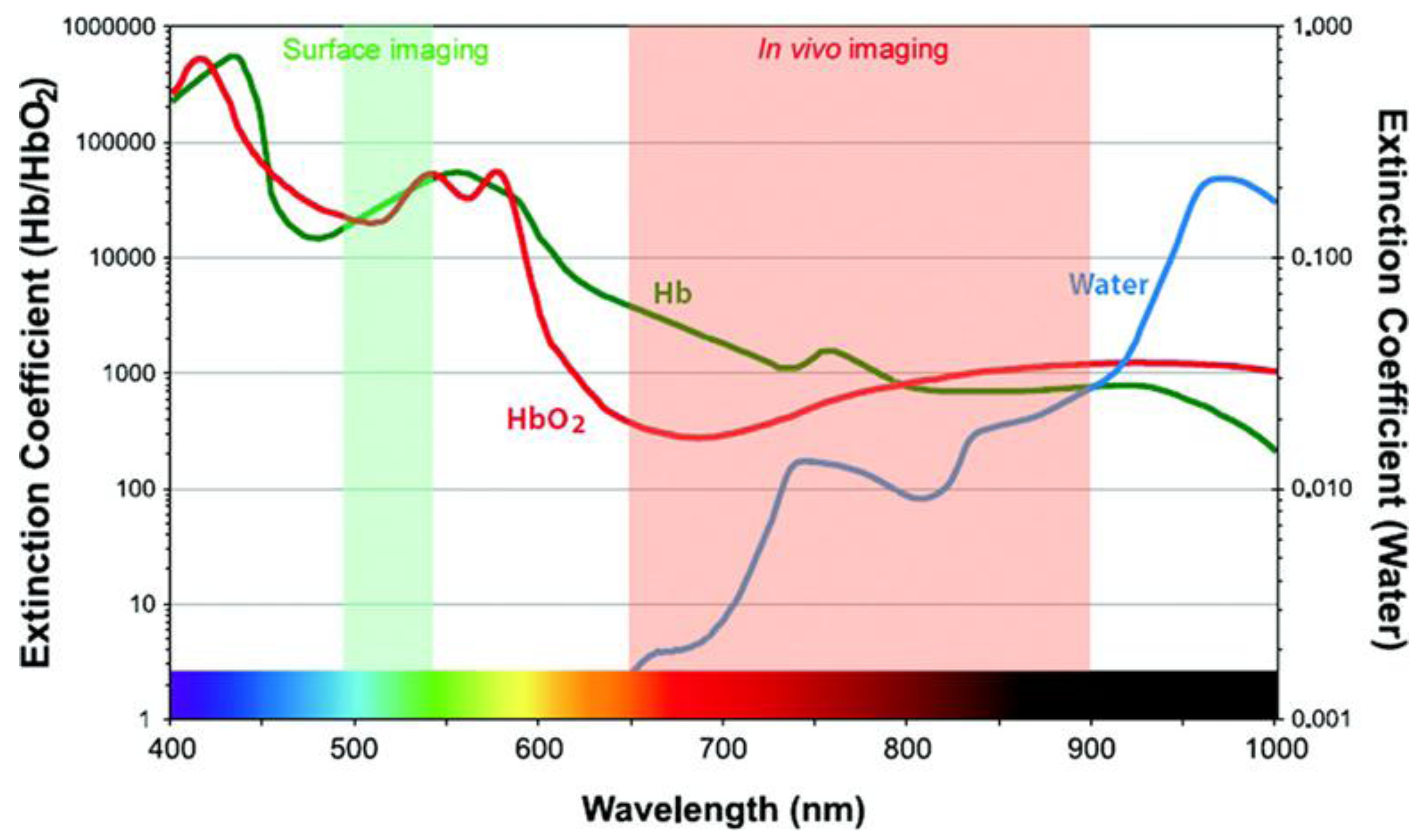
2.1. The Near-Infrared Window
2.2. Image-Guided Removal of Sentinel Lymph Node

2.2. Other Clinical Applications
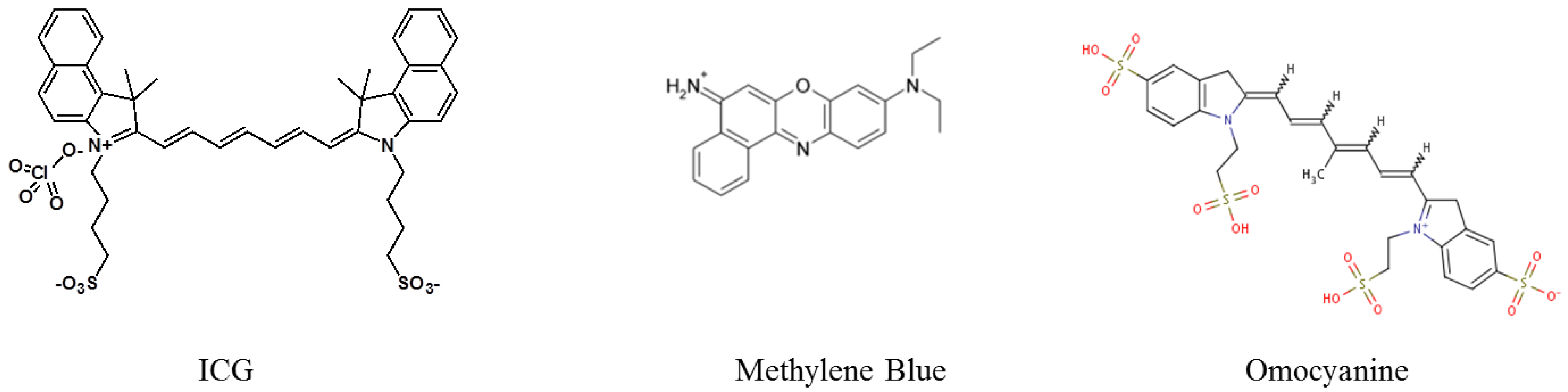
3. The Benefits of Nanotechnologies
3.1. Limitations of Low Molecular Weight Fluorophores for in Vivo Fluorescence Imaging
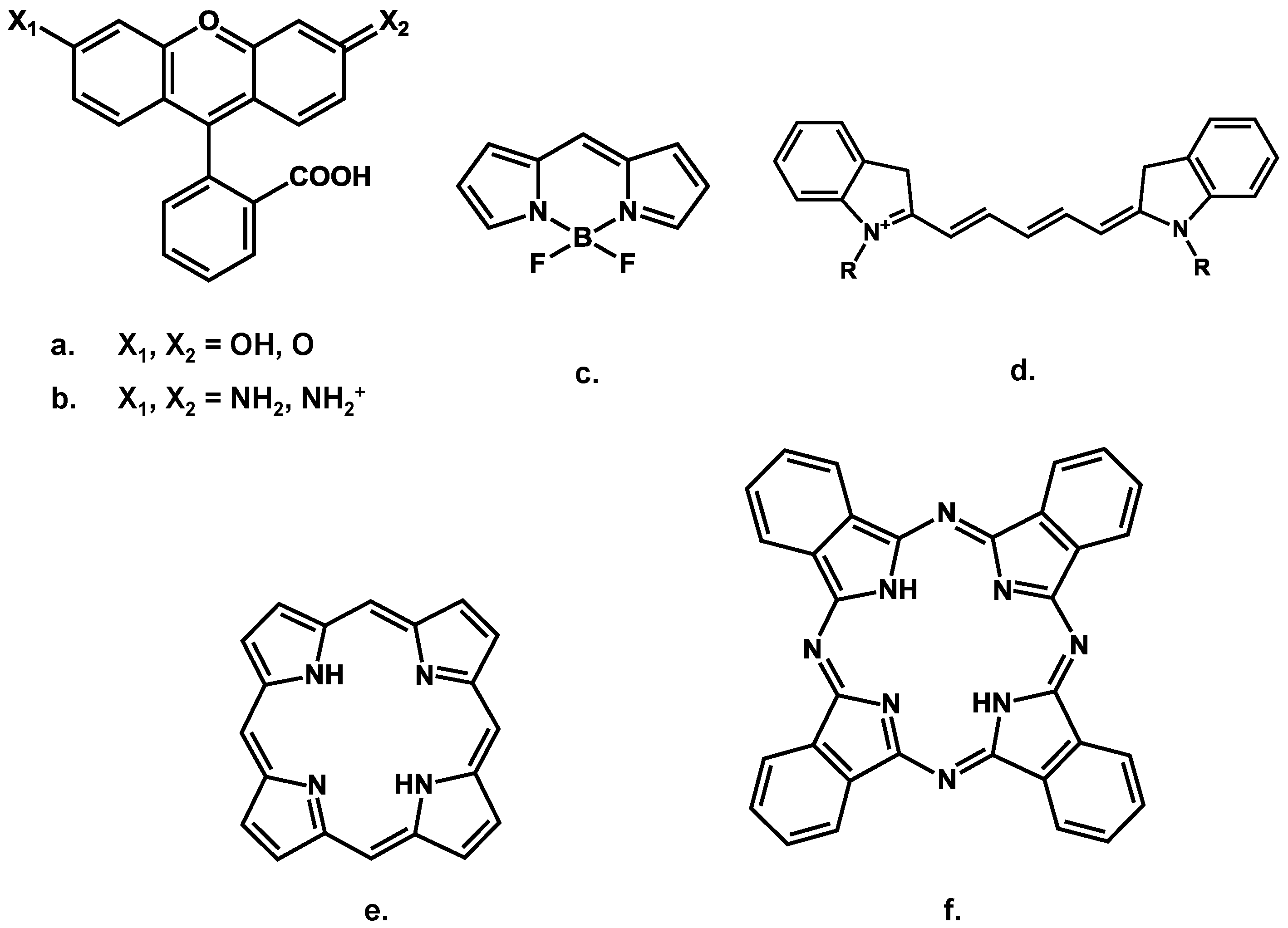
3.2. What Can Nanotechnologies Bring in the Design of Efficient Fluorescent Probes

4. Fluorescent Nanoprobes

4.1. Quantum Dots
4.2. Dye-Loaded Inorganic Nanocarriers
4.3. Dye-Loaded Organic Nanocarriers
4.3.1. Polymer-Based Nanoparticles

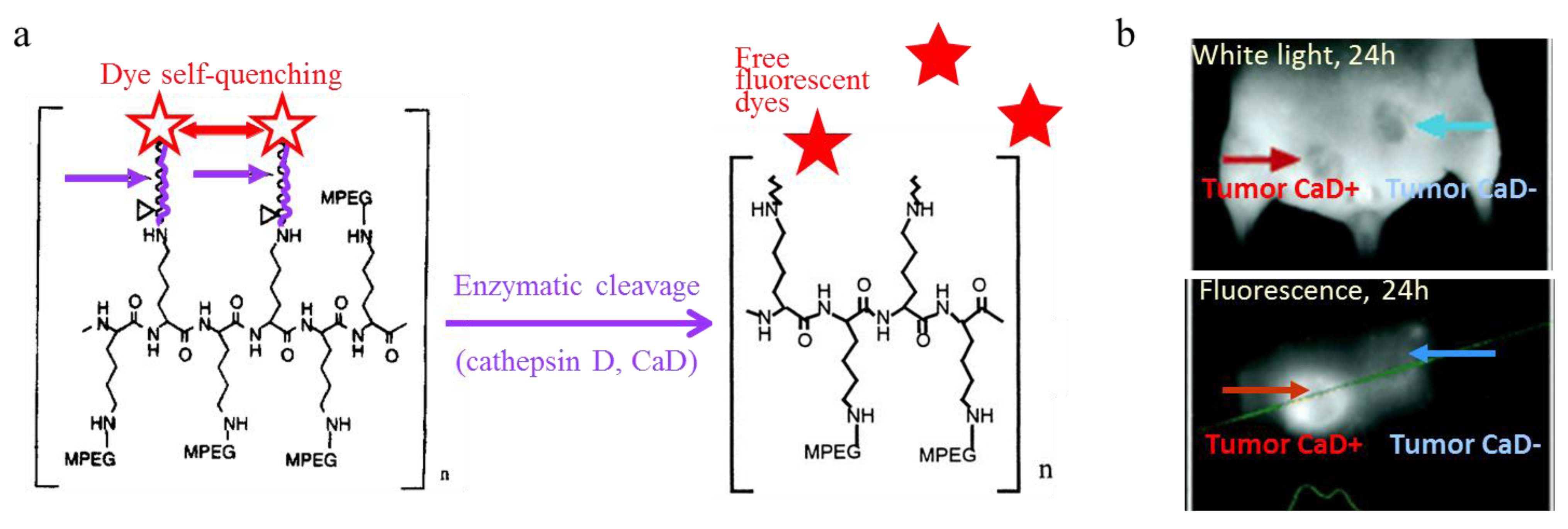
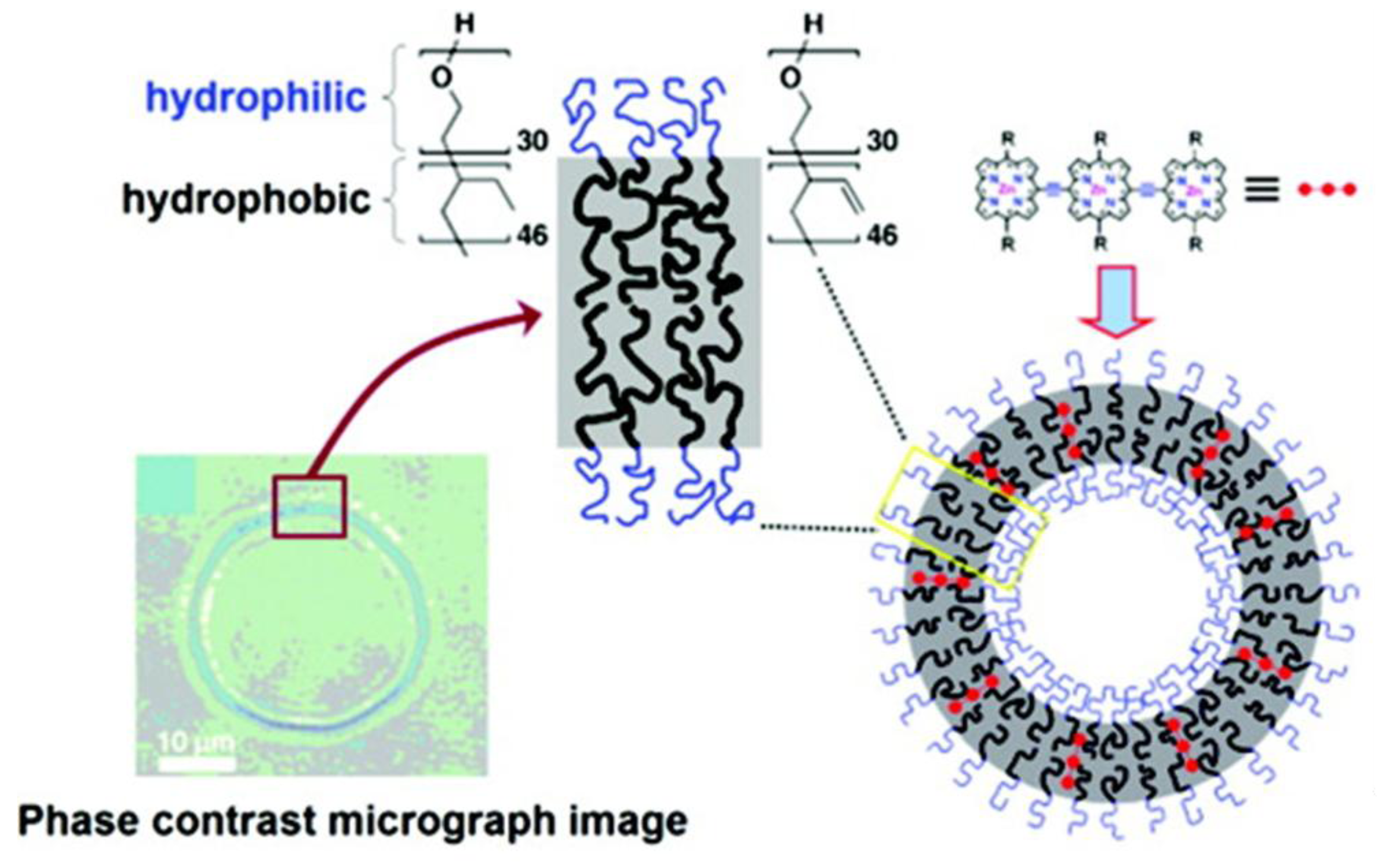
4.3.2. Lipid-Based Nanoparticles
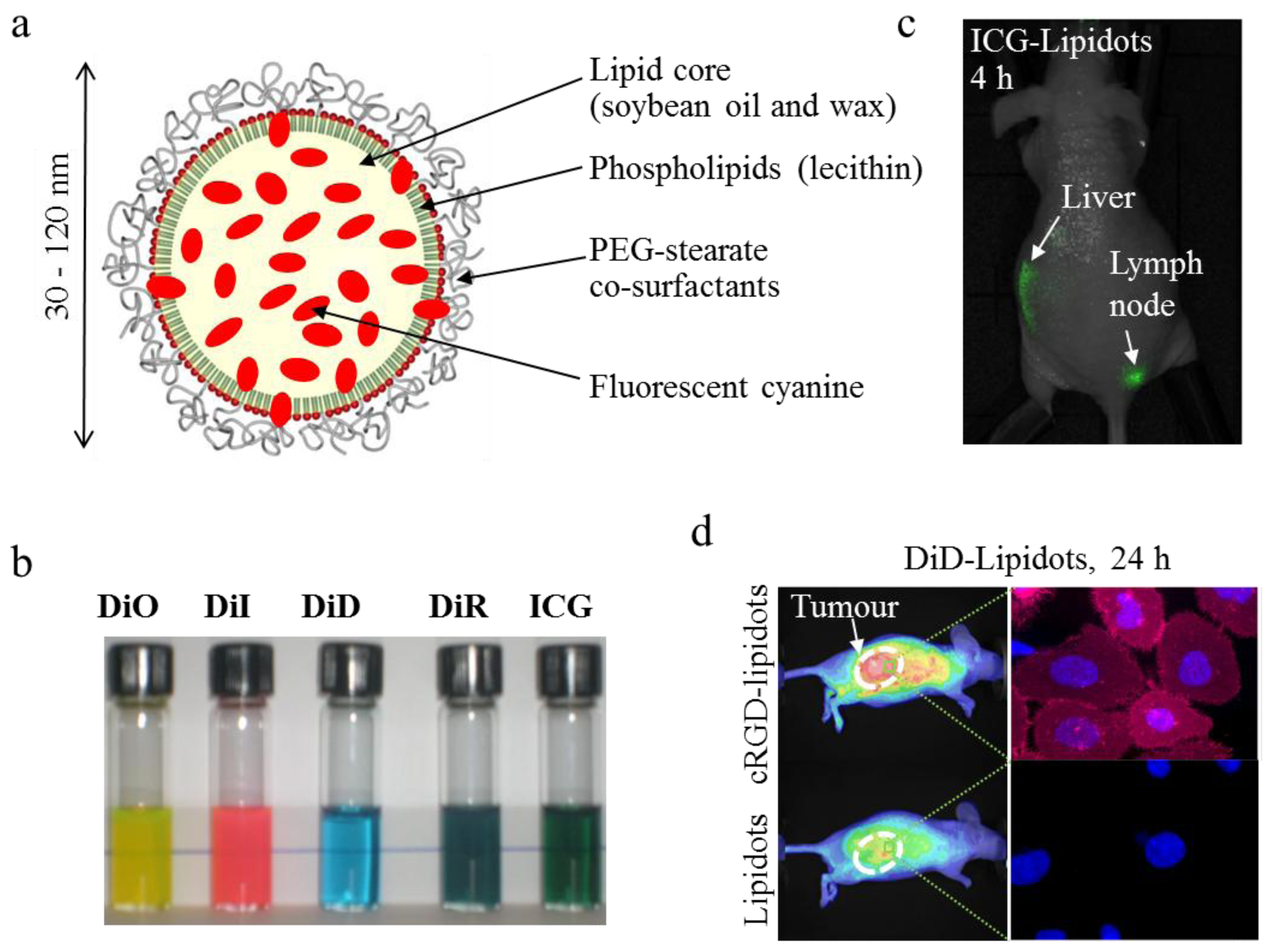
5. Conclusions and Perspectives: Transfer to the Clinic
Acknowledgments
Conflicts of Interests
References
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Develop. 2003, 17, 545–580. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Integrating noninvasive molecular imaging into molecular medicine: An evolving paradigm. Trends Mol. Med. 2007, 13, 183–191. [Google Scholar] [CrossRef]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef]
- Leblond, F.; Davis, S.C.; Valdès, P.A.; Pogue, B.W. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J. Photochem. Photobiol. B: Biol. 2010, 98, 77–94. [Google Scholar]
- Hassan, M.; Klaunberg, B.A. Biomedical applications of fluorescence imaging in vivo. Comp. Med. 2004, 54, 635–644. [Google Scholar]
- Koo, V.; Hamilton, P.W.; Williamson, K. Non invasive in vivo imaging in small animal research. Cellular Oncol. 2006, 28, 127–139. [Google Scholar]
- Licha, K.; Olbrich, C. Optical imaging in drug discovery and diagnostic applications. Adv. Drug Deliv. Rev. 2005, 57, 1087–1108. [Google Scholar] [CrossRef]
- Miyashiro, I.; Miyoshi, N.; Hiratsuka, M.; Kishi, K.; Yamada, T.; Ohue, M.; Ohigashi, H.; Yano, M.; Ishikawa, O.; Imaoka, S. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: Comparison with infrared imaging. Ann. Surg. Oncol. 2008, 15, 1640–1643. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Ikeda, H.; Takahashi, M.; Kawasaki, K.; Doihara, H. Evaluation of breast lymphatic pathways with indocyanine green fluorescence imaging in patients with breast cancers. Word J. Surg. 2008, 32, 1924–1929. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Sharma, R.; Rasmussen, J.C.; Marshall, M.V.; Wendt, J.A.; Pham, H.Q.; Bonefas, E.; Houston, J.P.; Sampath, L.; Adams, K.E.; Blanchard, D.K.; Fischer, R.E.; Chiang, S.B.; Elledge, R.; Mawad, M.E. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore. Radiology 2008, 246, 734–741. [Google Scholar] [CrossRef]
- Tagaya, N.; Yamazaki, R.; Nakagawa, A.; Abe, A.; Hamada, K.; Kubota, K.; Oyama, T. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am. J. Surg. 2008, 195, 850–853. [Google Scholar] [CrossRef]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Laidevant, A.; Hervé, L.; Debourdeau, M.; Boutet, J.; Grenier, N.; Dinten, J.-M. Fluorescence time-resolved imaging system embedded in an ultrasound prostate probe. Biomed. Opt. Express 2011, 2, 194–206. [Google Scholar] [CrossRef]
- Liu, Y.; Bauer, A.Q.; Akers, W.J.; Sudlow, G.; Liang, K.; Shen, D.; Berezin, M.Y.; Culver, J.P.; Achilefu, S. Hands-free, wireless goggles for near-infrared fluorescence and real-time image-guided surgery. Surgery 2011, 149, 689–698. [Google Scholar] [CrossRef]
- Qin, C.; Zhu, S.; Tian, J. New optical molecular imaging systems. Curr. Pharm. Biotechnol. 2010, 11, 620–627. [Google Scholar] [CrossRef]
- Pierce, M.C.; Javier, D.J.; Richards-Kortum, R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int. J. Cancer 2008, 123, 1979–1990. [Google Scholar] [CrossRef]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar]
- te Velde, E.A.; Veerman, T.; Subramaniam, V.; Ruers, T. The use of fluorescent dyes and probes in surgical oncology. Eur. J. Surg. Oncol. 2010, 36, 6–15. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Bremer, C.; Ntziachristos, V.; Weissleder, R. Optical-based molecular imaging: Contrast agents and potential medical applications. Eur. Radiol. 2003, 13, 231–243. [Google Scholar]
- Desmettre, T.; Devoisselle, J.-M.; Mordon, S. Fluorescence properties and metabolic features of Indocyanine Green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Nishioka, Y.; Yoshino, H. Lymphatic targeting with nanoparticulate system. Adv. Drug Deliv. Rev. 2001, 47, 55–64. [Google Scholar] [CrossRef]
- Gravier, J.; Navarro, F.; Delmas, T.; Mittler, F.; Couffin, A.C.; Vinet, F.; Texier, I. Lipidots: A biocompatible alternative to quantum dots for in vivo fluorescence imaging. J. Biomed. Opt. 2011, 16, 096013. [Google Scholar] [CrossRef]
- Onishi, S.; Lomnes, S.J.; Laurence, R.G.; Gobashian, A.; Mariani, G.; Frangioni, J.V. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol. Imaging 2005, 4, 172–181. [Google Scholar]
- Kim, S.; Lim, Y.T.; Soltesz, E.G.; De Grand, A.M.; Lee, J.; Nakayama, A.; Parker, J.A.; Mihaljevic, T.; Laurence, R.G.; Dor, D.M.; et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004, 22, 93–97. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hama, Y.; Koyama, Y.; Barett, T.; Regino, C.; Urano, Y.; Choyke, P.L. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007, 7, 1711–1716. [Google Scholar] [CrossRef]
- Jain, R.; Dandekar, P.; Patravale, V. Diagnostic nanocarriers for sentinel lymph node imaging. J. Control. Release 2009, 138, 90–102. [Google Scholar] [CrossRef]
- Hutteman, M.; Mieog, J.S.; van der Vorst, J.R.; Liefers, G.J.; Putter, H.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res. Treat. 2011, 127, 163–170. [Google Scholar] [CrossRef]
- Benson, R.C.; Kues, H.A. Fluorescence properties of indocyanine green as related to angiography. Phys. Med. Biol. 1978, 23, 159–163. [Google Scholar]
- Lee, B.T.; Matsui, A.; Hutteman, M.; Lin, S.J.; Winer, J.H.; Laurence, R.G.; Frangioni, J.V. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: Current research and early clinical experience. J. Reconstr. Microsurg. 2010, 26, 59–65. [Google Scholar] [CrossRef]
- Unno, N.; Suzuki, M.; Yamamoto, N.; Inuzuka, K.; Sagara, D.; Nishiyama, N.; Tanaka, H.; Konno, H. Indocyanine Green fluorescence angiography for intraoperative assessment of blood flow: A feasibility study. Eur. J. Vas. Endovasc. Surg. 2008, 35, 205–207. [Google Scholar] [CrossRef]
- Matsui, A.; Tanaka, E.; Choi, H.S.; Winer, J.H.; Kianzad, V.; Gioux, S.; Laurence, R.G.; Frangioni, J.V. Real-time intra-operative near-infrared fluorescence identification of the extra-hepatic bile ducts using clinically available contrast agents. Surgery 2010, 148, 87–95. [Google Scholar] [CrossRef]
- Aoki, T.; Yasuda, D.; Shimizu, Y.; Odaira, M.; Niiya, T.; Kusano, T.; Mitamura, K.; Hayashi, K.; Murai, N.; Koizumi, T.; et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J. Surg. 2008, 32, 1763–1767. [Google Scholar] [CrossRef]
- Matsui, A.; Tanaka, E.; Choi, H.K.; Kienzad, V.; Gioux, S.; Lomnes, S.J.; Frangioni, J.V. Real-time, near-infrared, fluorescence-guided identification of the ureters using methylene blue. Surgery 2010, 148, 78–86. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Tan, I.C.; Marshall, M.V.; Adams, K.E.; Kwon, S.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Covington, K.R.; Sevick-Muraca, E.M. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl. Oncol. 2010, 3, 362–372. [Google Scholar]
- Alacam, B.; Yazici, B.; Intes, X.; Nioka, S.; Chance, B. Pharmacokinetic-rate images of indocyanine green for breast tumors using near-infrared optical methods. Phys. Med. Biol. 2008, 53, 837–859. [Google Scholar] [CrossRef]
- Poellinger, A.; Persigehl, T.; Mahler, M.; Bahner, M.; Ponder, S.L.; Diekmann, F.; Bremer, C.; Moesta, T. Near-infrared imaging of the breast using omocianine as a fluorescent dye: Results of a placebo-controlled, clinical, multicenter trial. Investig. Radiol. 2011, 46, 697–704. [Google Scholar]
- van de Ven, S.; Wiethoff, A.; Nielsen, T.; Brendel, B.; van der Voort, M.; Nachabe, R.; Van der Mark, M.; Van Beek, M.; Bakker, L.; Fels, L.; et al. A novel fluorescent imaging agent for diffuse optical tomography of the breast: First clinical trial experience in patients. Mol. Imaging Biol. 2010, 12, 343–348. [Google Scholar] [CrossRef]
- Gonçalves, M.S.T. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Pham, W.; Medarova, Z.; Moore, A. Synthesis and application of a water-soluble near-infrared dye for cancer detection using optical imaging. Bioconjug. Chem. 2005, 16, 735–740. [Google Scholar] [CrossRef]
- Choi, H.S.; Nasr, K.; Alyabyev, S.; Feith, D.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Hyun, H.; Patonay, G.; Strekowski, L.; et al. Synthesis and in vivo Fate of Zwitterionic Near-Infrared Fluorophores. Angew. Chem. Int. Ed. 2011, 50, 6258–6263. [Google Scholar]
- Ye, Y.; Bloch, S.; Kao, J.; Achilefu, S. Multivalent carbocyanine molecular probes: Synthesis and applications. Bioconjug. Chem. 2005, 16, 51–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Achilefu, S. Synthesis and evaluation of polyhydroxylated near-infrared carbocyanine molecular probes. Org. Lett. 2004, 6, 2067–2070. [Google Scholar]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Jennings, L.E.; Long, N.J. ‘Two is better than one’-probes for dual-modality molecular imaging. Chem. Commun. 2009, 3511–3524. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Tian, M.; Zhang, H. Optical probes and the applications in multimodality imaging. Contrast Media Mol. Imaging 2011, 6, 169–177. [Google Scholar]
- Janib, S.M.; Moses, J.E.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Reiss, P.; Protiere, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Medintz, I.L.; Mattoussi, H.; Clapp, A.R. Potential clinical applications of quantum dots. Int. J. Nanomed. 2008, 3, 151–167. [Google Scholar]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Sena, M.; Gao, X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef]
- Xing, Y.; Rao, J. Quantum dot bioconjugates for in vitro diagnostics and in vivo imaging. Cancer Biomark. 2008, 4, 207–319. [Google Scholar]
- Wang, Y.M.; Chen, L. Quantum dots, lighting up the research and development of nanomedicine. Nanomed.: Nanotechnol. Biol. Med. 2011, 7, 385–402. [Google Scholar]
- Li, L.; Daou, T.J.; Texier, I.; Chi, T.T.K.; Liem, N.Q.; Reiss, P. Highly luminescent CuInS2/ZnS Core/Shell nanocrystals: Cadmium-free quantum dots for in vivo imaging. Chem. Mater. 2009, 21, 2422–2429. [Google Scholar] [CrossRef]
- Li, L.; Reiss, P. One-pot synthesis of highly luminescent InP/ZnS nanocrystals without precursor injection. J. Am. Chem. Soc. 2008, 130, 11588–11589. [Google Scholar] [CrossRef]
- Choi, H.S.; Ipe, B.I.; Misra, P.; Lee, J.H.; Bawendi, M.G.; Frangioni, J.V. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009, 9, 2354–2359. [Google Scholar] [CrossRef]
- Gao, J.; Chen, K.; Xie, R.; Xie, J.; Lee, S.-W.; Cheng, Z.; Peng, X.; Chen, X. Ultrasmall Near-infrared non-cadmium quantum dots for in vivo tumor imaging. Small 2010, 6, 256–261. [Google Scholar] [CrossRef]
- Pons, T.; Pic, E.; Lequeux, N.; Cassette, E.; Bezdetnaya, L.; Guillemin, F.; Marchal, F.; Dubertret, B. Cadmium-free CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano 2010, 4, 2531–2538. [Google Scholar] [CrossRef]
- Schipper, M.L.; Iyer, G.; Koh, A.L.; Cheng, Z.; Ebenstein, Y.; Aharoni, A.; Keren, S.; Bentolila, L.A.; Li, J.; Rao, J.; et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small 2009, 5, 126–134. [Google Scholar]
- Yong, K.-T.; Roy, I.; Ding, H.; Bergey, E.J.; Prasad, P. Biocompatible near-infrared quantum dots as ultrasensitive probes for long term in vivo imaging applications. Small 2009, 5, 1997–2004. [Google Scholar] [CrossRef]
- Zimmer, J.P.; Kim, S.W.; Ohnishi, S.; Tanaka, E.; Frangioni, J.V.; Bawendi, M.G. Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their applications to in vivo imaging. J. Am. Chem. Soc. 2006, 128, 2526–2527. [Google Scholar]
- Choi, H.S.; Liu, W.; Misra, W.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Yang, R.S.H.; Chang, L.W.; Wu, J.-P.; Tsai, M.-H.; Wang, H.-J.; Kuo, Y.-C.; Yeh, T.-K.; Yang, C.S.; Lin, P. Persistent tissues kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ. Health Perspect. 2007, 115, 1339–1343. [Google Scholar] [CrossRef]
- Schipper, M.L.; Cheng, Z.; Lee, S.-W.; Bentolila, L.A.; Iyer, G.; Rao, J.; Chen, X.; Wu, A.M.; Weiss, S.; Gambhir, S.S. micro-PET based biodistribution of quantum dots in living mice. J. Nucl. Med. 2007, 48, 1511–1518. [Google Scholar] [CrossRef]
- Gao, J.; Chen, K.; Luong, R.; Bouley, D.M.; Mao, H.; Qiao, T.; Gambhir, S.S.; Cheng, Z. A novel clinically translatable fluorescent nanoparticle for targeted molecular imaging of tumors in living subjects. Nano Lett. 2011, 12, 281–286. [Google Scholar]
- Hauck, T.S.; Anderson, R.E.; Fischer, H.C.; Newbigging, S.; Chan, W.C.W. In vivo Quantum-dot toxicity assessment. Small 2010, 6, 138–144. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Daou, T.J.; Li, L.; Reiss, P.; Josserand, V.; Texier, I. Effect of poly(ethylene glycol) length on the in vivo behavior of coated quantum dots. Langmuir 2009, 25, 3040–3044. [Google Scholar]
- Tavares, A.J.; Chong, L.; Petryayeva, E.; Algar, W.R.; Krull, U.J. Quantum dots as contrast agents for in vivo tumor imaging: Progress and issues. Anal. Bioanal. Chem. 2011, 399, 2331–2342. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Ballou, B.; Ernst, L.A.; Andreko, S.; Harper, T.; Fitzpatrick, J.A.; Waggoner, A.S.; Bruchez, M.P. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug. Chem. 2007, 18, 389–396. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kosaka, N.; Ogawa, M.; Morgan, N.Y.; Smith, P.D.; Murray, C.B.; Ye, X.C.; Collins, J.; Kumar, G.A.; Bell, H.; Choyke, P.L. In vivo multiple color lymphatic imaging using upconverting nanocrystals. J. Mater. Chem. 2009, 19, 6481–6484. [Google Scholar]
- Choi, J.; Burns, A.; Williams, R.M.; Zhou, Z.; Zipfel, W.R.; Wiesner, U.; Nikitin, A.Y. Core-shell silica nanoparticles as fluorescent labels for nanomedicine. J. Biomed. Opt. 2007, 12, 064007. [Google Scholar] [CrossRef]
- Friedman, R. Nano dot technology enters clinical trials. J. Natl. Cancer Inst. 2011, 103, 1428–1429. [Google Scholar] [CrossRef]
- Kumar, S.; Roy, I.; Ohulchanskyy, T.Y.; Goswani, L.N.; Bonoiu, A.C.; Bergey, E.J.; Tramposch, K.M.; Maitra, A.; Prasad, P. Covalently dye-linked, surface-controlled, and bioconjugated organically modified silica nanoparticles as targeted probes for optical imaging. ACS Nano 2008, 2, 449–456. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheng, S.H.; Wang, Y.J.; Chen, Y.-C.; Chen, N.-T.; Souris, J.; Chen, C.-T.; Mou, C.-Y.; Yang, C.-S.; Lo, L.-W. Near-Infrared Mesoporous Silica Nanoparticles for Optical Imaging: Characterization and in vivo Biodistribution. Adv. Func.Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Kumar, R.; Roy, I.; Ohulchansky, T.; Vathy, L.A.; Bergey, E.; Sajjad, M.; Prasad, P. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef]
- He, X.; Nie, H.; Wang, K.; Tan, W.; Wu, X.; Zhang, P. In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal. Chem. 2008, 80, 9597–9603. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for bioimaging. Adv. Colloid. Interf. Sci. 2006, 471–485. [Google Scholar]
- Larson, D.R.; Ow, H.; Vishwasrao, H.D.; Heikal, A.A.; Wiesner, U.; Webb, W.W. Silica nanoparticle architecture determines radiative properties of encapsulated fluorophores. Chem. Mater. 2008, 20, 2677–2684. [Google Scholar] [CrossRef]
- Rampazzo, E.; Bonacchi, S.; Montalti, M.; Prodi, L.; Zaccheroni, N. Self-organizing core-shell nanostructures: Spontaneous accumulation of dye in the core of doped silica nanoparticles. J. Am. Chem. Soc. 2007, 129, 14251–14256. [Google Scholar] [CrossRef]
- Bringley, J.F.; Penner, T.L.; Wang, R.; Harder, J.F.; Harrison, W.J.; Buonemani, L. Silica nanoparticles encapsulating near-infrared emissive cyanine dyes. J. Colloid. Interf. Sci. 2008, 320, 132–139. [Google Scholar] [CrossRef]
- Burns, A.; Vider, J.; Ow, H.; Herz, E.; Penate-Medina, O.; Baumgart, M.; Larson, S.M.; Wiesner, U.; Bradbury, M. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009, 9, 442–448. [Google Scholar]
- Altinoglu, E.I.; Russin, T.J.; Kaiser, J.M.; Barth, B.M.; Eklund, P.C.; Kester, M.; Adair, J.H. Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano 2008, 2, 2075–2084. [Google Scholar] [CrossRef]
- Barth, B.M.; Altinoglu, E.I.; Shanmugavelandy, S.S.; Kaiser, J.M.; Crespo-Gonzalez, D.; DiVittore, N.A.; McGovern, C.; Goff, T.M.; Keasey, N.R.; Adair, J.H.; Loughran, T.P.; Claxton, D.F.; Kester, M. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano 2011, 5, 5325–5337. [Google Scholar]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Schwiertz, J.; Wiehe, A.; Gräfe, S.; Gitter, B.; Epple, M. Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria. Biomaterials 2009, 30, 3324–3331. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cha, E.J.; Park, K.; Lee, S.Y.; Hong, J.K.; Sun, I.C.; Kim, S.Y.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.H. A near-infrared fluorescence quenched gold nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. 2008, 47, 2804–2807. [Google Scholar]
- Zhou, C.; Long, M.; Qin, Y.; Sun, X.; Zheng, J. Luminescent gold nanoparticles with efficient renal clearance. Angew. Chem. Int. Ed. 2011, 50, 3168–3172. [Google Scholar]
- Faure, A.-C.; Dufort, S.; Josserand, V.; Perriat, P.; Coll, J.L.; Roux, S.; Tillement, O. Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethyleneglycol) coatings. Small 2009, 5, 2565–2575. [Google Scholar] [CrossRef]
- le Masne de Chermont, Q.; Chanéac, C.; Seguin, J.; Pellé, F.; Maîtrejean, S.; Jolivet, J.P.; Gourier, D.; Bessodes, M.; Scherman, D. Nanoprobes with near infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 9266–9271. [Google Scholar]
- Texier, I.; Heinrich, E.; Berger, M.; Tillement, O.; Louis, C.; Peltié, P. Luminescent up-converting nano-crystals for in vivo imaging. Proc. SPIE 2007, 6449, 64490D:1–64490D:11. [Google Scholar]
- Hilderbrand, S.A.; Shao, F.; Salthouse, C.; Mahmood, U.; Weissleder, R. Upconverting luminescent nanomaterials: Application to in vivo bioimaging. Chem. Commun. 2009, 4188–4190. [Google Scholar]
- Xiong, L.; Chen, Z.; Tian, Q.; Cao, T.; Xu, C.; Li, F. High contrast upconversion luminescence targeted imaging in vivo using peptide-labeled nanophosphors. Anal. Chem. 2009, 81, 8687–8694. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Du, X.; Xiong, L.; Hu, H.; Li, F. Dual-modality in vivo imaging using rare-earth nanocrystals with near-infrared to near-infrared (NIR-to-NIR) upconversion luminescence and magnetic resonace properties. Biomaterials 2010, 31, 3287–3295. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomed.: Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar]
- Wagner, V.; Dullaart, A.; Bock, A.-K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Bricarello, D.A.; Smolowitz, J.T.; Zivkovic, A.M.; German, J.B.; Parikh, A.N. Reconstituted lipoprotein: A versatile class of biologically-inspired nanostructures. ACS Nano 2011, 5, 42–57. [Google Scholar]
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Constantinides, P.P.; Chaubal, M.V.; Shorr, R. Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv. Drug Deliv. Rev. 2008, 60, 757–767. [Google Scholar] [CrossRef]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef]
- Joshi, M.D.; Müller, R.H. Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 2009, 71, 161–172. [Google Scholar] [CrossRef]
- Sawant, K.K.; Dodiya, S.S. Recent advances and patents on solid lipid nanoparticles. Recent pat. Drug Deliv. Formul. 2008, 2, 120–135. [Google Scholar] [CrossRef]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Tung, C.H. Fluorescent peptide probes for in vivo diagnostic imaging. Biopolymers 2004, 76, 391–403. [Google Scholar] [CrossRef]
- Tung, C.-H.; Bredow, S.; Mahmood, U.; Weissleder, R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug. Chem. 1999, 10, 892–896. [Google Scholar] [CrossRef]
- Tung, C.-H.; Gerszten, R.E.; Jaffer, F.A.; Weissleder, R. A novel near infrared fluorescence sensor for detection of thrombin activation in blood. ChemBioChem 2002, 3, 207–211. [Google Scholar] [CrossRef]
- Tung, C.-H.; Mahmood, U.; Bredow, S.; Weissleder, R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000, 60, 4953–4958. [Google Scholar]
- Miki, K.; Oride, K.; Inoue, S.; Kuramochi, Y.; Nayak, R.R.; Matsuoka, H.; Harada, H.; Hiraoka, M.; Ohe, K. Ring-opening metathesis polymerization-based synthesis of polymeric nanoparticles for enhanced tumor imaging in vivo: Synergistic effect of folate-receptor targeting and PEGylation. Biomaterials 2010, 31, 934–942. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Enhanced photo-stability, thermal-stability and aqueous-stability of indocyanine green in polymeric nanoparticulate systems. J. Photochem. Photobiol. B: Biol. 2004, 74, 29–38. [Google Scholar]
- Saxena, V.; Sadoqi, M.; Shao, J. Polymeric nanoparticulate delivery system for indocyanine green: Biodistribution in healthy mice. Int. J. Pharm. 2006, 308, 200–204. [Google Scholar] [CrossRef]
- Larush, L.; Magdassi, S. Formation of near-infrared fluorescent nanoparticles for medical imaging. Nanomedicine 2011, 6, 233–240. [Google Scholar] [CrossRef]
- Schadlich, A.; Rose, C.; Kuntsche, J.; Caysa, H.; Mueller, T.; Gopferich, A.; Mader, K. How stealthy are PEG-PLA nanoparticles? An NIR in vivo study combined with detailed size measurements. Pharm. Res. 2011, 28, 1995–2007. [Google Scholar] [CrossRef]
- Tong, R.; Coyle, V.J.; Tang, L.; Barger, A.M.; Fan, T.M.; Cheng, J.J. Polylactide nanoparticles containing stably incorporated cyanine dyes for in vitro and in vivo imaging applications. Microsc. Res. Tech. 2010, 73, 901–909. [Google Scholar] [CrossRef]
- Longmire, M.R.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Biologically optimized nanosized molecules and particles: More than just size. Bioconjug. Chem. 2011, 22, 993–1000. [Google Scholar] [CrossRef]
- Almutairi, A.; Akers, W.J.; Berezin, M.Y.; Achilefu, S.; Fréchet, J.M.J. Monitoring the biodegradation of dendritic near-infrared nanoprobes by in vivo fluorescence imaging. Mol. Pharm. 2008, 5, 1103–1110. [Google Scholar] [CrossRef]
- Quadir, M.A.; Radowski, M.R.; Kratz, F.; Licha, K.; Hauff, P.; Haag, R. Dendritic multishell architectures for drug and dye transport. J. Control. Release 2008, 132, 289–294. [Google Scholar] [CrossRef]
- Rodriguez, V.B.; Henry, S.M.; Hoffman, A.S.; Stayton, P.S.; Li, X.; Pun, S.H. Encapsulation and stabilization of indocyanine green within poly(styrene-alt-maleic anhydride) block-poly(styrene) micelles for near-infrared imaging. J. Biomed. Opt. 2008, 13, 014025. [Google Scholar] [CrossRef]
- Zheng, X.; Xing, D.; Zhou, F.; Wu, B.; Chen, W.R. Indocyanine Green-containing nanostructures as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol. Pharm. 2011, 8, 447–456. [Google Scholar] [CrossRef]
- Tanisaka, H.; Kizaka-Kondoh, S.; Makino, A.; Tanaka, S.; Hiraoka, M.; Kimura, S. Near-infrared fluorescent labeled peptosome for application to cancer imaging. Bioconjug. Chem. 2008, 19, 109–117. [Google Scholar] [CrossRef]
- Wang, D.; Qian, J.; He, S.; Park, J.S.; Lee, K.-S.; Han, S.; Mu, Y. Aggregation-enhanced fluorescence in PEGylated phospholipid nanomicelles for in vivo imaging. Biomaterials 2011, 32, 5880–5888. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Kelly, K.; Niedre, M.; Weissleder, R. Near infrared fluorescence-based bacteriophage particles for ratiometric pH imaging. Bioconjug. Chem. 2008, 19, 1635–1639. [Google Scholar] [CrossRef]
- Ghoroghchian, P.; Frail, P.; Susumu, K.; Blessington, D.; Brannan, A.; Bates, F.; Chance, B.; Hammer, D.; Therien, M. Near IR emissive polymersome: Self-assembled soft matter for in vivo optical imaging. Proc. Natl. Acad. Sci. USA 2005, 102, 2922–2927. [Google Scholar]
- Duncan, T.V.; Ghoroghchian, P.; Rubtsov, I.V.; Hammer, D.; Therien, M. Ultrafast excited-state dynamics of nanoscale near-infrared emissive polymersomes. J. Am. Chem. Soc. 2008, 130, 9773–9784. [Google Scholar]
- Ghoroghchian, P.P.; Frail, P.R.; Li, G.; Zupancich, J.A.; Bates, F.S.; Hammer, D.A.; Therien, M.J. Controlling bulk optical properties of emissive polymersomes through intramembranous polymer-fluorophore interactions. Chem. Mater. 2007, 19, 1309–1318. [Google Scholar] [CrossRef]
- McNeil, S.E. Nanoparticle therapeutics: A personal perspective. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2009, 1, 264–271. [Google Scholar]
- Weyenberg, W.; Filev, P.; van den Plas, D.; Vandervoort, J.; De Smet, K.; Sollie, P.; Ludwiga, A. Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application. Int. J. Pharm. 2007, 337, 291–298. [Google Scholar] [CrossRef]
- Devoisselle, J.-M.; Soulié-Bégu, S.; Mordon, S.; Desmettre, T.; Maillols, H. A preliminary study of the in vivo behaviour of an emulsion formulation of Indocyanine Green. Lasers Med. Sci. 1998, 13, 279–282. [Google Scholar] [CrossRef]
- Mulder, W.; Strijkers, G.; van Tilborg, G.; Cormode, D.P.; Fayad, Z.A.; Nicolay, K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 2009, 42, 904–914. [Google Scholar] [CrossRef]
- Akers, W.J.; Kim, C.; Berezin, M.Y.; Guo, K.; Fuhrhop, R.; Lanza, G.M.; Fischer, G.M.; Daltrozzo, E.; Zumbusch, A.; Cai, X.; Wang, L.V.; Achilefu, S. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano 2011, 5, 173–182. [Google Scholar]
- Delmas, T.; Couffin, A.C.; Bayle, P.A.; de Crécy, F.; Neumann, E.; Vinet, F.; Bardet, M.; Bibette, J.; Texier, I. Preparation and characterisation of highly stable lipid nanoparticles with amorphous core of tuneable viscosity. J. Colloid Interf. Sci. 2011, 360, 471–481. [Google Scholar] [CrossRef]
- Delmas, T.; Piraux, H.; Couffin, A.C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How to prepare and stabilize very small nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar]
- Navarro, F.; Mittler, F.; Berger, M.; Josserand, V.; Gravier, J.; Vinet, F.; Texier, I. Cell tolerability and biodistribution in mice of Indocyanine Green-loaded lipid nanoparticles. J. Biomed. Nanotechnol. 2012, in press. [Google Scholar]
- Navarro, F.; Berger, M.; Guillermet, S.; Josserand, V.; Guyon, L.; Goutayer, M.; Neumann, E.; Rizo, P.; Vinet, F.; Texier, I. Lipid nanoparticle vectorization of IndoCyanin Green improves non invasive fluorescence imaging. J. Biomed. Nanotechnol. 2012, in press. [Google Scholar]
- Goutayer, M.; Dufort, S.; Josserand, V.; Royère, A.; Heinrich, E.; Vinet, F.; Bibette, J.; Coll, J.L.; Texier, I. Tumor targeting of functionalized lipid nanoparticles: Assessment by in vivo fluorescence imaging. Eur. J. Pharm. Biopharm. 2010, 75, 137–147. [Google Scholar] [CrossRef]
- Texier, I.; Goutayer, M.; Da Silva, A.; Guyon, L.; Djaker, N.; Josserand, V.; Neumann, E.; Bibette, J.; Vinet, F. Cyanine loaded lipid nanoparticles for improved in vivo fluorescence imaging. J. Biomed. Opt. 2009, 14, 054005. [Google Scholar] [CrossRef]
- Cormode, D.P.; Jarzyna, P.A.; Mulder, W.; Fayad, Z.A. Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Deliv. Rev. 2010, 62, 329–338. [Google Scholar] [CrossRef]
- Kenneth, K.N.G.; Lovell, J.F.; Zheng, G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc. Chem. Res. 2011, 44, 1105–1113. [Google Scholar] [CrossRef]
- Cao, W.; Kenneth, K; Corbin, I.; Zhang, Z.; Ding, L.; Chen, J.; Zheng, G. Synthesis and Evaluation of a Stable Bacteriochlorophyll-Analog and Its Incorporation into High-Density Lipoprotein Nanoparticles for Tumor Imaging. Bioconjug. Chem. 2009, 20, 2023–2031. [Google Scholar] [CrossRef]
- Corbin, I.R; Chen, J.; Cao, W.; Li, H.; Lund-Katz, S.; Zheng, G. Enhanced cancer-targeted delivery using engineered high-density lipoprotein-based nanocarriers. J. Biomed. Nanotechnol. 2007, 3, 367–376. [Google Scholar]
- Zhang, Z.; Chen, J.; Ding, L.; Jin, H.; Lovell, J.F.; Corbin, I.; Cao, W. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small 2010, 6, 430–437. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Li, H.; Glickson, J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl. Acad. Sci. USA 2005, 102, 17757–17762. [Google Scholar]
- Chen, J.; Corbin, I.; Li, H.; Cao, W.; Glickson, J.D.; Zheng, G. Ligand conjugated low-density lipoprotein nanoparticles for enhanced optical cancer imaging in vivo. J. Am. Chem. Soc. 2007, 129, 5798–5799. [Google Scholar] [CrossRef]
- Kirchherr, A.-K.; Briel, A.; Mäder, K. Stabilization of indocyanine green by encapsulation within micellar systems. Mol. Pharm. 2009, 6, 480–491. [Google Scholar] [CrossRef]
- Deissler, V.; Rüger, R.; Frank, W.; Fahr, A.; Kaiser, W.A.; Hilger, I. Fluorescent liposomes as contrast agents for in vivo optical imaging of edemas in mice. Small 2008, 4, 1240–1246. [Google Scholar] [CrossRef]
- Sandanaraj, B.S.; Gremlich, H.-U.; Kneuer, R.; Dawson, J.; Wacha, S. Fluorescent nanoprobes as a biomarker for increased vascular permeability: Implications in diagnosis and treatment of cancer and inflammation. Bioconjug. Chem. 2010, 21, 93–101. [Google Scholar] [CrossRef]
- Portnoy, E.; Lecht, S.; Lazarovici, P.; Danino, D.; Magdassi, S. Cetuximab-labeled liposomes containing near-infrared probe for in vivo imaging. Nanomed.: Nanotechnol. Biol. Med. 2011, 7, 480–488. [Google Scholar] [CrossRef]
- Proulx, S.T.; Luciani, P.; Derzsi, S.; Rinderknecht, M.; Mumprecht, V.; Leroux, J.-C.; Detmar, M. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010, 70, 7053–7062. [Google Scholar]
- Lovell, J.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.; Cao, W.; Whang, LV.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biphotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar]
- Frias, J.C.; Lipinski, M.J.; Lipinski, S.E.; Albeda, M.T. Modified lipoproteins as contrats agents for imaging atherosclerosis. Contrast Media Mol. Imaging 2007, 2, 16–23. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mérian, J.; Gravier, J.; Navarro, F.; Texier, I. Fluorescent Nanoprobes Dedicated to in Vivo Imaging: From Preclinical Validations to Clinical Translation. Molecules 2012, 17, 5564-5591. https://doi.org/10.3390/molecules17055564
Mérian J, Gravier J, Navarro F, Texier I. Fluorescent Nanoprobes Dedicated to in Vivo Imaging: From Preclinical Validations to Clinical Translation. Molecules. 2012; 17(5):5564-5591. https://doi.org/10.3390/molecules17055564
Chicago/Turabian StyleMérian, Juliette, Julien Gravier, Fabrice Navarro, and Isabelle Texier. 2012. "Fluorescent Nanoprobes Dedicated to in Vivo Imaging: From Preclinical Validations to Clinical Translation" Molecules 17, no. 5: 5564-5591. https://doi.org/10.3390/molecules17055564