Adsorption Behavior of Fe(II) and Fe(III) Ions on Thiourea Cross-Linked Chitosan with Fe(III) as Template
Abstract
:1. Introduction
2. Results and Discussion
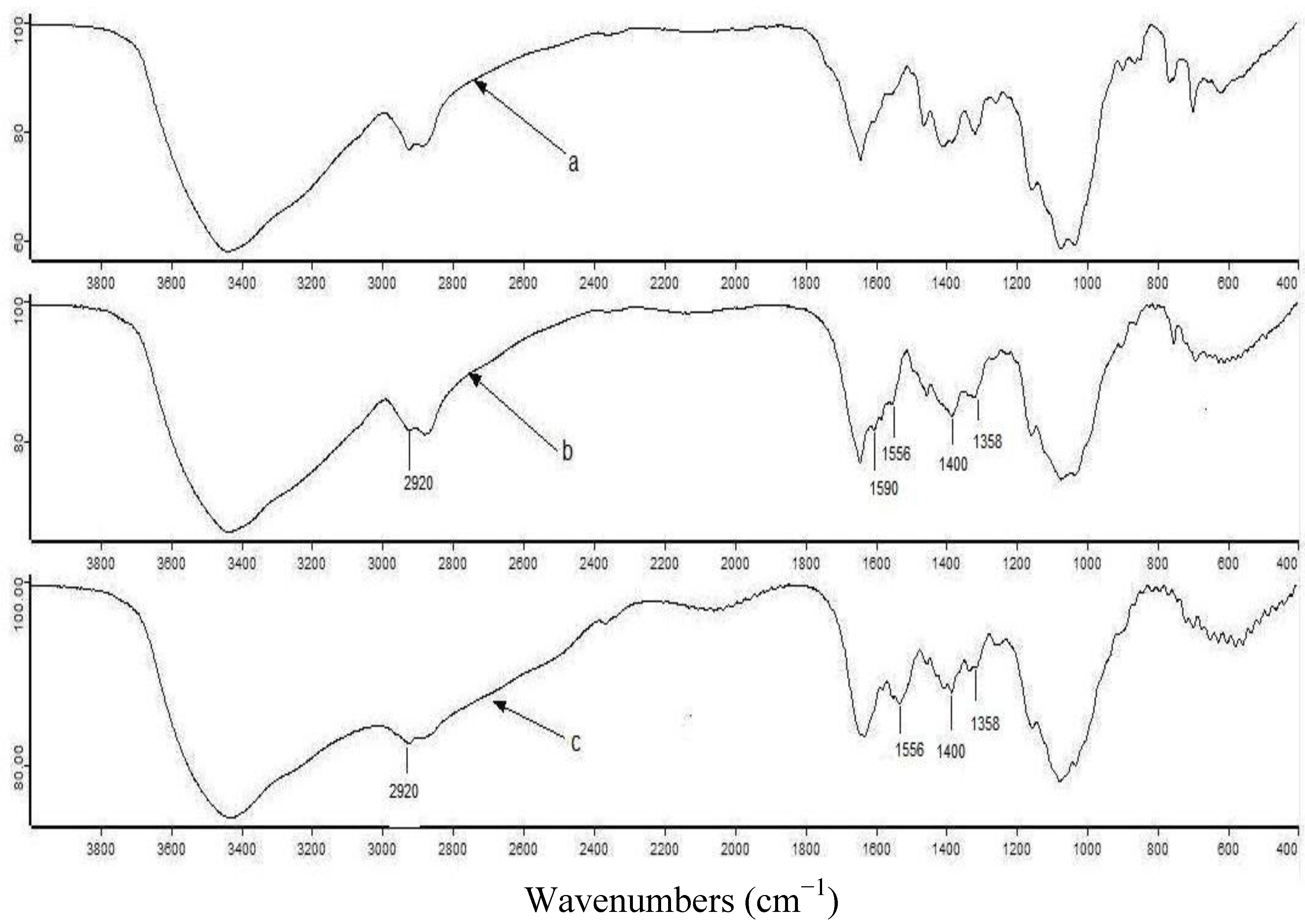
2.1. FTIR Analysis
2.2. Characterization by SEM

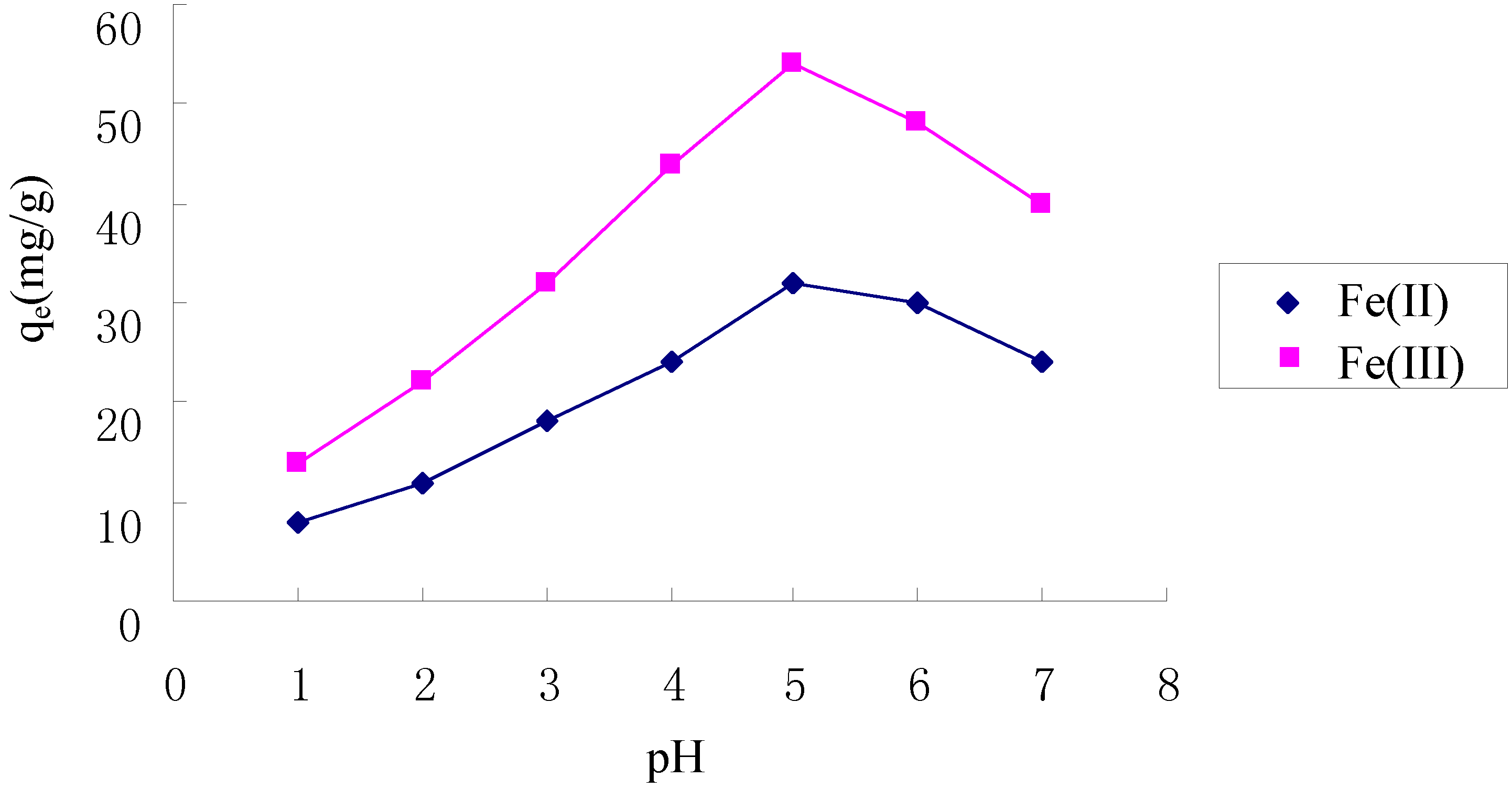
2.3. Effect of pH on Adsorption of Fe(II) and Fe(III)
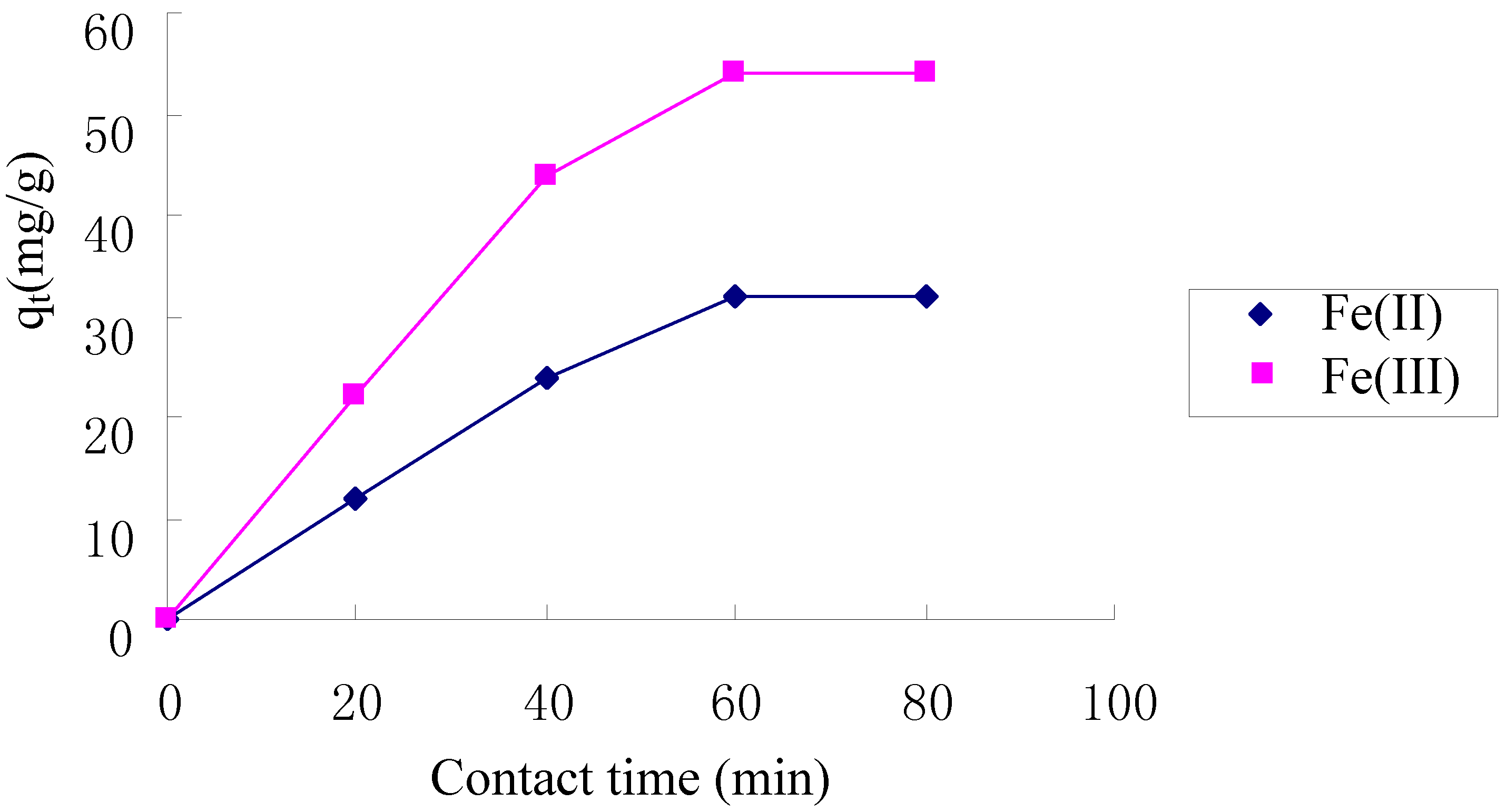
2.4. Kinetics of Adsorption


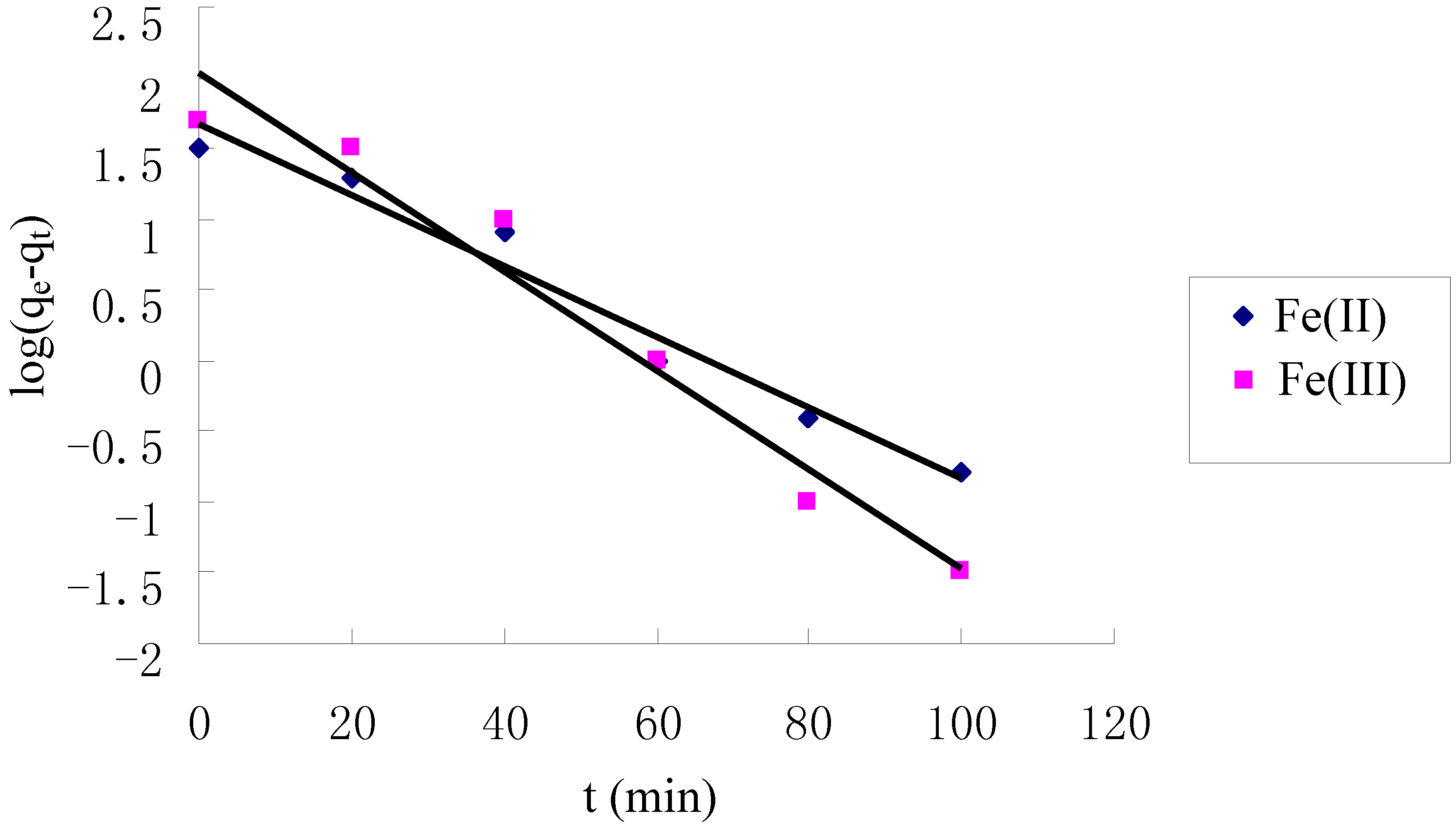
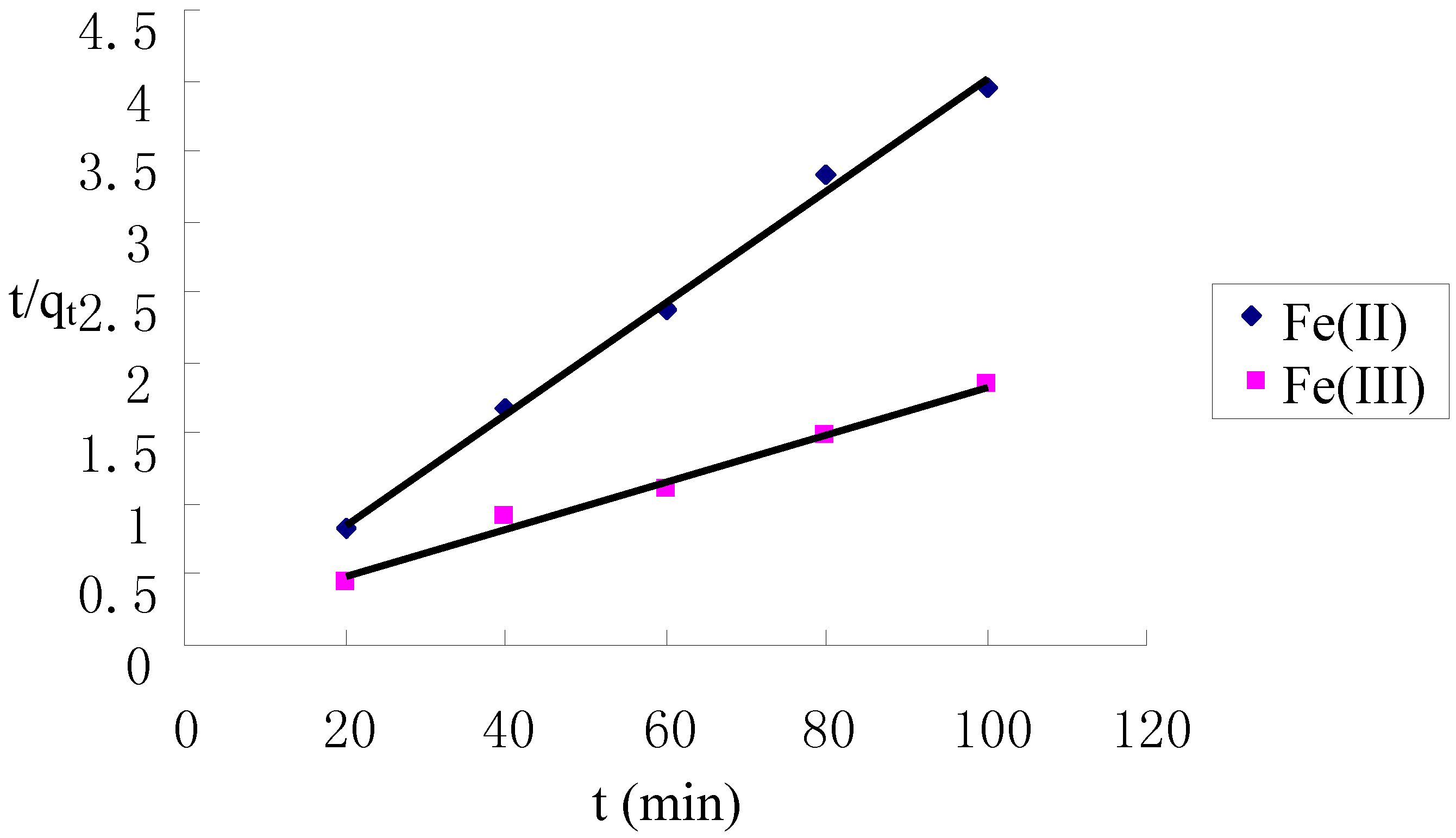
| Metal ion | Pseudo first-order | Pseudo second-order | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g·mg−1·min−1) | R2 | |
| Fe(II) | 0.058 | 0.970 | 0.028 | 0.996 |
| Fe(III) | 0.081 | 0.963 | 0.0019 | 0.990 |
2.5. Adsorption Isotherms


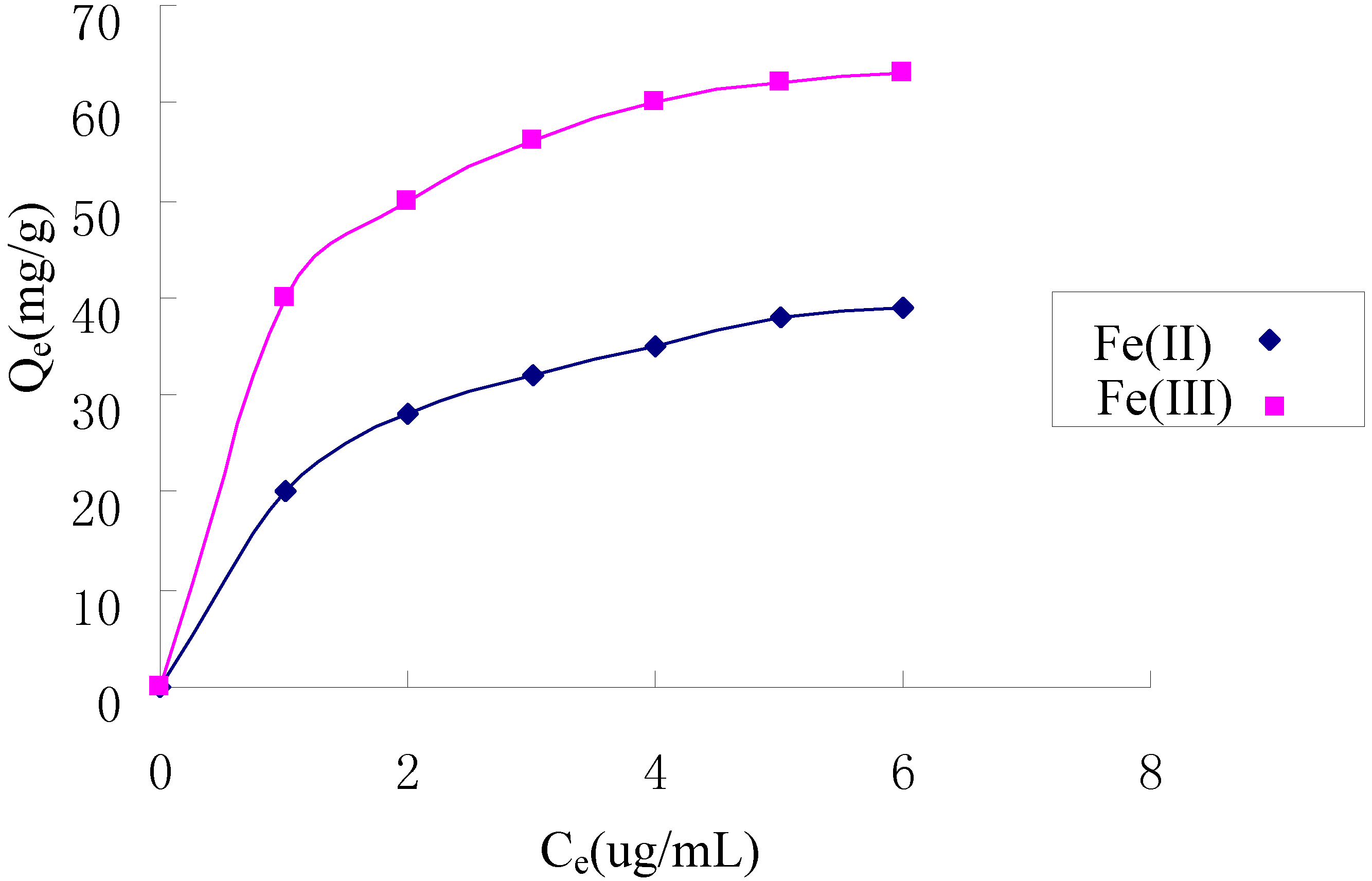

| Metal ion | Langmuir model | Pseudo second-order | ||||
|---|---|---|---|---|---|---|
| Q (mg/g) | b (mL/ug) | R2 | KF (mg/g) | n | R2 | |
| Fe(II) | 48.3 | 0.692 | 0.9988 | 20.5 | 2.52 | 0.9875 |
| Fe(III) | 71.9 | 1.19 | 0.9994 | 40.2 | 3.53 | 0.9872 |
2.6. Selective Adsorption of Fe(III)
| Metal ion | Adsorption capacity (mmol/g) |
|---|---|
| Fe(III) | 1.38 |
| Pb(II) | 0.35 |
| Zn(II) | 0.46 |
| Cd(II) | 0.18 |
| Ni(II) | 0.50 |
3. Experimental
3.1. General
| FAAS parameters | |
|---|---|
| Lamp current(mA) | 12 |
| Slit width(nm) | 0.2 |
| Flow rate of acetylene(L/min) | 2.2 |
| Flow rate of air(L/min) | 15.0 |
| Analytical wavelength(nm) | 248.3 |
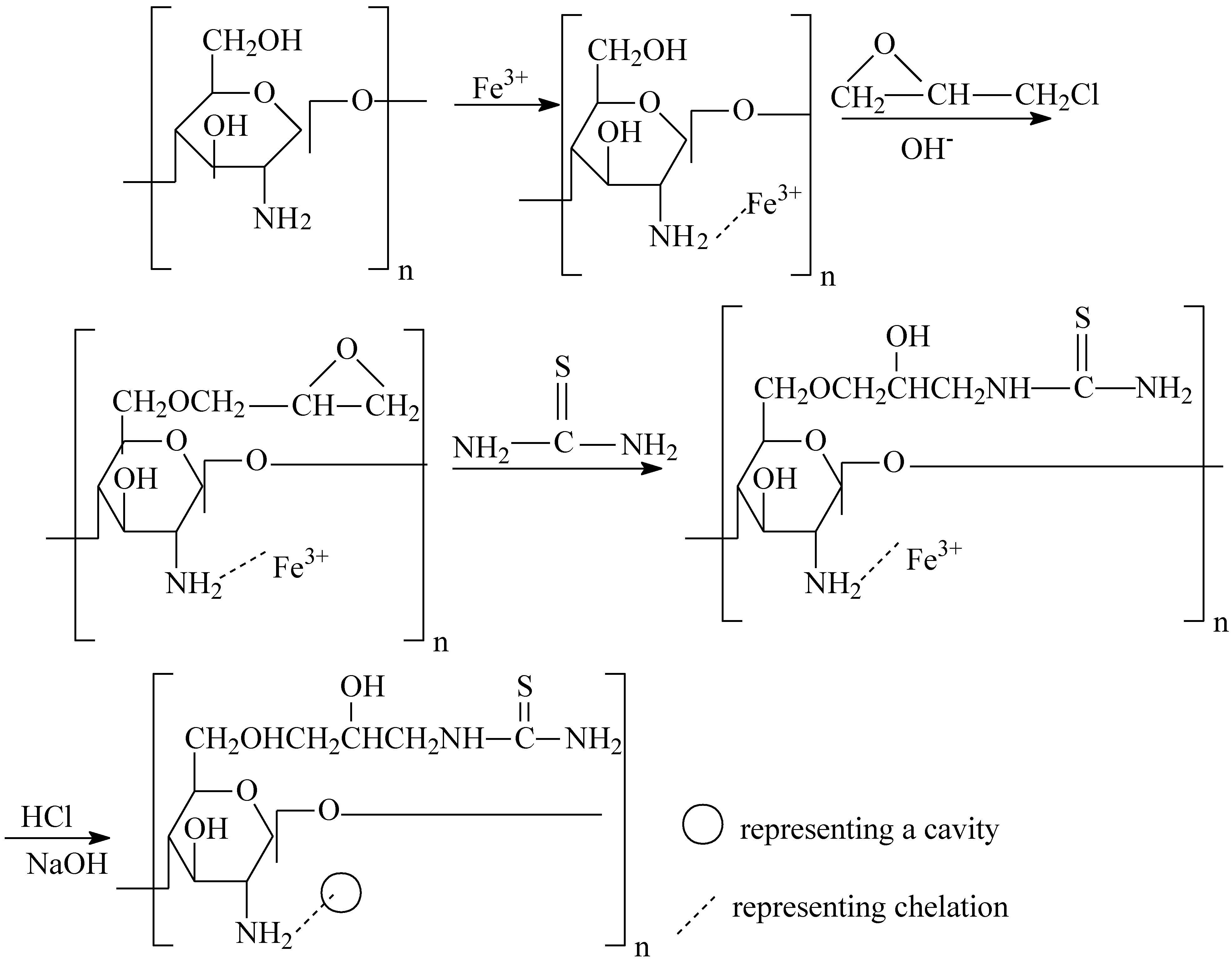
3.2. Preparation of TCCTS Template
- (1) CTS (6.0 g) was dissolved in 1% aqueous solution of acetic acid (60 mL) and then added to a 500 mL beaker containing iron trichloride solution (200 mL, 0.02 mol/L). The pH of the solution in beaker was adjusted to 5.5, the beaker was shaken for 24 h at 300 rpm, 25 °C until the product was obtained. The solid product was filtered under reduced pressure. The filtrate was washed with distilled water until no Fe(III) ion was detected by KSCN solution and then the product was dried at 60 °C in vacuum.
- (2) The product obtained from step (1) (4.5 g) was dissolved in 5% NaOH solution (100 mL). Epichlorohydrin (15 mL) was added to the solution in a three-necked flask. The mixture was heated on a water bath for 4 h at 70 °C. Thiourea (6.0 g) was dissolved in distilled water (100 mL), and the solution was then added to the mixture of the flask and heated for 5 h at 70 °C until the product was formed. The product was filtered under reduced pressure and washed several times with ethanol followed by distilled water. Subsequently the filtrate was put into a beaker containing 0.1 mol/L HCl (100 mL) and stirred for 2 h at 25 °C, then filtered. This process was repeated until no Fe(III) ion was detected in solution. The filtrate was treated with 0.1 mol/L NaOH solution for 5 h and then washed several times in turn with ethanol, acetone, distilled water. The TCCTS template was obtained after dried at 60 °C in vacuum for 5 h.
3.3. Effect of pH
3.4. Kinetics of Adsorption
3.5. Adsorption Isotherms

3.6. Selectivity for Adsorption of Fe(III)
4. Conclusions
Acknowledgements
References
- Gupta, V.K.; Sharma, S. Removal of cadmium and zinc from aqueous solutions using red mud. Environ. Sci. Technol. 2002, 36, 3612–3617. [Google Scholar]
- Safavi, A.; Abdollahi, H. Speciation of Fe(II) and Fe(III) with chromagenic mixed reagents by principal-component regression. Microchem. J. 1999, 63, 211–217. [Google Scholar]
- Kiber, R.J.; Williams, K.; Willey, J.D.; Skrabal, S. Iron speciation in coastal rainwater: Concentration and deposition to seawater. Mar. Chem. 2001, 73, 83–95. [Google Scholar]
- Aksu, Z.; Calik, A.; Dursun, A.Y.; Demircan, Z. Biosorption of iron(III)-cyanide complex anions to Rhizopus arrhizus: Application of adsorption isotherms. Process Biochem. 1999, 34, 483–491. [Google Scholar]
- Aydin, F.A.; Soylak, M. A novel multi-element coprecipitation technique for separation and enrichment of metal ions in environmental samples. Talanta 2007, 73, 134–141. [Google Scholar]
- Sarzanini, C.; Abolino, O.; Mentastri, E. Flow-injection preconcentration and electrothermal atomic absorption spectrometry determination of manganese in seawater. Anal. Chim. Acta 2001, 435, 343–350. [Google Scholar]
- Okamoto, Y.; Nomura, Y.; Iwamaru, K. High preconcentration of ultra-trace metal ions by liquid-liquid extraction using water/oil/water emulsions as liquid surfactant membranes. Microchem. J. 2000, 65, 341–346. [Google Scholar]
- Wan, W.S.; Ngah, S.; Ghani, A.; Kamari, A. Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 2005, 96, 443–450. [Google Scholar]
- El-Shafey, E.; Pichugin, M.; Appleton, A. Application of a carbon sorbent for the removal of cadmium and other heavy metal ions from aqueous solution. J. Chem. Technol. Biotechnol. 2002, 77, 429–436. [Google Scholar]
- Guibal, E.; Vincent, T.; Mendoza, R.N. Synthesis and characterization of thiorea derivative of chitosan for platinum recovery. J. Appl. Polym. Sci. 2000, 75, 119–134. [Google Scholar]
- Yang, Z.; Zhuang, L.; Tan, G. Preparation and adsorption behavior for metal of chitosan cross-linked by dihydroxy azacrown ether. J. Appl. Polym. Sci. 2002, 85, 530–535. [Google Scholar]
- Lu, G.; Yao, X.; Wu, X.; Zhan, T. Determiantion of the total iron by chitosan modified glassy carbon electrode. Microchem. J. 2001, 69, 81–87. [Google Scholar]
- Chassary, P.; Vincent, T.; Guibal, E. Metal anion sorption on chitosan and derivative materials: A strategy for polymer modification and optimum use. React. Funct. Polym. 2004, 60, 137–149. [Google Scholar]
- Ruiz, M.; Sastre, A.M.; Guibal, E. Palladium sorption on glutaraldehyde-crosslinked chitosan. React. Funct. Polym. 2000, 45, 155–173. [Google Scholar]
- Coelho, T.C.; Laus, R.; Mangrich, A.S. Effect of heparin coating on epichlorohydrin cross-linked chitosan microspheres on the adsorption of copper(II) ions. React. Funct. Polym. 2007, 67, 468–475. [Google Scholar]
- Birinci, E.; Gulfen, M.; Aydin, A.O. Separation and recovery of palladium(II) from base metal ions by melamine-formaldehyde-thiourea(MFT) chelating resin. Hydrometallurgy 2009, 95, 15–21. [Google Scholar]
- Donia, A.M.; Atia, A.A.; Elwakeel, K.Z. Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 2007, 87, 197–206. [Google Scholar]
- Gavilan, K.C.; Pestov, A.V.; Garcia, H.M. Mercury sorption on a thiocarbamoyl derivative of chitosan. J. Hazard. Mater. 2009, 165, 415–426. [Google Scholar]
- Lin, W.; Ronge, X.; Song, L. Studies on adsorption behavior of Pb(II) onto a thiourea-modified chitosan resin with Pb(II) as template. Carbohydr. Polym. 2010, 81, 305–310. [Google Scholar]
- Graciela, R.; Jorge, S.; Jaime, A.F. Adsorption of chromium onto crossed-linked chitosan. Sep. Purif. Technol. 2005, 44, 31–36. [Google Scholar]
- Chiou, M.S.; Li, H.Y. Adsorption behaviour of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar]
- McKay, G.; Ho, Y.S. Pseudo-second order model for sorption process. Process Biochem. 1999, 34, 4151–465. [Google Scholar]
- Sag, Y.; Aktay, Y. Kinetic studies on sorption of Cr(VI) and Cu(II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem. Eng. J. 2002, 12, 143–153. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dai, J.; Ren, F.; Tao, C. Adsorption Behavior of Fe(II) and Fe(III) Ions on Thiourea Cross-Linked Chitosan with Fe(III) as Template. Molecules 2012, 17, 4388-4399. https://doi.org/10.3390/molecules17044388
Dai J, Ren F, Tao C. Adsorption Behavior of Fe(II) and Fe(III) Ions on Thiourea Cross-Linked Chitosan with Fe(III) as Template. Molecules. 2012; 17(4):4388-4399. https://doi.org/10.3390/molecules17044388
Chicago/Turabian StyleDai, Jun, FengLian Ren, and ChunYuan Tao. 2012. "Adsorption Behavior of Fe(II) and Fe(III) Ions on Thiourea Cross-Linked Chitosan with Fe(III) as Template" Molecules 17, no. 4: 4388-4399. https://doi.org/10.3390/molecules17044388




