Protective Effect of Ischemic Postconditioning against Ischemia Reperfusion-Induced Myocardium Oxidative Injury in IR Rats
Abstract
:1. Introduction
2. Result
| Indexs | SO | IR | IPO |
|---|---|---|---|
| VTt (s) | 2.63 ± 4.61 | 64.53 ± 21.86 ** | 22.18 ± 25.15 ## |
| VFt (s) | 0 | 12.51 ± 20.15 | 0 |
| VAS | 0.62 ± 0.8 | 4.01 ± 1.19 ** | 2.43 ± 1.01 ## |
| Index | SO | IR | IPO | |
|---|---|---|---|---|
| LVSP (mmHg) | Base | 138.68 ± 14.29 | 141.05 ± 13.09 | 139.37 ± 12.17 |
| R15min | 136.35 ± 15.29 | 80.24 ± 5.92 | 95.11 ± 7.93 | |
| R30min | 135.79 ± 11.69 | 86.03 ± 4.82 ** | 110.44 ± 12.84 ## | |
| R60min | 127.82 ± 13.27 | 87.31 ± 9.03 ** | 112.73 ± 13.02 ## | |
| R120min | 125.72 ± 11.05 | 69.08 ± 5.39 ** | 101.74 ± 12.18 ## | |
| LVEDP (mmHg) | Base | −4.06 ± 2.31 | 5.13 ± 4.22 * | 6.22 ± 3.17 # |
| R15min | −3.21 ± 2.16 | 16.02 ± 8.51 ** | 6.03 ± 4.27 ## | |
| R30min | −2.59 ± 1.62 | 18.39 ± 7.39 ** | 7.35 ± 3.02 ## | |
| R60min | −1.94 ± 1.58 | 20.11 ± 8.92 ** | 7.42 ± 2.99 ## | |
| R120min | −1.73 ± 2.01 | 23.15 ± 11.73 ** | 9.73 ± 3.17 ## | |
| +dp/dtmax (mmHg/s) | Base | 6,281.9 ± 2,900.5 | 6,301.7 ± 3,011.3 | 6,316.4 ± 2,794.1 |
| R15min | 5,927.1 ± 2,176.4 | 3,517.2 ± 1,538.9 * | 4,016.3 ± 1,933.5 # | |
| R30min | 5,827.8 ± 1,276.3 | 2,718.4 ± 893.2 * | 4,083.2 ± 1,739.2 # | |
| R60min | 5,628.5 ± 2,007.4 | 2,561.7 ± 599.2 * | 4,217.3 ± 2,175.4 # | |
| R120min | 5,513.1 ± 2,166.3 | 2,381.4 ± 739.1 * | 3,977.2 ± 1,308.4 # | |
| −dp/dtmax (mmHg/s) | Base | 5,132.8 ± 1,694.3 | 5,124.6 ± 1,352.7 | 5,341.7 ± 1,595.4 |
| R15min | 5,044.4 ± 1,488.6 | 1,984.2 ± 674.4 ** | 3,106.3 ± 900.5 ## | |
| R30min | 5,051.2 ± 1,276.4 | 2,264.2 ± 832.1 ** | 3,582.9 ± 1,254.3 ## | |
| R60min | 4,885.9 ± 1,366.8 | 2,607.5 ± 927.7 ** | 3,606.3 ± 846.3 ## | |
| R120min | 4,633.5 ± 1,298.1 | 2,287.9 ± 715.6 ** | 3,364.7 ± 947.9 ## | |
| Indexes | SO | IR | IPO |
|---|---|---|---|
| LV | - | 525.66 ± 47.13 | 516.65 ± 44.57 |
| AAR | - | 259.46 ± 24.16 | 207.24 ± 29.11 |
| AAR/LV (%) | - | 49.64 ± 5.87 | 40.47 ± 4.96 |
| MIA | - | 68.24 ± 5.73 | 36.31 ± 3.78 ## |
| MIA/AAR (%) | - | 27.64 ± 1.98 | 17.68 ± 1.73 ## |
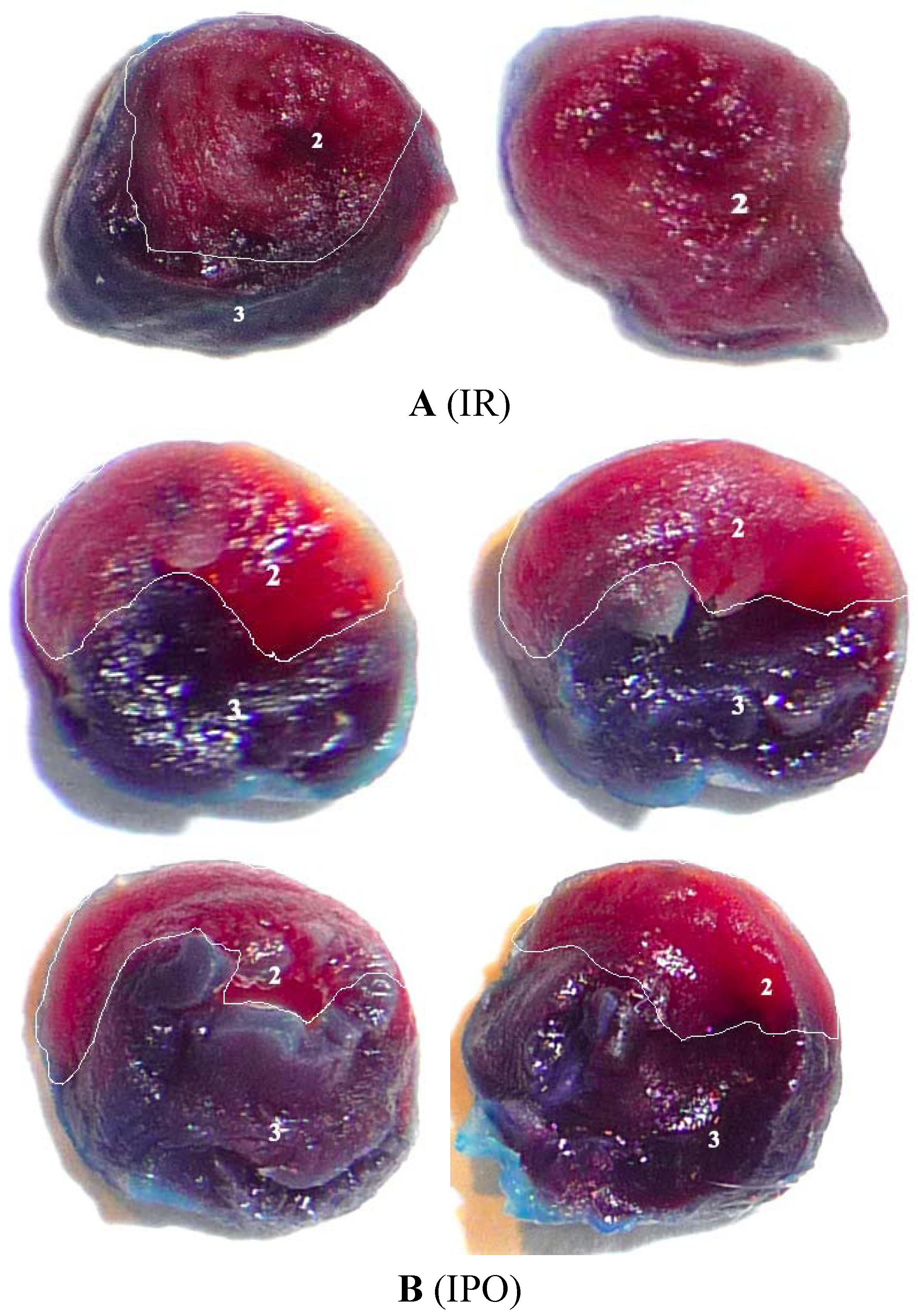
| Indexes | SO | IR | IPO |
|---|---|---|---|
| CK (U/L) | 3,186.3 ± 351.6 | 7,319.2 ± 485.1 ** | 4,287.5 ± 332.8 ## |
| LDH (U/L) | 1,528.4 ± 132.7 | 3,328.7 ± 295.3 ** | 1,832.8 ± 152.7 ## |
| AST (U/L) | 251.8 ± 22.9 | 406.1 ± 31.8 ** | 298.6 ± 25.4 ## |
| Indexes | SO | IR | IPO |
|---|---|---|---|
| Na+-K+-ATPase (μmol pi/mg prot/h) | 7.91 ± 0.77 | 4.82 ± 0.51 ** | 7.52 ± 0.67 ## |
| Ca2+-Mg2+-ATPase (μmol pi/mg prot/h) | 8.52 ± 0.72 | 5.99 ± 0.46 ** | 7.61 ± 0.81 ## |
| Indexes | SO | IR | IPO |
|---|---|---|---|
| MDA | 3.28 ± 0.26 | 6.82 ± 0.53 ** | 4.13 ± 0.29 ## |
| SOD | 287.32 ± 31.73 | 98.41 ± 7.08 ** | 241.63 ± 27.56 ## |
| CAT | 28.94 ± 2.54 | 12.62 ± 1.42 ** | 25.17 ± 2.64 ## |
| GSH-Px | 37.82 ± 1.89 | 21.65 ± 2.65 ** | 34.06 ± 2.16 ## |
| GR | 25.28 ± 1.66 | 10.63 ± 1.43 ** | 22.69 ± 1.98 ## |
3. Discussion
4. Experimental
4.1. Cardiac Ischemic Reperfusion Models
4.2. Myocardial Infarct Size Determination
4.3. Tissue Preparation
4.4. Biochemical Analysis
5. Conclusions
Conflict of Interest
References and Notes
- Kloner, R.A.; Rezkalla, S.H. Preconditioning, postconditioning and their application to clinical cardiology. Cardiovasc. Res. 2006, 70, 297–307. [Google Scholar]
- Hausenloy, D.J.; Baxter, G.; Bell, R.; Bøtker, H.E.; Davidson, S.M.; Downey, J.; Heusch, G.; Kitakaze, M.; Lecour, S.; Mentzer, R.; et al. Translating novel strategies for cardioprotection: The Hatter Workshop Recommendations. Basic Res. Cardiol. 2010, 105, 677–686. [Google Scholar] [CrossRef]
- Schwartz Longacre, L.; Kloner, R.A.; Arai, A.E.; Baines, C.P.; Bolli, R.; Braunwald, E.; Downey, J.; Gibbons, R.J.; Gottlieb, R.A.; Heusch, G.; et al. New horizons in cardioprotection: Recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 2011, 124, 1172–1179. [Google Scholar]
- Zhao, Z.Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H579–H588. [Google Scholar]
- Heusch, G.; Buchert, A.; Feldhaus, S.; Schulz, R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res. Cardiol. 2006, 101, 354–356. [Google Scholar]
- Kaljusto, M.L.; Mori, T.; Mohammad Husain Rizvi, S.; Galagudza, M.; Frantzen, M.L.; Valen, G.; Vaage, J. Postconditioning in rats and mice. Scand. Cardiovasc. J. 2006, 40, 334–341. [Google Scholar]
- Kin, H.; Zhao, Z.Q.; Sun, H.Y.; Wang, N.P.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Guyton, R.A.; Vinten-Johansen, J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc. Res. 2004, 62, 74–85. [Google Scholar]
- Tang, X.L.; Sato, H.; Tiwari, S.; Dawn, B.; Bi, Q.; Li, Q.; Shirk, G.; Bolli, R. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions 45 min. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2308–H2317. [Google Scholar]
- Tsang, A.; Hausenloy, D.J.; Mocanu, M.M.; Yellon, D.M. Postconditioning: A form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ. Res. 2004, 95, 230–232. [Google Scholar] [CrossRef]
- Argaud, L.; Gateau-Roesch, O.; Raisky, O.; Loufouat, J.; Robert, D.; Ovize, M. Postconditioning inhibits mitochondrial permeability transition. Circulation 2005, 111, 194–197. [Google Scholar]
- Couvreur, N.; Lucats, L.; Tissier, R.; Bize, A.; Berdeaux, A.; Ghaleh, B. Differential effects of postconditioning on myocardial stunning and infarction: A study in conscious dogs and anesthetized rabbits. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1345–H1350. [Google Scholar]
- Darling, C.E.; Jiang, R.; Maynard, M.; Whittaker, P.; Vinten-Johansen, J.; Przyklenk, K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: Role of ERK1/2. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1618–H1626. [Google Scholar]
- Yang, X.M.; Proctor, J.B.; Cui, L.; Krieg, T.; Downey, J.M.; Cohen, M.V. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J. Am. Coll. Cardiol. 2004, 44, 1103–1110. [Google Scholar]
- Iliodromitis, E.K.; Georgiadis, M.; Cohen, M.V.; Downey, J.M.; Bofilis, E.; Kremastinos, D.T. Protection from post-conditioning depends on the number of short ischemic insults in anesthetized pigs. Basic Res. Cardiol. 2006, 101, 502–507. [Google Scholar]
- Skyschally, A.; van Caster, P.; Boengler, K.; Gres, P.; Musiolik, J.; Schilawa, D.; Schulz, R.; Heusch, G. Ischemic postconditioning in pigs: No causal role for RISK activation. Circ. Res. 2009, 104, 15–18. [Google Scholar]
- Heusch, G.; Musiolik, J.; Gedik, N.; Skyschally, A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ. Res. 2011, 109, 1302–1308. [Google Scholar]
- Darling, C.E.; Solari, P.B.; Smith, C.S.; Furman, M.I.; Przyklenk, K. “Postconditioning” the human heart: Multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res. Cardiol. 2007, 102, 274–278. [Google Scholar] [CrossRef]
- Sivaraman, V.; Mudalagiri, N.R.; di Salvo, C.; Kolvekar, S.; Hayward, M.; Yap, J.; Keogh, B.; Hausenloy, D.J.; Yellon, D.M. Postconditioning protects human atrial muscle through the activation of the RISK pathway. Basic Res. Cardiol. 2007, 102, 453–459. [Google Scholar]
- Staat, P.; Rioufol, G.; Piot, C.; Cottin, Y.; Cung, T.T.; L’Huillier, I.; Aupetit, J.F.; Bonnefoy, E.; Finet, G.; Andre-Fouet, X.; et al. Postconditioning the human heart. Circulation 2005, 112, 2143–2148. [Google Scholar]
- Ferdinandy, P.; Schulz, R.; Baxter, G.F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007, 59, 418–458. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar]
- Heusch, G.; Boengler, K.; Schulz, R. Cardioprotection: Nitric oxide, protein kinases, and mitochondria. Circulation 2008, 118, 1915–1919. [Google Scholar]
- Xi, L.; Das, A.; Zhao, Z.Q.; Merino, V.F.; Bader, M.; Kukreja, R.C. Loss of myocardial ischemic postconditioning in adenosine A1 and bradykinin B2 receptors gene knockout mice. Circulation 2008, 118, S32–S37. [Google Scholar]
- Guo, H.T.; Zhang, R.H.; Zhang, Y.; Zhang, L.J.; Li, J.; Shi, Q.X.; Wang, Y.M.; Fan, R.; Bi, H.; Yin, W.; et al. Endogenous κ-opioid peptide mediates the cardioprotection induced by ischemic postconditioning. J. Cardiovasc. Pharmacol. 2011, 58, 207–215. [Google Scholar] [CrossRef]
- Tsang, A.; Hausenloy, D.J.; Mocanu, M.M.; Yellon, D.M. Postconditioning: A form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ. Res. 2004, 95, 230–232. [Google Scholar]
- Kim, K.B.; Chung, H.H.; Kim, M.S.; Rho, J.R. Changes in the antioxidative defensive system during open heart operations in humans. Ann. Thorac. Surg. 1994, 58, 170–175. [Google Scholar]
- Heusch, G. Postconditioning: Old wine in a new bottle? J. Am. Coll. Cardiol. 2004, 44, 1111–1112. [Google Scholar] [CrossRef]
- Ovize, M.; Baxter, G.F.; di Lisa, F.; Ferdinandy, P.; Garcia-Dorado, D.; Hausenloy, D.J.; Heusch, G.; Vinten-Johansen, J.; Yellon, D.M.; Schulz, R. Working group of cellular biology of heart of european society of cardiology. postconditioning and protection from reperfusion injury: Where do we stand? Position paper from the working group of cellular biology of the heart of the European society of cardiology. Cardiovasc. Res. 2010, 87, 406–423. [Google Scholar] [CrossRef]
- Rhun, L.Y.; Kirkland, J.B.; Shah, G.M. Cellular responses to DNA damage in the absence of poly (ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 1998, 245, 1–10. [Google Scholar]
- Koç, H. A comparison of blood lipid levels of physical education and sports school students attending to different curriculum. Afr. J. Pharm. Pharmacol. 2010, 4, 890–894. [Google Scholar]
- özdağ, S. Effects of short-term exercise on heart-rate blood pressure oxidative stress paraoxonase activity and lipid hydroperoxide. Afr. J. Pharm Pharmacol. 2010, 4, 658–661. [Google Scholar]
- Almogren, A. Pulmonary tuberculosis associated with increased number and percentage of natural killer and B cells in the peripheral blood. Afr. J. Microbiol. Res. 2011, 5, 2048–2052. [Google Scholar]
- di Napoli, P.; Taccardi, A.A.; de Caterina, R.; Barsotti, A. Pathophsiology of ischemia-reperfusion injury: Experimental data. Ital. Heart J. 2002, 3, 24–28. [Google Scholar]
- Fang, Y.; Wang, X.-P.; Lin, H.-P.; Wang, Q.-X. Correlation between clinicopathology and expression of heat shock protein 72 in human primary lung carcinomas. Afr. J. Pharm. Pharmacol. 2011, 5, 1310–1314. [Google Scholar]
- Gharibi, S.; Tajbakhsh, S.; Zandi, K.; Yaghobi, R. Evaluation of fluorescent in situ hybridization for rapid diagnosis of enterococcal wound infection. Afr. J. Microbiol. Res. 2010, 4, 2498–2502. [Google Scholar]
- Bolli, R.; Jeroudi, M.O.; Patel, S.B. Direct evidence that oxygenderived free radicals contribute to post ischemic myocardial dysfunction in the intact dog. Proc. Natl. Acad. Sci. USA 1989, 86, 4695–4699. [Google Scholar]
- Maulik, S.K.; Seth, S.D.; Maulik, M.; Manchanda, S.C. Oxyfedrine in myocardial stunning. Indian J. Exp. Biol. 1997, 35, 1214–1217. [Google Scholar]
- Gauthaman, K.; Banerjee, S.K.; Dinda, A.K.; Ghosh, C.C.; Maulik, S.K. Terminalia arjuna (Roxb.) protects rabbit heart against ischemic-reperfusion injury: Role of antioxidant enzymes and heat shock protein. J. Ethnopharmacol. 2005, 96, 403–409. [Google Scholar] [CrossRef]
- Shabanzadeh, A.P.; Shuaib, A.; Wang, C.X. Simvastatin reduced ischemic brain injury and perfusion deficits in an embolic model of stroke. Brain Res. 2005, 1042, 1–5. [Google Scholar] [CrossRef]
- Galagudza, M.; Kurapeev, D.; Minasian, S.; Valen, G.; Vaage, J. Ischemic postconditioning: Brief ischemia during reperfusion converts persistent ventricular fibrillation into regular rhythm. Eur. J. Cardiothorac. Surg. 2004, 25, 1006–1010. [Google Scholar]
- Panteghini, M.; Cuccia, C.; Calarco, M.; Gei, P.; Bozzetti, E.; Visioli, O. Serum enzymes in acute myocardial infarction after intracoronary thrombolysis. Clin. Biochem. 1986, 19, 294–297. [Google Scholar]
- Bhayana, V.; Ralph Henderson, A. Biochemical markers of myocardial damage. Clin. Biochem. 1995, 28, 1–29. [Google Scholar]
- Szabados, E.; Literati-Nagy, P.; Farkas, B.; Sumegi, B. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem. Pharmacol. 2000, 59, 937–945. [Google Scholar]
- Rajadurai, M.; Stanely Mainzen Prince, P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology 2007, 230, 178–188. [Google Scholar]
- Yorozuya, T.; Adachi, N.; Dote, K.; Nakanishi, K.; Takasaki, Y.; Arai, T. Enhancement of Na+, K+-ATPase and Ca2+-ATPase activities in multi-cycle ischemic preconditioning in rabbit hearts. Eur. J. Cardiothorac. Surg. 2004, 26, 981–987. [Google Scholar]
- Tian, D.; Dmitrieva, R.I.; Doris, P.A.; Crary, J.F.; Sondhi, R.; Sacktor, T.C.; Bergold, P.J. Protein kinase M zeta regulation of Na/K ATPase: A persistent neuroprotective mechanism of ischemic preconditioning in hippocampal slice cultures. Brain Res. 2008, 1213, 127–139. [Google Scholar] [CrossRef]
- de Souza Wyse, A.T.; Streck, E.L.; Worm, P.; Wajner, A.; Ritter, F.; Netto, C.A. Preconditioning prevents the inhibition of Na+, K+-ATPase activity after brain ischemia. Neurochem. Res. 2000, 25, 971–975. [Google Scholar]
- Chan, K.M.; Delfert, D.; Junger, K.D. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 1986, 157, 375–380. [Google Scholar]
- Halow, J.M.; Figueredo, V.M.; Shames, D.M.; Camacho, S.A.; Baker, A.J. Role of slowed Ca (2+) transient decline in slowed relaxation during myocardial ischemia. J. Mol. Cell. Cardiol. 1999, 31, 1739–1748. [Google Scholar]
- Samouilidou, E.C.; Karli, J.N.; Levis, G.M.; Darsinos, J.T. The sarcolemmal Ca2+-ATPase of the ischemic-reperfused myocardium: Protective effect of hypocalcemia on calmodulin-stimulated activity. Life Sci. 1998, 62, 29–36. [Google Scholar]
- Maupoil, V.; Rochette, L. Evaluation of free radical and lipid peroxide formation during global ischemia and reperfusion in isolated perfused rat heart. Cardiovasc. Drugs Ther. 1988, 2, 615–621. [Google Scholar]
- Baker, J.E.; Felix, C.C.; Blinger, G.N.; Kalyanaraman, B. Myocardial ischemia and reperfusion: Direct evidence for free radical generation by electron spin resonance spectroscopy. Proc. Natl. Acad. Sci. USA 1988, 85, 2786–2789. [Google Scholar]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; di Venosa, N.; Serena, D.; Ruggiero, F.M. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic. Biol. Med. 1999, 27, 42–50. [Google Scholar]
- Jha, N.; Jurma, O.; Lalli, G.; Liu, Y.; Pettus, E.H.; Greenamyre, J.T.; Liu, R.M.; Forman, H.J.; Andersen, J.K. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. J. Biol. Chem. 2000, 275, 26096–26101. [Google Scholar]
- Lesnefsky, E.J.; Moghaddas, S.; Tandler, B.; Kerner, J.; Hoppel, C.L. Mitochondrial dysfunction in cardiac disease: Ischemiareperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001, 33, 1065–1089. [Google Scholar] [CrossRef]
- Heusch, G.; Boengler, K.; Schulz, R. Inhibition of mitochondrial permeability transition pore opening: The holy grail of cardioprotection. Basic Res. Cardiol. 2010, 105, 151–154. [Google Scholar]
- Jiang, Y.R.; Yin, H.J.; Zhang, B.; Shi, D.Z. Effects of gross saponins of tribulus on Na~+-K~+-ATPase and Ca~(2+)-Mg~(2+)-ATPase activity and lactic acid content in hyperlipemic rats with acute myocardial infarction. Tradit. Chin. Drug Res. Clin. Pharmacol. 2006, 17, 248–250. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1984, 105, 302–310. [Google Scholar]
- McCord, J.M.; Fridowich, I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968, 243, 5753–5760. [Google Scholar]
- Clairbone, A.I.; Green-Wald, R.A. Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; p. 283. [Google Scholar]
- Lee, D.S.; Steinbaugh, G.E.; Quarrie, R.; Yang, F.C.; Hassan Talukder, M.A.; Zweier, J.L.; Crestanello, J.A. Ischemic postconditioning does not provide cardioprotection from long-term ischemic injury in isolated male or female rat hearts. J. Surg. Res. 2010, 164, 175–181. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar]
- Skyschally, A.; van Caster, P.; Iliodromitis, E.K.; Schulz, R.; Kremastinos, D.T.; Heusch, G. Ischemic postconditioning: Experimental models and protocol algorithms. Basic Res. Cardiol. 2009, 104, 469–483. [Google Scholar]
- Boengler, K.; Schulz, R.; Heusch, G. Loss of cardioprotection with ageing. Cardiovasc. Res. 2009, 83, 247–261. [Google Scholar]
- Abtahi, H.; Ghazavi, A.; Karimi, M. Antimicrobial activities of ethanol extract of black grape. Afr. J. Microbiol. Res. 2011, 5, 4446–4448. [Google Scholar]
- Kouhpayeh, H.; Hashemi, M.; Naderi, M.; Mozafar, S.H. The status of serum procalcitonin in sepsis and non-infectious systemic inflammatory response syndrome. Afr. J. Microbiol. Res. 2010, 4, 2632–2634. [Google Scholar]
- Hayrullah, Y.; Mehmet, B.; Mustafa, B.K.; Yesim, G.A.; Sadik, B. The effects of dialysers on some blood biochemical parameters in hemodialysis patients. Afr. J. Pharm. Pharmacol. 2011, 5, 2513–2516. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, L.; Ma, J.; Liu, H. Protective Effect of Ischemic Postconditioning against Ischemia Reperfusion-Induced Myocardium Oxidative Injury in IR Rats. Molecules 2012, 17, 3805-3817. https://doi.org/10.3390/molecules17043805
Zhang L, Ma J, Liu H. Protective Effect of Ischemic Postconditioning against Ischemia Reperfusion-Induced Myocardium Oxidative Injury in IR Rats. Molecules. 2012; 17(4):3805-3817. https://doi.org/10.3390/molecules17043805
Chicago/Turabian StyleZhang, Li, Jiangwei Ma, and Huajin Liu. 2012. "Protective Effect of Ischemic Postconditioning against Ischemia Reperfusion-Induced Myocardium Oxidative Injury in IR Rats" Molecules 17, no. 4: 3805-3817. https://doi.org/10.3390/molecules17043805




