A New Norisoprenoid and Other Compounds from Fuzhuan Brick Tea
Abstract
:1. Introduction
2. Results and Discussion
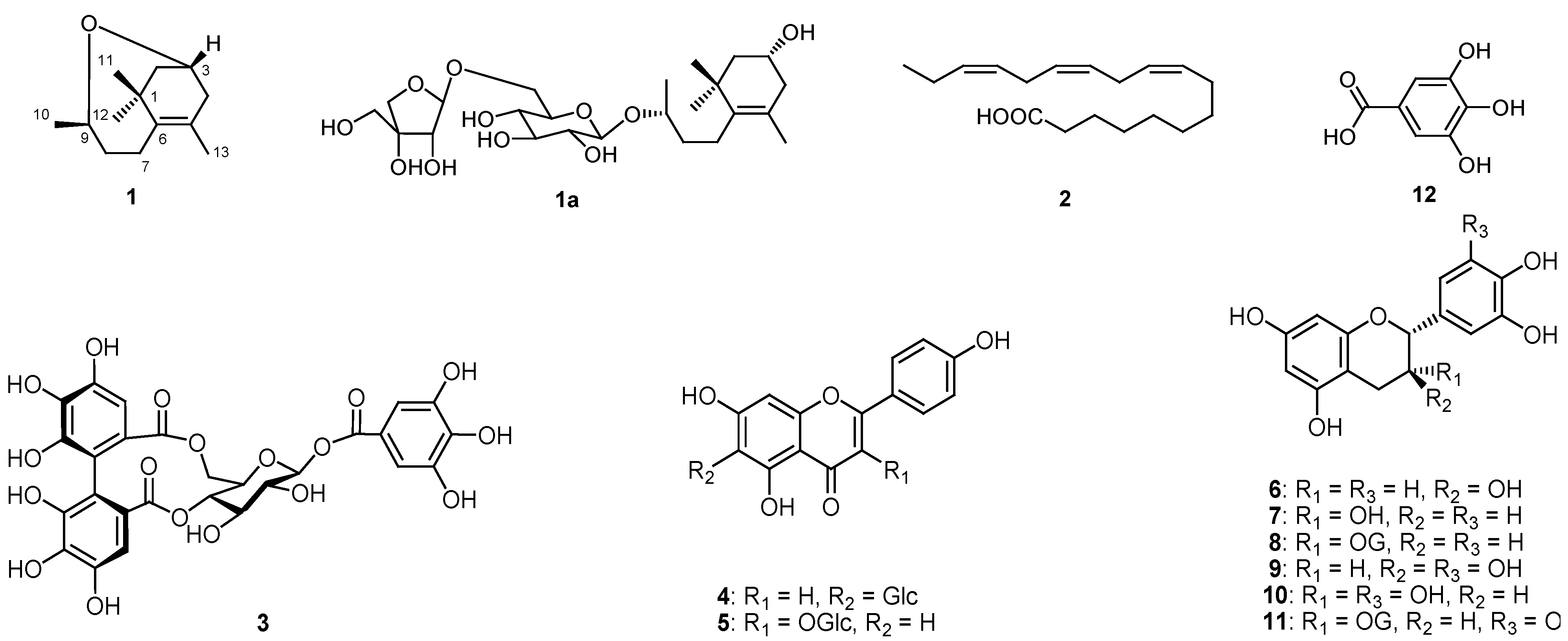

 ) correlations of compound 1.
) correlations of compound 1.
3. Experimental
3.1. General
3.2. Materials
3.3. Extraction and Isolation
3.4. 3,9-Oxido-5-megastigmene
| Positions | δH | δC | HMBC (1H to 13C) |
|---|---|---|---|
| 1 | ─ | 37.97 | |
| 2 | 2.260 (1H, br d, 9.6, H-2a) | 46.37 | C-1, 3, 4, 6, 11, 12 |
| 1.675 (1H, m, H-2b) a | C-1, 3, 4, 6, 11, 12 | ||
| 3 | 5.144 (1H, m) | 72.14 | |
| 4 | 2.727 (1H, dd, 12.8, 3.6, H-4a) | 40.44 | C-2, 3, 5, 6, 13 |
| 2.337 (1H, m, H-4b) b | C-2, 3, 5, 6, 13 | ||
| 5 | ─ | 124.58 | |
| 6 | ─ | 137.55 | |
| 7 | 2.337 (1H, m, H-7a) b | 24.56 | C-1, 5, 6, 8, 9 |
| 2.182 (1H, ddd, 10.4, 10.4, 3.6, H-7b) | C-1, 5, 6, 8, 9 | ||
| 8 | 1.861 (1H, m, H-8a) | 38.22 | C-7, 9, 10 |
| 1.675 (1H, m, H-8b) a | C-7, 9, 10 | ||
| 9 | 4.947 (1H, m) | 75.31 | C-7, 8 |
| 10 | 1.518 (3H, d, 4.8) | 21.39 | C-8, 9 |
| 11 | 0.974 (3H, s) c | 28.53 d | C-1, 2, 6, 12 |
| 12 | 0.974 (3H, s) c | 29.81 d | C-1, 2, 6, 11 |
| 13 | 1.490 (3H, s) | 19.84 | C-4, 5, 6 |
3.5. Antibacterial Assays
Supplementary Materials
Acknowledgements
- Samples Availability: Samples of the compounds 1–12 are available from the authors.
References and Notes
- Wan, X.C. Tea Biochemistry (in Chinese), 3rd ed; China Agriculture Press: Beijing, China, 2003; pp. 268–275. [Google Scholar]
- Mo, H.Z.; Zhu, Y.; Chen, Z.M. Microbial fermented tea-A potential source of natural food preservatives. Trends Food Sci. Tech. 2008, 19, 124–130. [Google Scholar] [CrossRef]
- Xu, A.Q.; Wang, Y.L.; Wen, J.Y.; Liu, P.; Liu, Z.Y.; Li, Z.J. Fungal community associated with fermentation and storage of Fuzhuan brick-tea. Int. J. Food Microbiol. 2011, 146, 14–22. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Huang, J.A.; Yang, M.Z.; Zhang, Y.; Ou-Yang, M.; Fu, D.H. Research of the anti-diarrhea function of Fuzhuan Tea (in Chinese). Tea Sci. 2009, 29, 465–469. [Google Scholar]
- Fu, D.H.; Ryan, E.P.; Huang, J.A.; Liu, Z.H.; Weir, T.L.; Snook, R.L.; Ryan, T.P. Fermented Camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Res. Int. 2011, 44, 2999–3005. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Ding, L.; Xia, H.L.; Tu, Y.Y. Analysis of the major chemical compositions in Fuzhuan brick-tea and its effect on activities of pancreatic enzymes in vitro. Afr. J. Biotechnol. 2010, 9, 6748–6754. [Google Scholar]
- Mo, H.Z.; Zhang, H.; Li, Y.Q.; Zhu, Y. Antimicrobial activity of the indigenously microbial fermented Fuzhuan brick-tea. J. Biotechnol. 2008, 136 (Suppl. 1), S722. [Google Scholar]
- Huang, Y.H.; Chen, J.H.; Zhou, Y.; Chen, X.Y. Differences in sensory quality and chemical composition of Fuzhuan Tea of different storage ages (in Chinese). Food Sci. 2010, 31, 228–232. [Google Scholar]
- Ling, T.J.; Wan, X.C.; Ling, W.W.; Zhang, Z.Z.; Xia, T.; Li, D.X.; Hou, R.Y. New triterpenoids and other constituents from a special microbial-fermented tea-Fuzhuan Brick Tea. J. Agric. Food Chem. 2010, 58, 4945–4950. [Google Scholar]
- Jiang, H.Y.; Shii, T.; Matsuo, Y.; Tanaka, T.; Jiang, Z.H.; Kouno, I. A new catechin oxidation product and polymeric polyphenols of post-fermented tea. Food Chem. 2011, 129, 830–836. [Google Scholar] [CrossRef]
- Sandri, J.; Viala, J. Direct preparation of (Z,Z)-1,4-dienic units with a new C6 homologating agent: Synthesis of α-linolenic acid. Synthesis 1995, 3, 271–275. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Okuda, T.; Memon, M.U.; Shigu, T.; Inoue, K. Spectral and chromatographic analysis of tannins. I. 13C nuclear magnetic resonance spectra of hydrolysable tannins. Chem. Pharm. Bull. 1984, 32, 1790–1799. [Google Scholar] [CrossRef]
- Jia, Z.S.; Zhou, B.; Yang, L.; Wu, L.M.; Liu, Z.L. 2D NMR study on tea polyphenols (in Chinese). Chin. J. Magn. Reson. 1998, 15, 23–30. [Google Scholar]
- Ramarathnam, N.; Osawa, T.; Namiki, M.; Kawakishi, S. Chemical studies on novel rice hull antioxidants. 2. Identification of isovitexin, a C-glycosyl flavonoid. J. Agric. Food Chem. 1989, 37, 316–319. [Google Scholar]
- Li, J.B.; Ding, Y. Studies on the chemical constituents from Myristica yunnanensis (in Chinese). Zhongguo Zhong Yao Za Zhi 2001, 26, 479–481. [Google Scholar]
- Davis, A.L.; Cai, Y.; Davis, A.P.; Lewis, J.R. 1H and 13C-NMR assignments of some green tea polyphenols. Magn. Reson. Chem. 1996, 34, 887–890. [Google Scholar]
- Achenbach, H.; Lottes, M.; Waibel, R.; Karikas, G.A.; Correa, A.M.D.; Gupta, M.P. Alkaloids and other compounds from Psychotria correae. Phytochemistry 1995, 38, 1537–1545. [Google Scholar]
- Lin, T.C.; Fang, J.M.; Cheng, Y.S. Terpenes and lignans from leaves of Chamaecyparis formosensis. Phytochemistry 1999, 51, 793–801. [Google Scholar]
- Ma, S.J.; Watanabe, N.; Yagi, A.; Sakata, K. The (3R,9R)-3-hydroxy-7,8-dihydro-β-ionol disaccharide glycoside is an aroma precursor in tea leaves. Phytochemistry 2001, 56, 819–825. [Google Scholar]
- Otsuka, H.; Zhong, X.N.; Hirata, E.; Shinzato, T.; Takeda, Y. Myrsinionosides A-E: Megastigmane glycosides from the leaves of Myrsine seguinii. Chem. Pharm. Bull. 2001, 49, 1093–1097. [Google Scholar] [CrossRef]
- Otsuka, H.; Tamaki, A. Platanionosides D-J: Megastigmane glycosides from the leaves of Alangium platanifolium (Sieb. Et Zucc.) Harms var. platanifolium Sieb. Et Zucc. Chem. Pharm. Bull. 2002, 50, 390–394. [Google Scholar]
- Otsuka, H. Linarionosides A-C and acyclic monoterpene diglucosides from Linaria japonica. Phytochemistry 1994, 37, 461–465. [Google Scholar]
- Xu, X.Q.; Mo, H.Z.; Yan, M.C.; Zhu, Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. J. Sci. Food Agric. 2007, 87, 1502–1504. [Google Scholar] [CrossRef]
- Winterhalter, P.; Schreier, P. Free and bound C13 norisoprenoids in quince (Cydonia oblonga, Mill.) fruit. J. Agric. Food Chem. 1988, 36, 1251–1256. [Google Scholar]
- Humpf, H.U.; Wintoch, H.; Schreier, P. 3,4-dihydroxy-7,8-dihydro-β-ionol β-D-glucopyranoside: Natural precursor of isomeric vitispiranes form Gooseberry (Ribes uva crispa L.) and Whitebeam (Sorbus aria) leaves. J. Agric. Food Chem. 1992, 40, 2060–2062. [Google Scholar]
- Winterhalter, P.; Schreier, P. C13-norisoprenoid glycosides in plant tissues: An overview on their occurrence, composition and role as flavour precursors. Flavour. Fragr. J. 1994, 9, 281–287. [Google Scholar] [CrossRef]
- Lin, L.Z.; Chen, P.; Harnly, J.M. New phenolic components and chromatographic profiles of green and fermented teas. J. Agric. Food Chem. 2008, 56, 8130–8140. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luo, Z.-M.; Ling, T.-J.; Li, L.-X.; Zhang, Z.-Z.; Zhu, H.-T.; Zhang, Y.-J.; Wan, X.-C. A New Norisoprenoid and Other Compounds from Fuzhuan Brick Tea. Molecules 2012, 17, 3539-3546. https://doi.org/10.3390/molecules17033539
Luo Z-M, Ling T-J, Li L-X, Zhang Z-Z, Zhu H-T, Zhang Y-J, Wan X-C. A New Norisoprenoid and Other Compounds from Fuzhuan Brick Tea. Molecules. 2012; 17(3):3539-3546. https://doi.org/10.3390/molecules17033539
Chicago/Turabian StyleLuo, Zhen-Mei, Tie-Jun Ling, Li-Xiang Li, Zheng-Zhu Zhang, Hong-Tao Zhu, Ying-Jun Zhang, and Xiao-Chun Wan. 2012. "A New Norisoprenoid and Other Compounds from Fuzhuan Brick Tea" Molecules 17, no. 3: 3539-3546. https://doi.org/10.3390/molecules17033539




