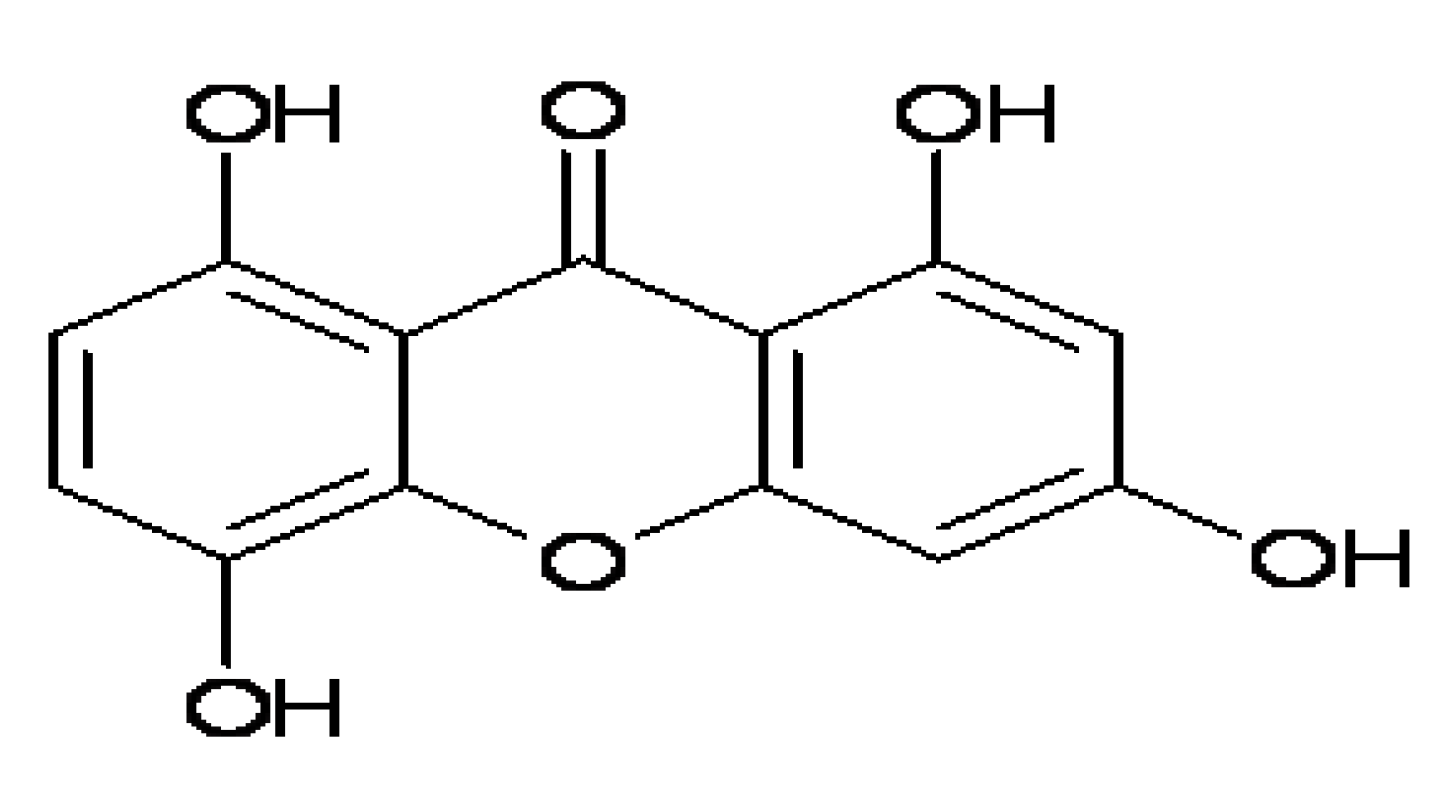
IMB2026791, a Xanthone, Stimulates Cholesterol Efflux by Increasing the Binding of Apolipoprotein A-I to ATP-Binding Cassette Transporter A1
Abstract
:1. Introduction
2. Results and Discussion
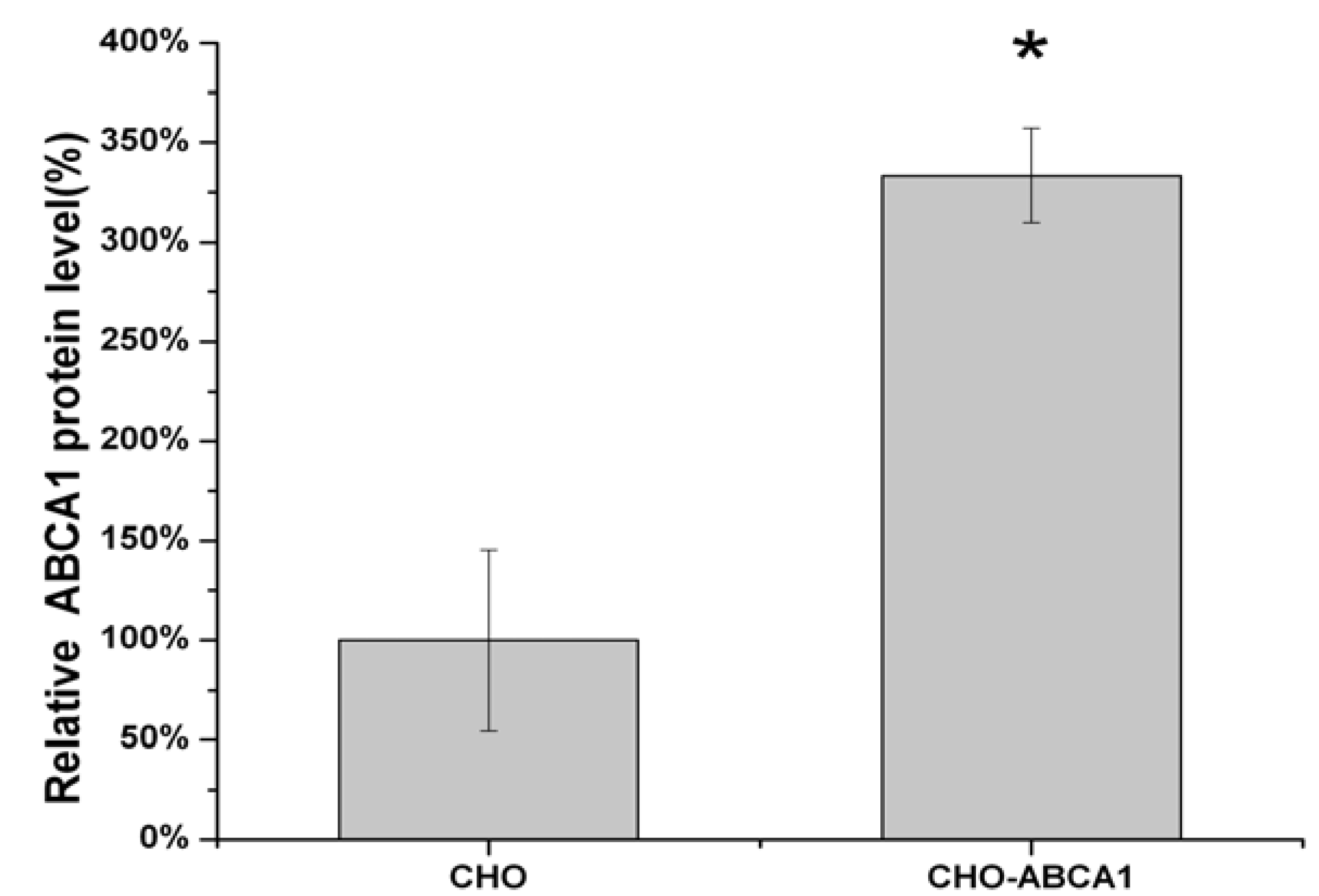
2.1. Construction of pIRES2-EGFP-ABCA1 and Evaluation of apoA-I-binding Activity of ABCA1
2.2. Cell-Based HTS Assay Optimization

2.3. High-Throughput Screening for Regulators of ABCA1 Activity
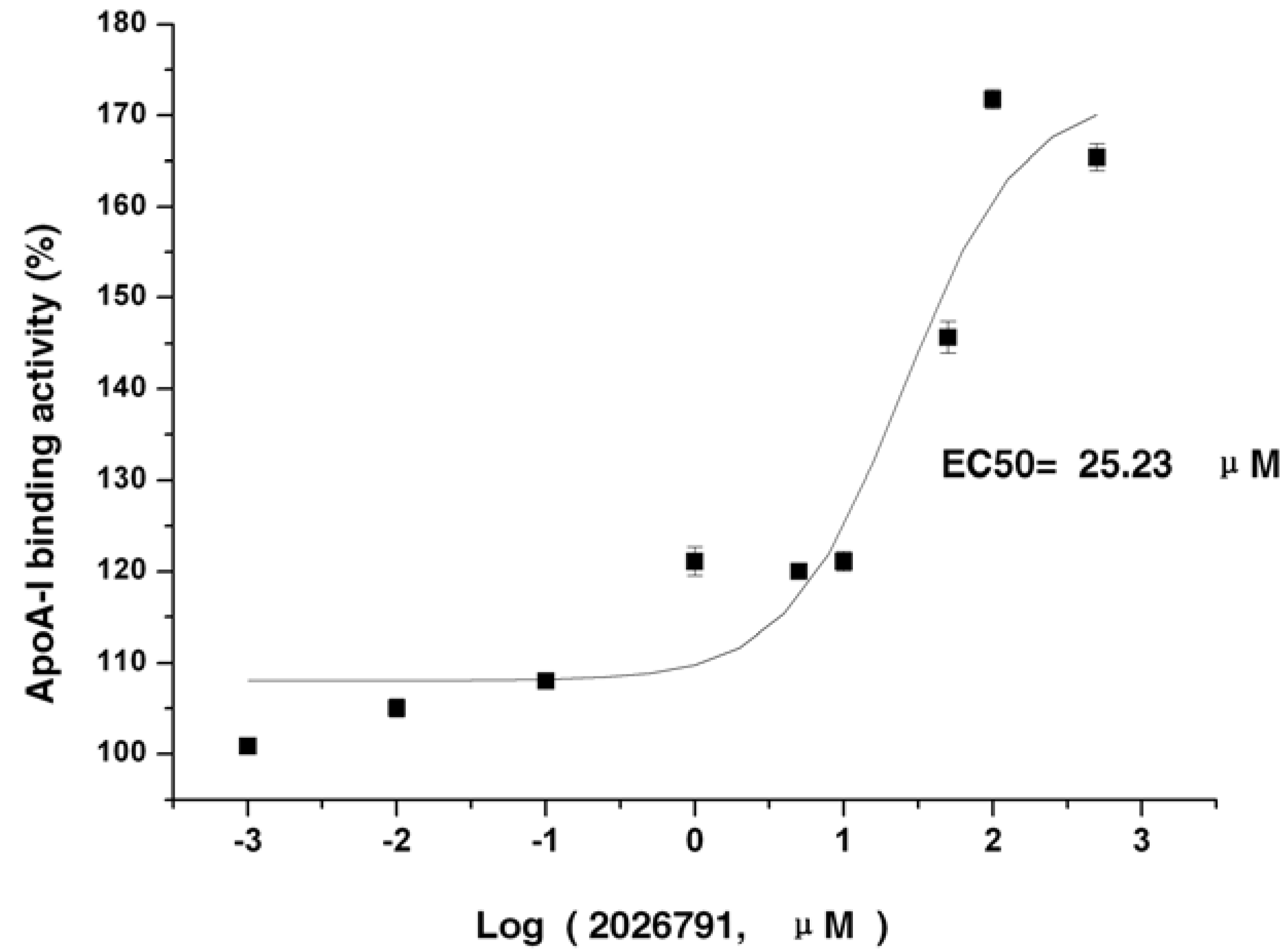
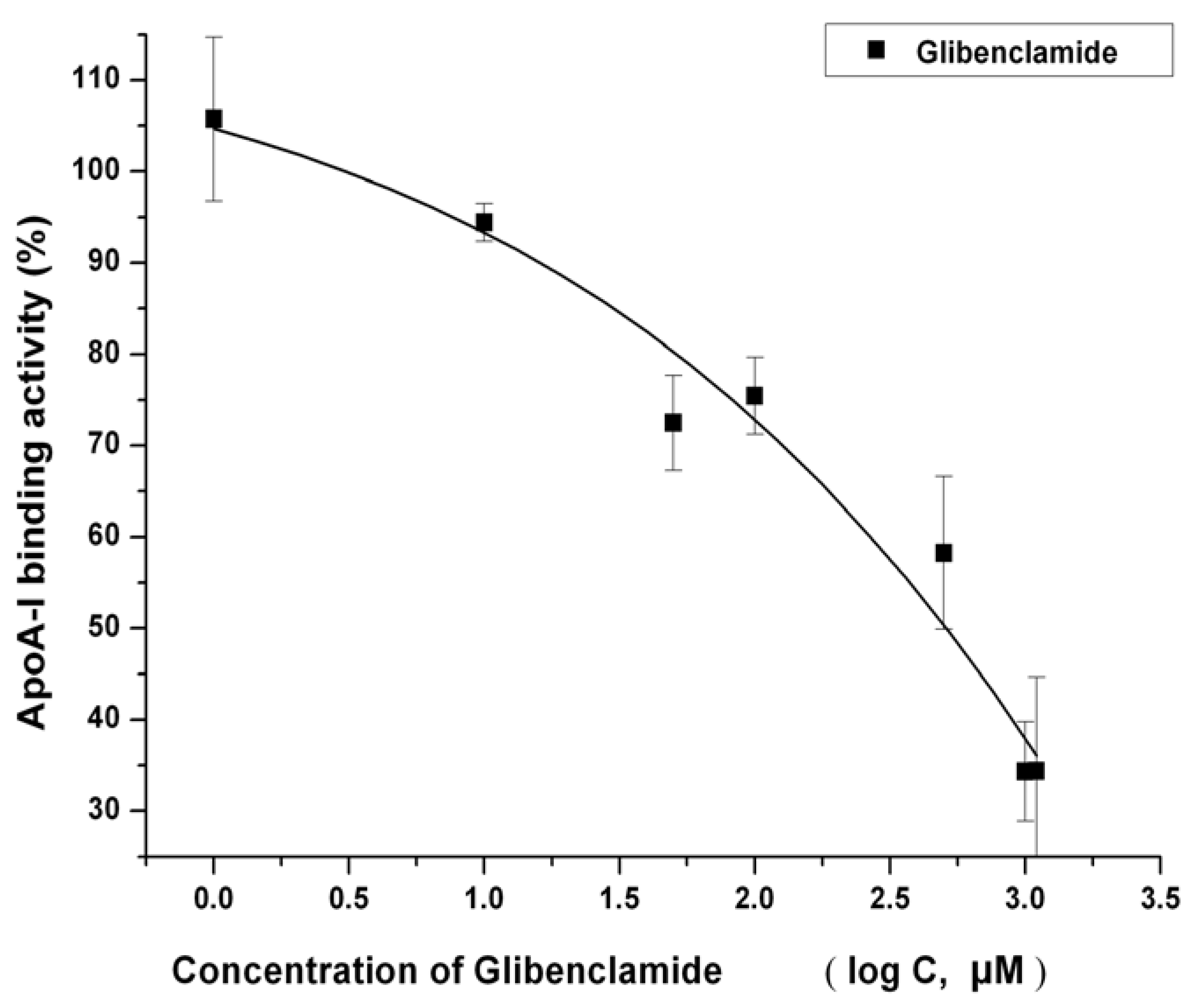
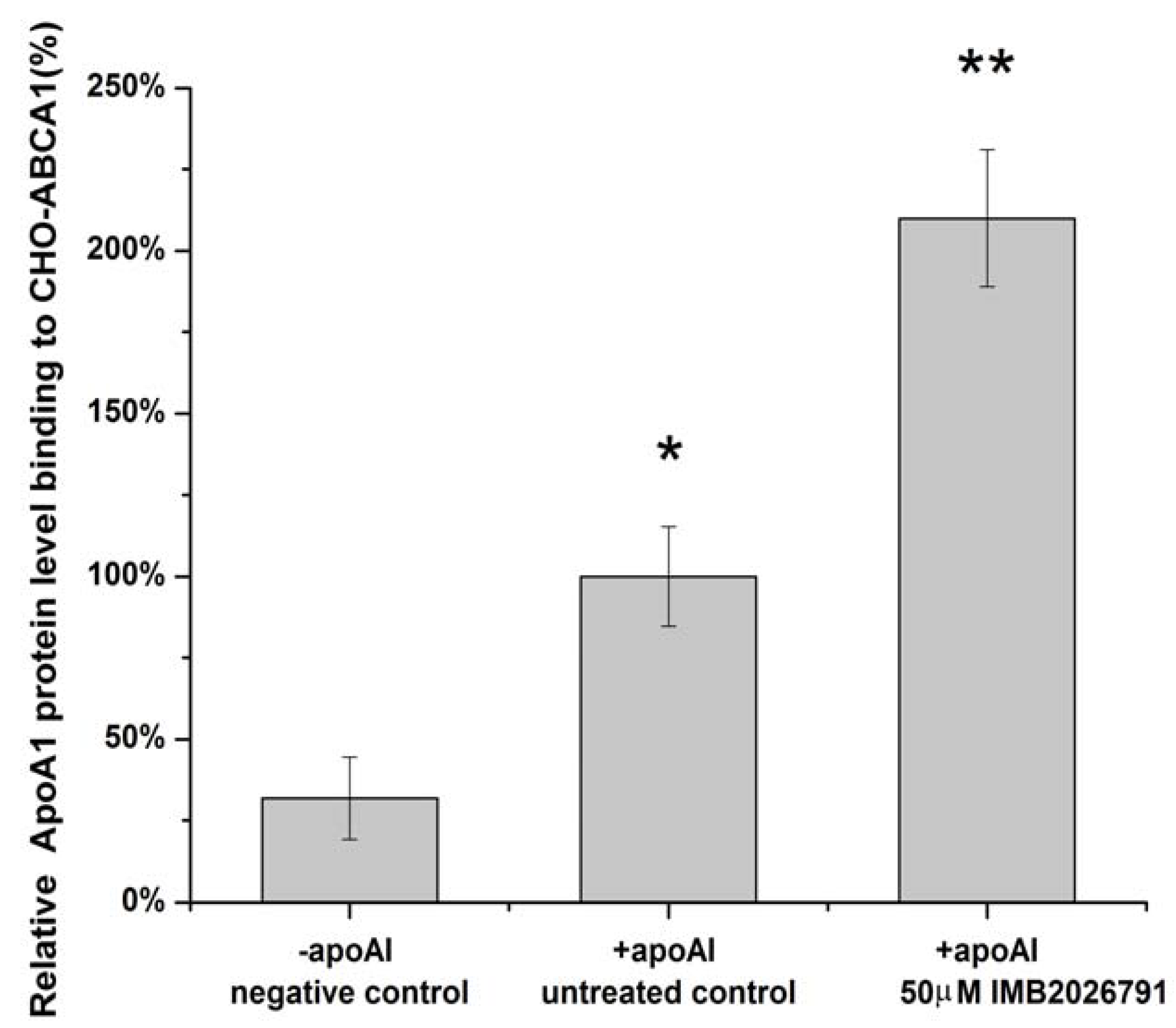
2.4. IMB2026791 Enhanced Binding of apoA-I to ABCA1

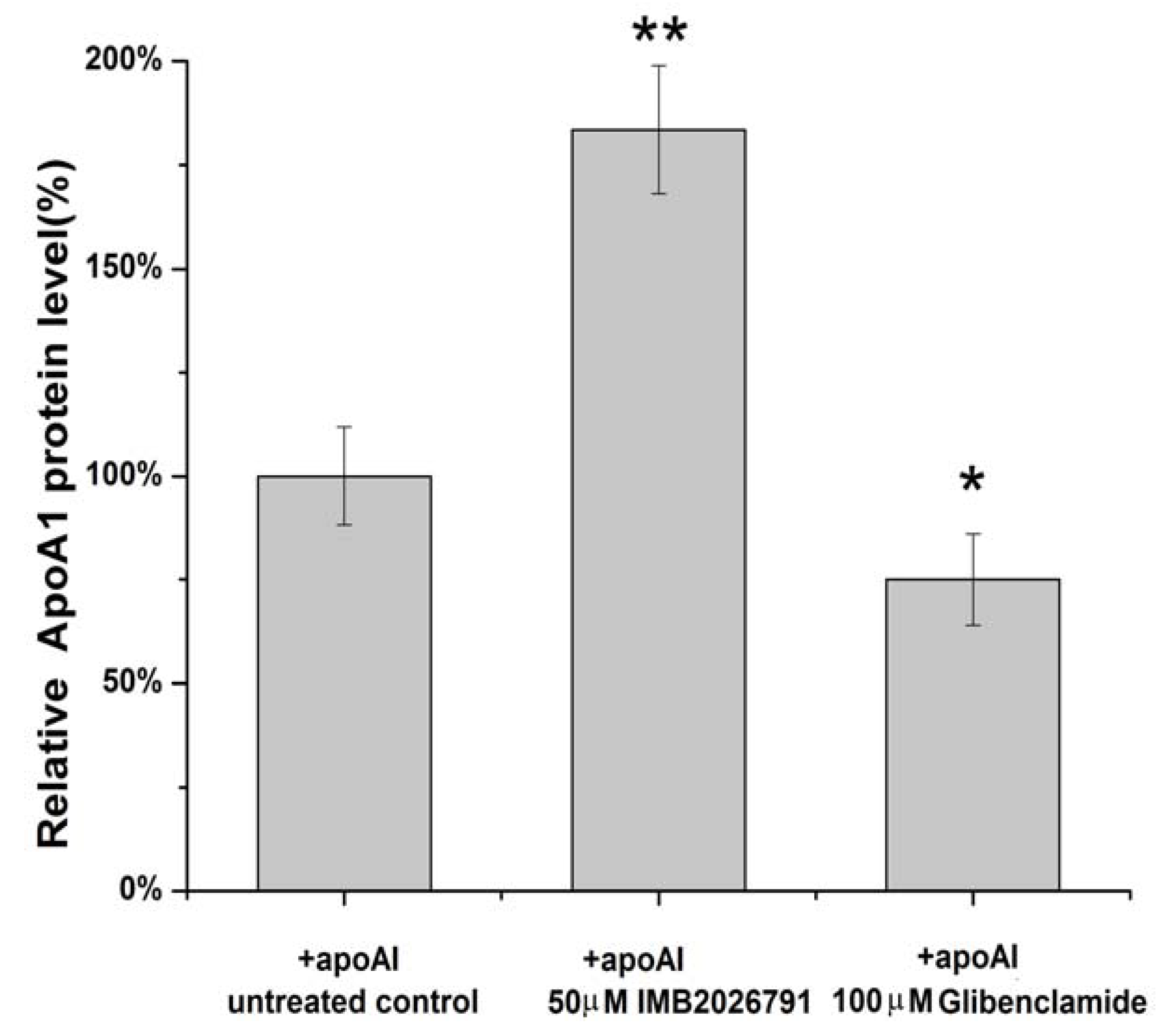
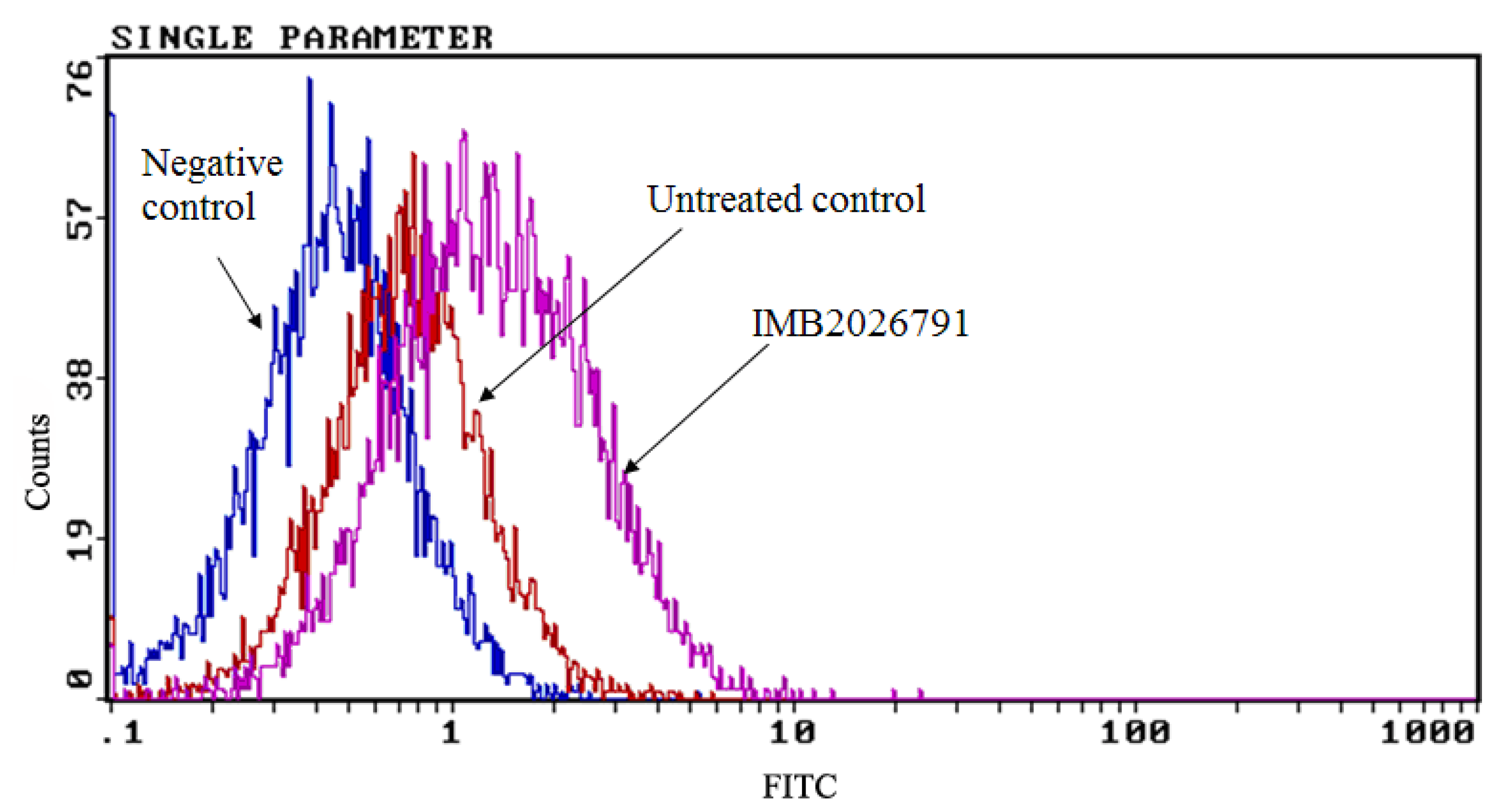
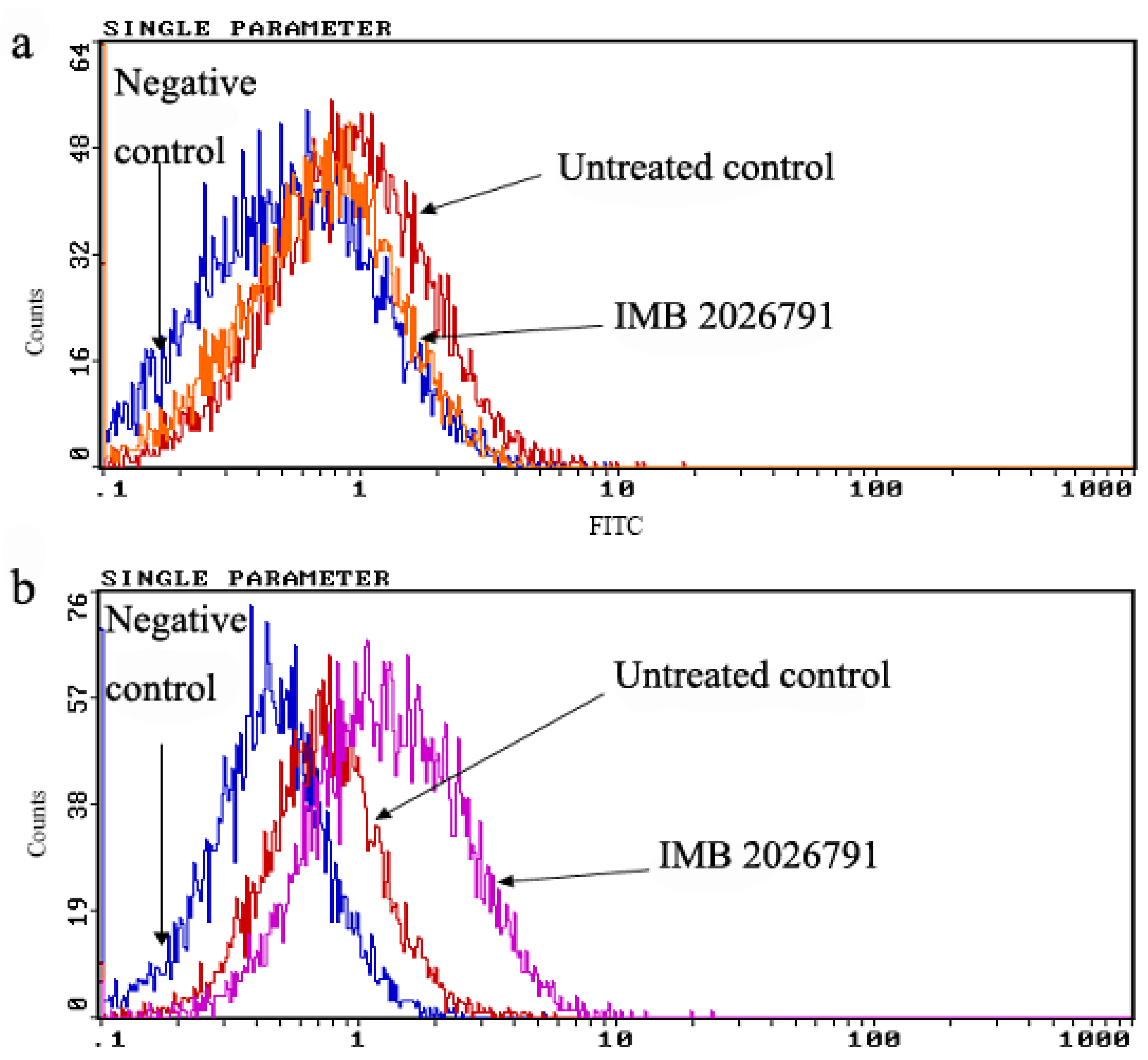
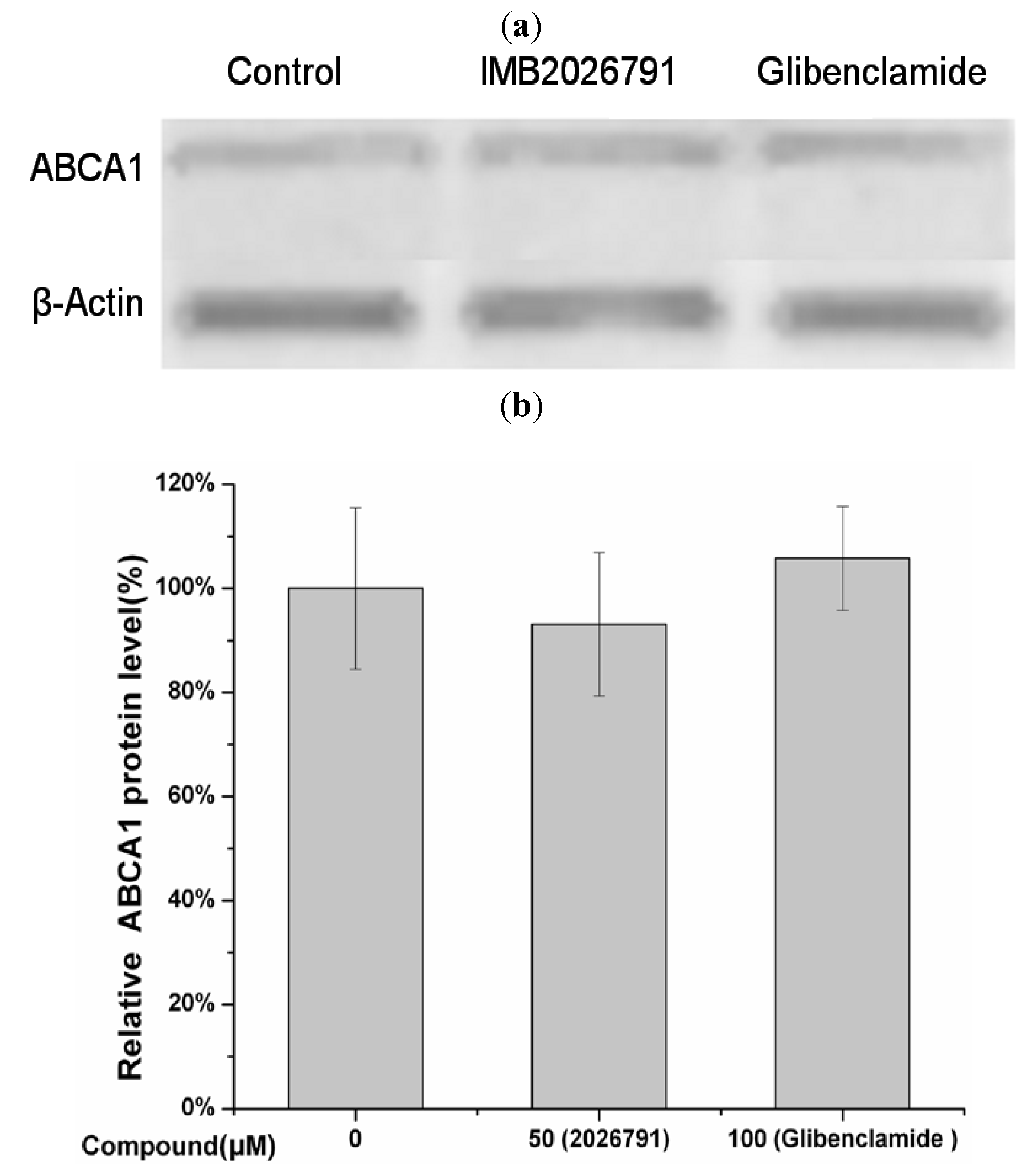
2.5. IMB2026791 and Glibenclamide Did Not Change the Surface and Total Expression of ABCA1

2.6. Effect of IMB2026791 and Glibenclamide on ABCA1-Mediated [3H] Cholesterol Efflux to apoA-I

2.7. Effects of IMB2026791 on the Viability of Cell Line A549

2.8. Discussion
3. Experimental
3.1. Materials
3.2. Cell Culture
3.3. Expression Plasmid Construction
3.4. Transfection and Selection of Stably Transfected Cell Line
3.5. HTS Assay
3.6. Western Blot Analysis
3.7. Flow Cytometry Analysis
3.8. Cholesterol Efflux Assay
3.9. Cell Growth Assay
3.10. Data Analysis and Statistical Analysis
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds are available from the authors and commercially available.
References and Notes
- Chambenoit, O.; Hamon, Y.; Marguet, D.; Rigneault, H.; Rosseneu, M.; Chimini, G. Specific docking of apolipoprotein A-I at the cell surface requires a functional ABCA1 transporter. J. Biol. Chem. 2001, 276, 9955–9960. [Google Scholar]
- Wang, N.; Silver, D.L.; Thiele, C.; Tall, A.R. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 2001, 276, 23742–23747. [Google Scholar]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of apoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef]
- Brooks-Wilson, A.; Marcil, M.; Clee, S.M.; Zhang, L.H.; Roomp, K.; van Dam, M.; Yu, L.; Brewer, C.; Collins, J.A.; Molhuizen, H.O.; et al. Mutations in abc1 in tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999, 22, 336–345. [Google Scholar] [CrossRef]
- Bodzioch, M.; Orso, E.; Klucken, J.; Langmann, T.; Bottcher, A.; Diederich, W.; Drobnik, W.; Barlage, S.; Buchler, C.; Porsch-Ozcurumez, M.; et al. The gene encoding ATP-binding cassette transporter 1 is mutated in tangier disease. Nat. Genet. 1999, 22, 347–351. [Google Scholar] [CrossRef]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.C.; Deleuze, J.F.; Brewer, H.B.; Duverger, N.; Denefle, P.; et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef]
- Lawn, R.M.; Wade, D.P.; Garvin, M.R.; Wang, X.; Schwartz, K.; Porter, J.G.; Seilhamer, J.J.; Vaughan, A.M.; Oram, J.F. The tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 1999, 104, R25–31. [Google Scholar] [CrossRef]
- Brousseau, M.E.; Schaefer, E.J.; Dupuis, J.; Eustace, B.; van Eerdewegh, P.; Goldkamp, A.L.; Thurston, L.M.; FitzGerald, M.G.; Yasek-McKenna, D.; O’Neill, G.; et al. Novel mutations in the gene encoding ATP-binding cassette 1 in four tangier disease kindreds. J. Lipid Res. 2000, 41, 433–441. [Google Scholar]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 1735, 1–19. [Google Scholar]
- Remaley, A.T.; Thomas, F.; Stonik, J.A.; Demosky, S.J.; Bark, S.E.; Neufeld, E.B.; Bocharov, A.V.; Vishnyakova, T.G.; Patterson, A.P.; Eggerman, T.L.; et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 2003, 44, 828–836. [Google Scholar] [CrossRef]
- Witting, S.R.; Maiorano, J.N.; Davidson, W.S. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter a1. J. Biol. Chem. 2003, 278, 40121–40127. [Google Scholar]
- Haidar, B.; Denis, M.; Marcil, M.; Krimbou, L.; Genest, J., Jr. Apolipoprotein A-I activates cellular cAMP signaling through the ABCA1 transporter. J. Biol. Chem. 2004, 279, 9963–9969. [Google Scholar]
- Nofer, J.R.; Remaley, A.T.; Feuerborn, R.; Wolinnska, I.; Engel, T.; von Eckardstein, A.; Assmann, G. Apolipoprotein A-I activates Cdc42 signaling through the ABCA1 transporter. J. Lipid Res. 2006, 47, 794–803. [Google Scholar] [CrossRef]
- Tang, C.; Vaughan, A.M.; Oram, J.F. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. J. Biol. Chem. 2004, 279, 7622–7628. [Google Scholar]
- Yamauchi, Y.; Hayashi, M.; Abe-Dohmae, S.; Yokoyama, S. Apolipoprotein A-I activates protein kinase c alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J. Biol. Chem. 2003, 278, 47890–47897. [Google Scholar]
- Nieland, T.J.; Chroni, A.; Fitzgerald, M.L.; Maliga, Z.; Zannis, V.I.; Kirchhausen, T.; Krieger, M. Cross-inhibition of sr-bi- and ABCA1-mediated cholesterol transport by the small molecules blt-4 and glyburide. J. Lipid Res. 2004, 45, 1256–1265. [Google Scholar] [CrossRef]
- Wang, N.; Tall, A.R. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscl. Throm. Vas. 2003, 23, 1178–1184. [Google Scholar] [CrossRef]
- Brewer, H.B., Jr.; Santamarina-Fojo, S. New insights into the role of the adenosine triphosphate-binding cassette transporters in high-density lipoprotein metabolism and reverse cholesterol transport. Am. J. Cardiol. 2003, 91, 3E–11E. [Google Scholar]
- Marcil, M.; Bissonnette, R.; Vincent, J.; Krimbou, L.; Genest, J. Cellular phospholipid and cholesterol efflux in high-density lipoprotein deficiency. Circulation 2003, 107, 1366–1371. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Morris, A.L.; Rhee, J.S.; Andersson, L.P.; Mendez, A.J.; Freeman, M.W. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J. Biol. Chem. 2002, 277, 33178–33187. [Google Scholar]
- Panagotopulos, S.E.; Witting, S.R.; Horace, E.M.; Hui, D.Y.; Maiorano, J.N.; Davidson, W.S. The role of apolipoprotein A-I helix 10 in apolipoprotein-mediated cholesterol efflux via the ATP-binding cassette transporter ABCA1. J. Biol. Chem. 2002, 277, 39477–39484. [Google Scholar]
- Sheppard, D.N.; Welsh, M.J. Effect of ATP-sensitive k+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J. Gen. Physiol. 1992, 100, 573–591. [Google Scholar] [CrossRef]
- Feng, B.; Tabas, I. ABCA1-mediated cholesterol efflux is defective in free cholesterol-loaded macrophages. Mechanism involves enhanced ABCA1 degradation in a process requiring full npc1 activity. J. Biol. Chem. 2002, 277, 43271–43280. [Google Scholar] [CrossRef]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar]
- von Eckardstein, A.; Langer, C.; Engel, T.; Schaukal, I.; Cignarella, A.; Reinhardt, J.; Lorkowski, S.; Li, Z.; Zhou, X.; Cullen, P.; et al. ATP binding cassette transporter ABCA1 modulates the secretion of apolipoprotein E from human monocyte-derived macrophages. FASEB J. 2001, 15, 1555–1561. [Google Scholar] [CrossRef]
- Xiao, H.; Li, N.; Jia, S.; Yang, Z.; Guo, R.; Hu, G.; Li, Y. Suppression of 3,4,5,6-tetrahydroxyxanthone on atherogenesis and its mechanisms in apolipoprotein E deficient mice, in Chinese. J. Int. Pathol. Clin. Med. 2006, 26, 185–189. [Google Scholar]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Y.; Yang, Y.; Zheng, Z.; Jiang, W.; Hong, B.; Yan, X.; Si, S. Identification of upregulators of human ATP-binding cassette transporter A1 via high-throughput screening of a synthetic and natural compound library. J. Biomol. Screen. 2008, 13, 648–656. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Lu, L.; Liu, H.; Peng, J.; Gan, L.; Shen, L.; Zhang, Q.; Li, L.; Zhang, L.; Su, C.; Jiang, Y. Regulations of the key mediators in inflammation and atherosclerosis by aspirin in human macrophages. Lipids Health Dis. 2010, 9, 16. [Google Scholar] [CrossRef]
- Fu, D.; Jiang, W.; Zheng, J.; Zhao, G.; Li, Y.; Yi, H.; Li, Z.; Jiang, J.; Yang, K.; Wang, Y.; Si, S. Jadomycin b, an aurora-b kinase inhibitor discovered through virtual screening. Mol. Cancer Ther. 2008, 7, 2386–2393. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, J.; Zhang, Z.; Xu, Y.; Feng, T.; Jiang, W.; Li, Z.; Hong, B.; Xie, Z.; Si, S. IMB2026791, a Xanthone, Stimulates Cholesterol Efflux by Increasing the Binding of Apolipoprotein A-I to ATP-Binding Cassette Transporter A1. Molecules 2012, 17, 2833-2854. https://doi.org/10.3390/molecules17032833
Liu J, Zhang Z, Xu Y, Feng T, Jiang W, Li Z, Hong B, Xie Z, Si S. IMB2026791, a Xanthone, Stimulates Cholesterol Efflux by Increasing the Binding of Apolipoprotein A-I to ATP-Binding Cassette Transporter A1. Molecules. 2012; 17(3):2833-2854. https://doi.org/10.3390/molecules17032833
Chicago/Turabian StyleLiu, Jikai, Zhongbing Zhang, Yanni Xu, Tingting Feng, Wei Jiang, Zhuorong Li, Bin Hong, Zijian Xie, and Shuyi Si. 2012. "IMB2026791, a Xanthone, Stimulates Cholesterol Efflux by Increasing the Binding of Apolipoprotein A-I to ATP-Binding Cassette Transporter A1" Molecules 17, no. 3: 2833-2854. https://doi.org/10.3390/molecules17032833



