Comparative Studies of the (Anti) Mutagenicity of Baccharis dracunculifolia and Artepillin C by the Bacterial Reverse Mutation Test
Abstract
:1. Introduction

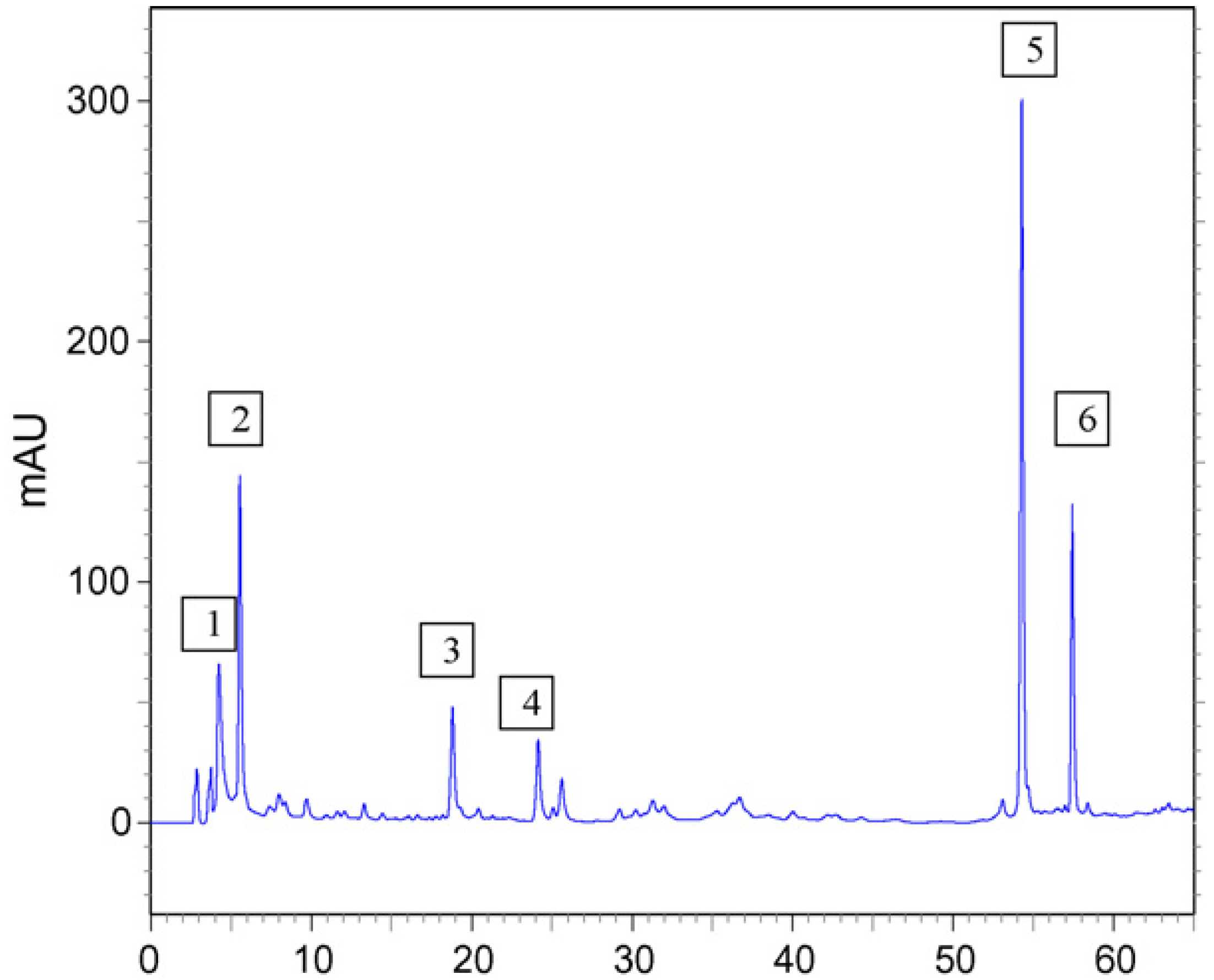
2. Results
2.1. Mutagenic Activity
| Treatments | Number of revertants (M ± SD)/ plate and MI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TA 98 | TA 100 | TA 102 | TA 97 | ||||||
| µg/plate | − S9 | + S9 | − S9 | + S9 | − S9 | + S9 | − S9 | + S9 | |
| Bd-EAE | 0.00 a | 20 ± 2 | 27 ± 6 | 154 ± 10 | 210 ± 14 | 255 ± 11 | 285 ± 24 | 132 ± 7 | 263 ± 7 |
| 11.4 | 19 ± 1 (0.9) | 26 ± 2 (1.1) | 137 ± 8 (0.8) | 211 ± 3 (0.9) | 282 ± 17 (1.0) | 327 ± 10 (1.1) | 137 ± 11 (0.9) | 280 ± 29 (1.1) | |
| 22.8 | 21 ± 5 (1.0) | 32 ± 2 (1.4) | 150 ± 11 (0.9) | 210 ± 3 (0.9) | 272 ± 11 (0.9) | 306 ± 6 (1.0) | 132 ± 12 (0.9) | 292 ± 28 (1.1) | |
| 45.6 | 19 ± 3 (0.9) | 32 ± 5 (1.4) | 150 ± 8 (0.9) | 215 ± 8 (1.0) | 283 ± 11 (1.0) | 304 ± 9 (1.0) | 133 ± 15 (0.9) | 275 ± 21 (1.0) | |
| 91.4 | 22 ± 3 (1.0) | 30 ± 4 (1.3) | 142 ± 11 (0.9) | 203 ± 8 (0.9) | 234 ± 3 (0.8) | 322 ± 13 (1.1) | 135 ± 6 (0.9) | 255 ± 15 (1.0) | |
| 182.8 | 21 ± 5 (1.0) | 23 ± 3 (1.0) | 138 ± 13 (0.8) | 200 ± 9 (0.9) | 196 ± 6 (0.7) | 341 ± 13 (1.1) | 91 ± 17 (0.6) | 238 ± 27 (0.9) | |
| Ctrol + | 1347 ± 88 b | 1567 ± 115 e | 1582 ± 98 c | 1456 ± 78 e | 1656 ± 60 d | 1932 ± 97 f | 1766.0 ± 49 b | 1789 ± 89 e | |
| ArtC | 0.00 a | 20 ± 3 | 30 ± 3 | 152 ± 2 | 164 ± 4 | 216 ± 2 | 216 ± 2 | 150 ± 15 | 155 ± 2 |
| 0.69 | 21 ± 3 (1.0) | 28 ± 3 (0.9) | 179 ± 4 (1.2) | 171 ± 1 (1.0) | 204 ± 4 (0.9) | 204 ± 4 (0.9) | 207 ± 3 (1.4) | 194 ± 3 (1.2) | |
| 1.37 | 24 ± 4 (1.2) | 32 ± 1 (1.1) | 174 ± 4 (1.1) | 171 ± 2 (1.0) | 213 ± 3 (1.0) | 213 ± 3 (1.0) | 221 ± 6 (1.5) | 174 ± 1 (1.1) | |
| 2.75 | 28 ± 5 (1.4) | 29 ± 3 (1.0) | 169 ± 6 (1.1) | 144 ± 2 (0.9) | 187 ± 5 (0.9) | 187 ± 5 (0.9) | 246 ± 5 (1.6) | 175 ± 4 (1.1) | |
| 5.49 | 29 ± 1 (1.5) | 32 ± 2 (1.1) | 158 ± 7 (1.0) | 153 ± 2 (0.9) | 215 ± 5 (1.0) | 215 ± 5 (1.0) | 207 ± 5 (1.4) | 175 ± 1 (1.1) | |
| 10.99 | 27 ± 2 (1.3) | 31 ± 1 (1.0) | 153 ± 3 (1.0) | 129 ± 1 (0.8) | 203 ± 6 (0.9) | 203 ± 6 (0.9) | 192 ± 6 (1.3) | 176 ± 3 (1.1) | |
| Ctrol + | 842 ± 34 b | 1498 ± 33 e | 2189 ± 73 c | 1072 ± 25 e | 1139 ± 42 d | 1099 ± 27 f | 1106 ± 23 b | 1861 ± 23 e | |
2.2. Antimutagenic Activity
3. Discussion
| Treatments | Number of revertants (M ± SD)/ plate and % of inhibition | ||||||||
| TA 98 | TA 100 | ||||||||
| µg/plate | − S9 | % inhibition | + S9 | % inhibition | − S9 | % inhibition | + S9 | % inhibition | |
| Bd-EAE | Ctrol + | NOPD | B[a]P | AS | AFB1 | ||||
| 638 ± 30 | 444 ± 14 | 1115 ± 36 | 2200 ± 140 | ||||||
| 11.4 | 482 ± 41 | 24 * | 312 ± 9 | 30 ** | 1126 ± 57 | - | 1517 ± 78 | 31 ** | |
| 22.8 | 453 ± 19 | 29 ** | 247 ± 10 | 44 *** | 1178 ± 61 | - | 1571 ± 86 | 29 ** | |
| 45.6 | 466 ± 35 | 27 ** | 218 ± 5 | 51 *** | 1152 ± 77 | - | 1467 ± 85 | 33 ** | |
| 91.4 | 449 ± 10 | 30 ** | 196 ± 4 | 56 *** | 1123 ± 17 | - | 741 ± 62 | 66 *** | |
| 182.8 | 480 ± 27 | 25 * | 175 ± 4 | 60 *** | 1186 ± 53 | - | 327 ± 44 | 85 *** | |
| ArtC | Ctrol + | 842 ± 34 | 498 ± 33 | 2189 ± 73 | 2171 ± 33 | ||||
| 0.69 | 526 ± 12 | 37 ** | 309 ± 17 | 38 ** | 1833 ± 48 | 16 * | 1595 ± 37 | 26 ** | |
| 1.37 | 520 ± 18 | 38 ** | 282 ± 23 | 43 *** | 1957 ± 36 | 11 * | 1639 ± 33 | 24 * | |
| 2.75 | 484 ± 31 | 42 *** | 218 ± 24 | 56 *** | 1813 ± 24 | 17 * | 1575 ± 11 | 27 ** | |
| 5.49 | 532 ± 27 | 37 ** | 217 ± 27 | 56 *** | 1727 ± 39 | 21 * | 1600 ± 38 | 26 ** | |
| 10.99 | 499 ± 26 | 41 *** | 189 ± 26 | 62 *** | 1885 ± 25 | 14 * | 1507 ± 43 | 30 ** | |
| TA 102 | TA 97a | ||||||||
| µg/plate | − S9 | % inhibition | + S9 | % inhibition | − S9 | % inhibition | + S9 | % inhibition | |
| Bd-EAE | Ctrol + | MMC | AF | NOPD | AA | ||||
| 935 ± 18 | 1336 ± 24 | 884 ± 34 | 2312 ± 81 | ||||||
| 11.4 | 1666 ± 87 | - | 1302 ± 8 | 2* | 672± 11 | 24 * | 1816 ± 76 | 21 * | |
| 22.8 | 1309 ± 56 | - | 1344 ± 29 | - | 613 ± 68 | 31 ** | 1731 ± 64 | 25 ** | |
| 45.6 | 1317 ± 72 | - | 1363 ± 27 | - | 633 ± 55 | 28 ** | 1659 ± 82 | 28 ** | |
| 91.4 | 1011 ± 76 | - | 1357 ± 9 | - | 486 ± 35 | 45 *** | 1603 ± 27 | 31 ** | |
| 182.8 | 891 ± 48 | - | 1209 ± 5 | 9 * | 488 ± 65 | 45 *** | 1481 ± 63 | 36 ** | |
| ArtC | Ctrol + | 1072 ± 25 | 1099 ± 27 | 1106 ± 23 | 1861 ± 23 | ||||
| 0.69 | 1096 ± 19 | - | 645 ± 23 | 41 *** | 876 ± 8 | 21 * | 1603 ± 26 | 14 * | |
| 1.37 | 1022 ± 18 | 5 * | 595 ± 25 | 46 *** | 862 ± 14 | 22 * | 1617 ± 16 | 13 * | |
| 2.75 | 994 ± 25 | 7 * | 566 ± 17 | 48 *** | 885 ± 19 | 20 * | 1669 ± 19 | 10 * | |
| 5.49 | 943 ± 17 | 12 * | 488 ± 14 | 56 *** | 933 ± 7 | 16 * | 1620 ± 21 | 13 * | |
| 10.99 | 879 ± 11 | 18 * | 529 ± 20 | 52 *** | 891 ± 17 | 19 * | 1602 ± 17 | 14 * | |
4. Experimental
4.1. Chemicals and Culture Media
4.2. Preparation of B. dracunculifolia Extracts and Isolation of Artepillin C
4.3. HPLC Analysis
4.4. Metabolic Activation System (S9 mixture)
4.5. Mutagenicity Assay
4.6. Antimutagenicity Assay
5. Conclusions
Acknowledgements
Conflict of Interest Statement
- Sample Availability: Samples of the compounds Bd-EAE and ArtC are available from the authors.
References and Notes
- Arriaga-Alba, M.; Blasco, J.L.; Ruíz-Pérez, N.J.; Sánchez-Navarrete, J.; Rivera-Sánchez, R.; González-Avila, M. Antimutagenicity mechanisms of the Rhoeo discolor ethanolic extract. Exp. Toxicol. Pathol. 2011, 63, 243–248. [Google Scholar] [CrossRef]
- Lemos, M.; de Barros, M.P.; Sousa, J.P.; Silva Filho, A.A.; Bastos, J.K.; de Andrade, S.F. Baccharis dracunculifolia, the main botanical source of Brazilian green propolis, displays antiulcer activity. J. Pharm. Pharmacol. 2007, 59, 603–608. [Google Scholar]
- Silva Filho, A.A.; Resende, D.O.; Fukui, M.J.; Santos, F.F.; Pauletti, P.M.; Cunha, W.R.; Silva, M.L.; Gregório, L.E.; Bastos, J.K.; Nanayakkara, N.P. In vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D.C. (Asteraceae). Fitoterapia 2009, 80, 478–482. [Google Scholar]
- Sousa, J.P.; Silva Filho, A.A.; Bueno, P.C.; Gregório, L.E.; Furtado, N.A.; Jorge, R.F.; Bastos, J.K. A validated reverse-phase HPLC analytical method for the quantification of phenolic compounds in Baccharis dracunculifolia. Phytochem. Anal. 2009, 20, 24–32. [Google Scholar] [CrossRef]
- Leitão, D.P.; Filho, A.A.; Polizello, A.C.; Bastos, J.K.; Spadaro, A.C. Comparative evaluation of in vitro effects of Brazilian green propolis and Baccharis dracunculifolia extracts on cariogenic factors of Streptococcus mutans. Biol. Pharm. Bull. 2004, 27, 1834–1839. [Google Scholar] [CrossRef]
- Silva Filho, A.A.; Bueno, P.C.P.; Gregório, L.E.; Silva, M.L.A.; Albuquerque, S.; Bastos, J.K. In vitro trypanocidal activity evaluation of crude extract and isolated compounds from Baccharis dracunculifolia D.C. (Asteraceae). J. Pharm. Pharmacol. 2004, 56, 1195–1199. [Google Scholar]
- Resende, F.A.; Alves, J.M.; Munari, C.C.; Senedese, J.M.; Sousa, J.P.B.; Bastos, J.K.; Tavares, D.C. Inhibition of doxorubicin-induced mutagenicity by Baccharis dracunculifolia. Mut. Res. 2007, 634, 112–118. [Google Scholar] [CrossRef]
- Missima, F.; da Silva Filho, A.A.; Nunes, G.A.; Bueno, P.C.; de Sousa, J.P.; Bastos, J.K.; Sforcin, J.M. Effect of Baccharis dracunculifolia D.C. (Asteraceae) extracts and its isolated compounds on macrophage activation. J. Pharm. Pharmacol. 2007, 59, 463–468. [Google Scholar]
- Park, Y.K.; Paredes-Guzman, J.F.; Aguiar, C.L.; Alencar, S.M.; Fujiwara, F.Y. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of Southeastern Brazilian propolis. J. Agric. Food Chem. 2004, 52, 1100–1103. [Google Scholar]
- Shimizu, K.; Ashida, H.; Matsuura, Y.; Kanazawa, K. Antioxidative bioavailability of artepillin C in Brazilian propolis. Arch. Biochem. Biophys. 2004, 424, 181–188. [Google Scholar] [CrossRef]
- Shimizu, K.; Das, S.K.; Baba, M.; Matsuura, Y.; Kanazawa, K. Dietary artepillin C suppresses the formation of aberrant crypt foci induced by azoxymethane in mouse colon. Cancer Lett. 2006, 240, 135–142. [Google Scholar] [CrossRef]
- Nakanishi, I.; Uto, Y.; Ohkubo, K.; Miyazaki, K.; Yakumaru, H.; Urano, S.; Okuda, H.; Ueda, J.; Ozawa, T.; Fukuhara, K.; Fukuzumi, S.; Nagasawa, H.; Hori, H.; Ikota, N. Efficient radical scavenging ability of artepillin C, a major component of Brazilian propolis, and the mechanism. Org. Biomol. Chem. 2003, 1, 1452–1454. [Google Scholar] [CrossRef]
- Salomão, K.; Dantas, A.P.; Borba, C.M.; Campos, L.C.; Machado, D.G.; Aquino Neto, F.R.; De Castro, S.L. Chemical composition and microbicidal activity of extracts from Brazilian and Bulgarian propolis. Lett. Appl. Microbiol. 2004, 38, 87–92. [Google Scholar] [CrossRef]
- Messerli, S.M.; Ahn, M.R.; Kunimasa, K.; Yanagihara, M.; Tatefuji, T.; Hashimoto, K.; Mautner, V.; Uto, Y.; Hori, H.; Kumazawa, S.; Kaji, K.; Ohta, T.; Maruta, H. ArtepillinC (ARC) in BraziliangreenpropolisselectivelyblocksoncogenicPAK1signaling and suppresses the growth of NFtumors in mice. Phytother. Res. 2009, 23, 423–427. [Google Scholar] [CrossRef]
- Paulino, N.; Abreu, S.R.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatoryeffects of a bioavailablecompound, artepillinC, in Brazilianpropolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef]
- Kimoto, T.; Arai, S.; Kohguchi, M.; Aga, M.; Nomura, Y.; Micallef, M.J.; Kurimoto, M.; Mito, K. Apoptosis and suppression of tumor growth by artepillin C extracted from Brazilian propolis. Cancer Detect. Prev. 1998, 22, 506–515. [Google Scholar] [CrossRef]
- Simões, L.M.; Gregório, L.E.; Silva Filho, A.A.; Souza, M.L.; Azzolini, A.E.; Bastos, J.K.; Lucisano-Valim, Y.M. Effect of Braziliangreenpropolis on the production of reactive oxygen species by stimulatedneutrophils. J. Ethnopharmacol. 2004, 94, 59–65. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Prasain, J.K.; Matsushige, K.; Saiki, I.; Kadota, S. Chemical constituents of Brazilian propolis and their cytotoxic activities. J. Nat. Prod. 1998, 61, 896–900. [Google Scholar] [CrossRef]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical origin and chemical composition of Brazilian propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar]
- Simões-Ambrosio, L.M.; Gregório, L.E.; Sousa, J.P.; Figueiredo-Rinhel, A.S.; Azzolini, A.E.; Bastos, J.K.; Lucisano-Valim, Y.M. The role of seasonality on the inhibitoryeffect of Braziliangreenpropolis on the oxidative metabolism of neutrophils. Fitoterapia 2010, 81, 1102–1108. [Google Scholar] [CrossRef]
- Chitme, H.R.; Chandra, R.; Kaushik, S. Studies on anti-diarrheal activity of Calotropis gigantea R. Br. in experimental animals. J. Pharm. Pharm. Sci. 2004, 7, 70–75. [Google Scholar]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Alternat. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Varanda, E.A.; Lima, L.M.; Chin, C.M. Mutagenicity of new lead compounds to treat sickle cell disease symptoms in a Salmonella/ microsome assay. Int. J. Mol. Sci. 2010, 11, 779–788. [Google Scholar] [CrossRef]
- Andrade, N.S.; Perazzo, F.F.; Maistro, E.L. Lack of clastogenic/ genotoxic effects of Baccharis dracunculifolia extract on Swiss mouse peripheral blood cells. Genet. Mol. Res. 2008, 7, 1414–1421. [Google Scholar] [CrossRef]
- Monteiro Neto, M.A.B.; Souza Lima, I.M.; Furtado, R.A.; Bastos, J.K.; Silva Filho, A.A.; Tavares, D.C. Antigenotoxicity of artepillin C in vivo evaluated by the micronucleus and comet assays. J. Appl. Toxicol. 2011, 31, 714–719. [Google Scholar] [CrossRef]
- Loh, D.S.; Er, H.M.; Chen, Y.S. Mutagenic and antimutagenic activities of aqueous and methanol extracts of Euphorbia hirta. J. Ethnopharmacol. 2009, 126, 406–414. [Google Scholar] [CrossRef]
- Munari, C.C.; Resende, F.A.; Alves, J.M.; de Sousa, J.P.; Bastos, J.K.; Tavares, D.C. Mutagenicity and antimutagenicity of Baccharis dracunculifolia extract in chromosomal aberration assays in Chinese hamster ovary cells. Planta Med. 2008, 74, 1363–1367. [Google Scholar] [CrossRef]
- Munari, C.C.; Alves, J.M.; Bastos, J.K.; Tavares, D.C. Evaluation of the genotoxic and antigenotoxic potential of Baccharis dracunculifolia extract on V79 cells by the comet assay. J. Appl. Toxicol. 2010, 30, 22–28. [Google Scholar] [CrossRef]
- Izuta, H.; Narahara, Y.; Shimazawa, M.; Mishima, S.; Kondo, S.; Hara, H. 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of bee products and their constituents determined by ESR. Biol. Pharm. Bull. 2009, 32, 1947–1951. [Google Scholar] [CrossRef]
- Zahin, M.; Aqil, F.; Ahmad, I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mut. Res. 2010, 703, 99–107. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mut. Res. 2000, 455, 29–60. [Google Scholar]
- Ajith, T.A.; Soja, M.A. Comparative study on the antimutagenicity of atorvastatin and lovastatin against directly acting mutagens. Cell Biol. Toxicol. 2006, 22, 269–274. [Google Scholar] [CrossRef]
- Sghaier, M.B.; Boubaker, J.; Neffati, A.; Limem, I.; Skandrani, I.; Bhouri, W.; Bouhlel, I.; Kilani, S.; Chekir-Ghedira, L.; Ghedira, K. Antimutagenic and antioxidant potentials of Teucrium ramosissimum essential oil. Chem. Biodivers. 2010, 7, 1754–1763. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, S.; He, J.; Liu, X.; Tang, Y.; Yuan, H.; He, L.; Lu, T.; Tu, B.; Wang, Y. Effects of subchronic exposure to benzo[a]pyrene (B[a]P) on learning and memory, and neurotransmitters in male Sprague-Dawley rat. Neurotoxicology 2011, 32, 188–198. [Google Scholar]
- Schwarz, D.; Kisselev, P.; Cascorbi, I.; Schunck, W.H.; Roots, I. Differential metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1 variants. Carcinogenesis 2001, 22, 453–459. [Google Scholar]
- Ross, J.; Nelson, G.; Kligerman, A.; Erexson, G.; Bryant, M.; Earley, K.; Gupta, R.; Nesnow, S. Formation and persistence of novel benzo[a]pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res. 1990, 50, 5088–5094. [Google Scholar]
- Chakravarti, D.; Venugopal, D.; Mailander, P.C.; Meza, J.L.; Higginbotham, S.; Cavalieri, E.L.; Rogan, E.G. The role of polycyclic aromatic hydrocarbon-DNA adducts in inducing mutations in mouse skin. Mut. Res. 1990, 649, 161–178. [Google Scholar]
- Chen, L.; Devanesan, P.D.; Higginbotham, S.; Ariese, F.; Jankowiak, R.; Small, G.J.; Rogan, E.G.; Cavalieri, E.L. Expanded analysis of benzo[a]pyrene-DNA adducts formed in vitro and in mouse skin: Their significance in tumor initiation. Chem. Res. Toxicol. 1996, 9, 897–903. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Cao, P.; Cai, J.; Gupta, R.C. Effect of green tea catechins and hydrolyzable tannins on benzo[a]pyrene-induced DNA adducts and structure-activity relationship. Chem. Res. Toxicol. 2010, 23, 771–777. [Google Scholar] [CrossRef]
- Wang, J.; Ogata, M.; Hirai, H.; Kawagishi, H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2011, 314, 164–169. [Google Scholar] [CrossRef]
- Mirzayans, R.; Liuzzi, M.; Paterson, M.G. Methyl methanesulfonate induced DNA damage and its repair in culture human fibroblasts: normal rates of induction and removal of alkali - labile sites in Xeroderma pigmentosum (group A) cells. Carcinogenesis 1988, 12, 2257–2263. [Google Scholar]
- Resende, F.A.; Tomazella, I.M.; Barbosa, L.C.; Ponce, M.; Furtado, R.A.; Pereira, A.C.; Bastos, J.K.; Andrade e Silva, M.L.; Tavares, D.C. Effect of the dibenzylbutyrolactonelignan (−)-hinokinin on doxorubicin and methyl methanesulfonate clastogenicity in V79 Chinese hamster lung fibroblasts. Mut. Res. 2010, 700, 62–66. [Google Scholar] [CrossRef]
- Quiles, J.L.; Huertas, J.R.; Battino, M.; Mataix, J.; Ramirez-Tortosa, M.C. Antioxidant nutrients and adriamycin toxicity. Toxicology 2002, 180, 79–95. [Google Scholar] [CrossRef]
- Pan, M.H.; Ghai, G.; Ho, C.T. Food bioactives, apoptosis and cancer. Mol. Nutr. Food Res. 2008, 52, 43–52. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mut. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Bernstein, L.; Kaldor, J.; Mccann, J.; Pike, M.C. An empirical approach to the statistical analysis of mutagenesis data from the Salmonella test. Mut. Res. 1982, 97, 267–281. [Google Scholar] [CrossRef]
- Santos, F.V.; Colus, I.M.S.; Silva, M.A.; Vilegas, W.; Varanda, E.A. Assessment of DNA damage induced by extracts and fractions of Strychnos pseudoquina, a Brazilian medicinal plant with antiulcerogenic activity. Food Chem. Toxicol. 2006, 44, 1585–1589. [Google Scholar] [CrossRef]
- Lira, W.M.; dos Santos, F.V.; Sannomiya, M.; Rodrigues, C.M.; Vilegas, W.; Varanda, E.A. Modulatory effect of Byrsonima basiloba extracts on the mutagenicity of certain direct and indirect-acting mutagens in Salmonella typhimurium assays. J. Med. Food 2008, 11, 111–119. [Google Scholar] [CrossRef]
- Neigi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Vargas, V.M.; Mota, V.E.; Henriques, J.A. Mutagenic activity detected by the Ames test in river water under the influence of petrochemical industries. Mut. Res. 1993, 319, 31–45. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Resende, F.A.; Munari, C.C.; De Azevedo Bentes Monteiro Neto, M.; Tavares, D.C.; Bastos, J.K.; Da Silva Filho, A.A.; Varanda, E.A. Comparative Studies of the (Anti) Mutagenicity of Baccharis dracunculifolia and Artepillin C by the Bacterial Reverse Mutation Test. Molecules 2012, 17, 2335-2350. https://doi.org/10.3390/molecules17032335
Resende FA, Munari CC, De Azevedo Bentes Monteiro Neto M, Tavares DC, Bastos JK, Da Silva Filho AA, Varanda EA. Comparative Studies of the (Anti) Mutagenicity of Baccharis dracunculifolia and Artepillin C by the Bacterial Reverse Mutation Test. Molecules. 2012; 17(3):2335-2350. https://doi.org/10.3390/molecules17032335
Chicago/Turabian StyleResende, Flávia Aparecida, Carla Carolina Munari, Moacir De Azevedo Bentes Monteiro Neto, Denise Crispim Tavares, Jairo Kenupp Bastos, Ademar Alves Da Silva Filho, and Eliana Aparecida Varanda. 2012. "Comparative Studies of the (Anti) Mutagenicity of Baccharis dracunculifolia and Artepillin C by the Bacterial Reverse Mutation Test" Molecules 17, no. 3: 2335-2350. https://doi.org/10.3390/molecules17032335




