2,3,5,4′- Tetrahydroxystilbene-2-O-β-D-glycoside Biosynthesis by Suspension Cells Cultures of Polygonum multiflorum Thunb and Production Enhancement by Methyl Jasmonate and Salicylic Acid
Abstract
:Abbreviations
| 6-BA | 6-benzylaminopurine 2,4-D; 2,4-dichlorophenoxyacetic acid |
| NAA | α-naphtalene acetic acid |
| KT | Kinetin |
| HPLC | high performance liquid chromatography |
| THSG | 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glycoside |
| MeJA | Methyl jasmonate |
| SA | salicylic acid |
| DW | dry weight |
1. Introduction
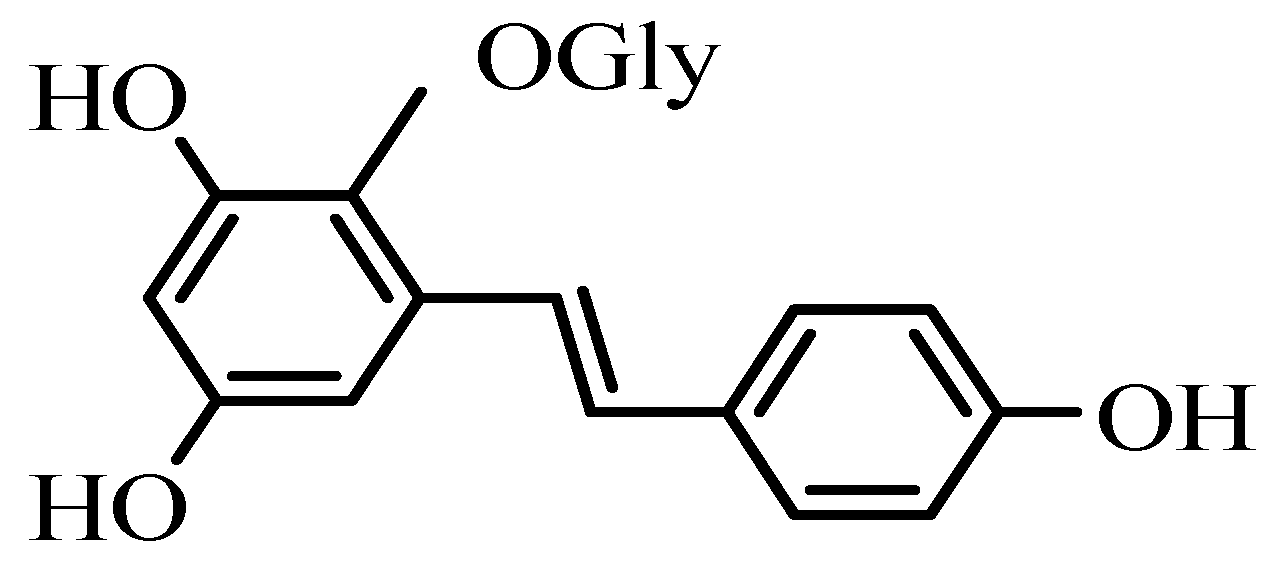
2. Results and Discussion
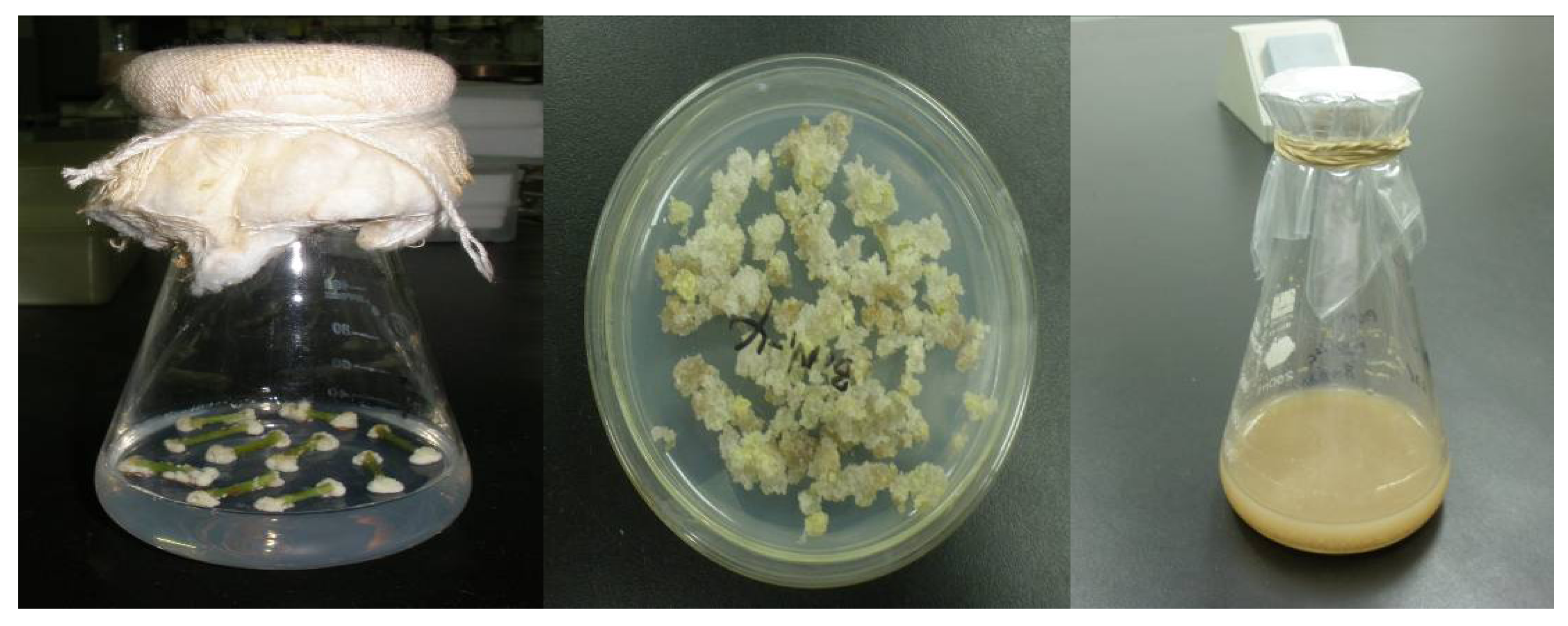
2.1. Friable Calli Induction and Suspension Cells Culture Establishment
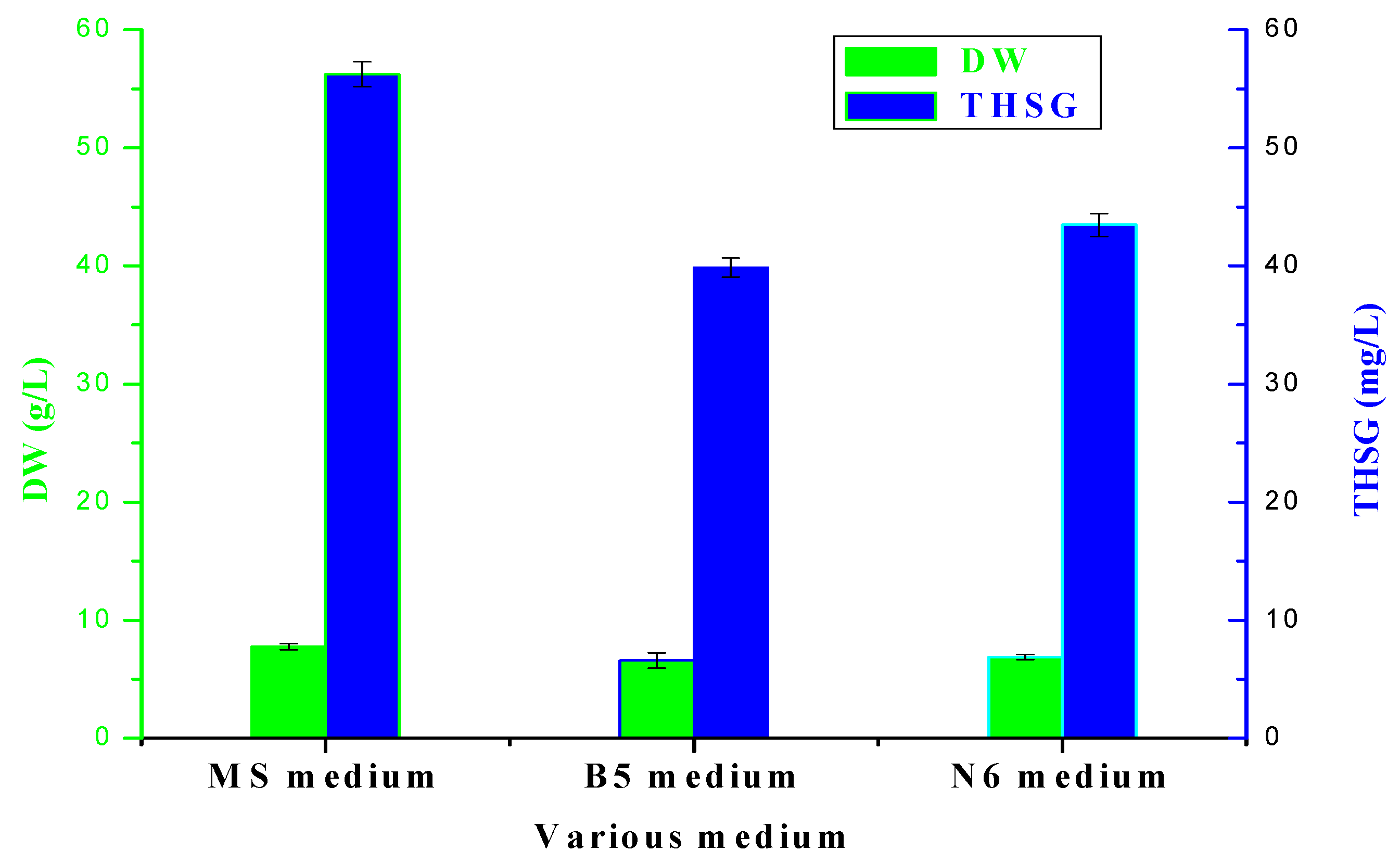
2.2. Cell Growth and THSG Production on MS, B5 and N6 Medium
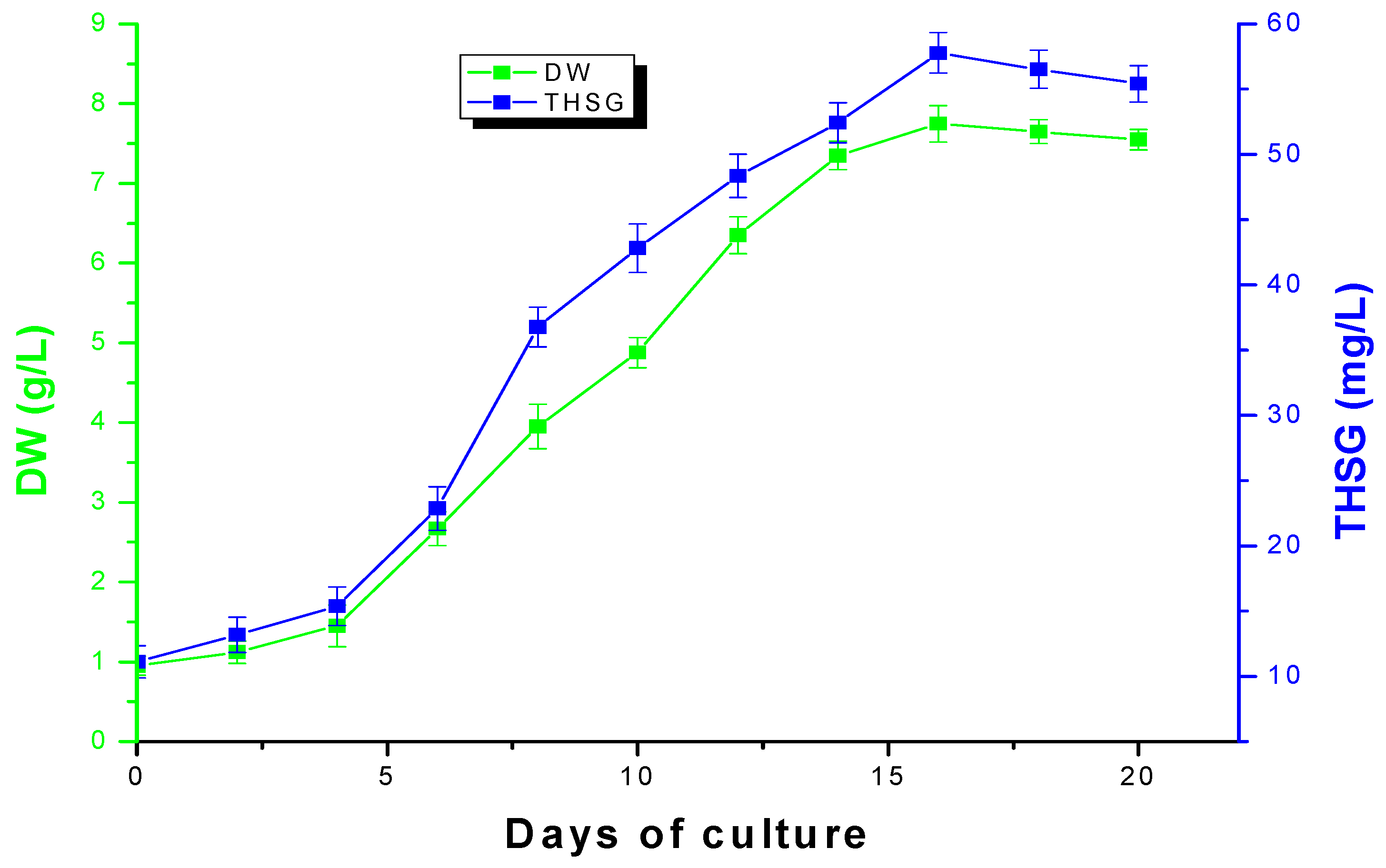
2.3. Time Course of Suspension Cells Cultures of Polygonum multiflorum Thunb
2.4. Different Concentrations of MeJA and SA on Cells DW and THSG Content in Suspension Cells

3. Experimental
3.1. Calli Induction and Culture
3.2. Elicitation of Suspension Cells by MeJA and SA
3.3. THSG Extraction and HPLC Analysis
4. Conclusions
Acknowledgements
References and Notes
- Zhang, Y.Z.; Shen, J.F.; Xu, J.Y.; Xiao, J.H.; Wang, J.L. Inhibitory effects of 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside on experimental inflammation and cyclo- oxygenase 2 activity. J. Asian Nat. Prod. Res. 2007, 9, 355–363. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhao, L.B.; Han, T.Z.; Wang, J.L. Protective effects of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur. J. Pharmacol. 2008, 578, 339–348. [Google Scholar] [CrossRef]
- Zeng, C.; Xiao, J.H.; Chang, M.J.; Wang, J.L. Beneficial effects of THSG on acetic acid-induced experimental colitis: Involvement of upregulation of PPAR-γ and inhibition of the Nf-Κb inflammatory pathway. Molecules 2011, 16, 8552–8568. [Google Scholar] [CrossRef]
- Lv, L.S.; Gu, X.H.; Tang, J.; Ho, C.T.; Tang, J. Stilbene glycoside from the roots of Polygonum multiflorum Thunb and their in vitro antioxidant activities. J. Food Lipids 2006, 13, 131–144. [Google Scholar] [CrossRef]
- Lv, L.S.; Gu, X.H.; Tang, J.; Ho, C.T. Antioxidant activity of stilbene glycoside from Polygonum multiflorum Thunb in vivo. Food Chem. 2007, 104, 1678–1681. [Google Scholar] [CrossRef]
- Liu, Q.L.; Xiao, J.H.; Ma, R.; Ban, Y.; Wang, J.L. Effect of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-d-glucoside on lipoprotein oxidation and proliferation of coronary arterial smooth cells. J.Asian Nat. Prod. Res. 2007, 9, 689–697. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Huang, G.H.; Ho, Y.L.; Hsieh, P.C.; Chung, H.P.; Chou, F.I.; Chang, Y.S. Influence of gamma irradiation on microbial load and antioxidative characteristics of Polygoni Multiflori Radix. Process Biochem. 2011, 46, 777–782. [Google Scholar] [CrossRef]
- Um, M.Y.; Choi, W.H.; Aan, J.Y.; Kim, S.R.; Ha, T.Y. Protective effect of Polygonum multiflorum Thunb on amyloid β-peptide 25-35 induced cognitive deficits in mice. J. Ethnopharmacol. 2006, 104, 144–148. [Google Scholar] [CrossRef]
- Wang, R.; Tang, Y.; Feng, B.; Ye, C.; Fang, L.; Zhang, L.; Li, L. Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience 2007, 149, 739–746. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.F.; Rosen, R.T.; Ho, C.T. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J. Agric. Food Chem. 1999, 47, 2226–2228. [Google Scholar]
- Luo, A.X.; Fan, Y.J.; Luo, A.S. In vitro free radicals scavenging activities of polysaccharide from Polygonum multiflorum Thunb. J. Med. Plants Res. 2011, 5, 966–972. [Google Scholar]
- Guan, S.Y.; Su, W.W.; Wang, N.; Li, P.B.; Wang, Y.G. A potent tyrosinase activator from Radix Polygoni Multiflori and its melanogenesis stimulatory effect in B16 melanoma cells. Phytother. Res. 2008, 22, 660–663. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Xu, J.M.; Long, M.H.; Tu, Z.M.; Yang, G.X.; He, G.Y. 2, 3, 5, 4’-tetrahydroxystilbene-2-O-β-D-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef]
- Park, H.J.; Zhang, N.N.; Park, D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011, 135, 369–375. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Khodakovskaya, M.V.; Glazunov, V.P.; Radchenko, S.V.; Zvereva, E.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yuan, Y.J.; Wu, J.C. Induction studies of methyl jasmonate and salicylic acid on taxane production in suspension cultures of Taxus chinensis var. mairei. Biochem. Eng. J. 2004, 19, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wu, J.C.; Yuan, Y.J. Salicylic acid-induced taxol production and isopentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinensis var. mairei. Cell Biol. Int. 2007, 31, 1179–1183. [Google Scholar] [CrossRef]
- Conceicão, L.F.R.; Ferreres, F.; Tavares, R.M.; Dias, A.C.P. Induction of phenolic compounds in Hypericum perforatum L. cells by Colletotrichum gloeosporioides elicitation. Phytochemistry 2006, 67, 149–155. [Google Scholar]
- Kang, S.M.; Jung, H.Y.; Kang, Y.M.; Yun, D.J.; Bahk, J.D.; Yang, J.K.; Choi, M.S. Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci. 2004, 166, 745–751. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J.; Filella, I. Methyl salicylate fumigation increases monoterpene emission rates. Biol. Plant. 2007, 51, 372–376. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds THSG are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shao, L.; Zhao, S.-J.; Cui, T.-B.; Liu, Z.-Y.; Zhao, W. 2,3,5,4′- Tetrahydroxystilbene-2-O-β-D-glycoside Biosynthesis by Suspension Cells Cultures of Polygonum multiflorum Thunb and Production Enhancement by Methyl Jasmonate and Salicylic Acid. Molecules 2012, 17, 2240-2247. https://doi.org/10.3390/molecules17022240
Shao L, Zhao S-J, Cui T-B, Liu Z-Y, Zhao W. 2,3,5,4′- Tetrahydroxystilbene-2-O-β-D-glycoside Biosynthesis by Suspension Cells Cultures of Polygonum multiflorum Thunb and Production Enhancement by Methyl Jasmonate and Salicylic Acid. Molecules. 2012; 17(2):2240-2247. https://doi.org/10.3390/molecules17022240
Chicago/Turabian StyleShao, Li, Shu-Jin Zhao, Tang-Bing Cui, Zhong-Yu Liu, and Wei Zhao. 2012. "2,3,5,4′- Tetrahydroxystilbene-2-O-β-D-glycoside Biosynthesis by Suspension Cells Cultures of Polygonum multiflorum Thunb and Production Enhancement by Methyl Jasmonate and Salicylic Acid" Molecules 17, no. 2: 2240-2247. https://doi.org/10.3390/molecules17022240



