A New Bromoallene-Producing Chemical Type of the Red Alga Laurencia nangii Masuda
Abstract
:1. Introduction
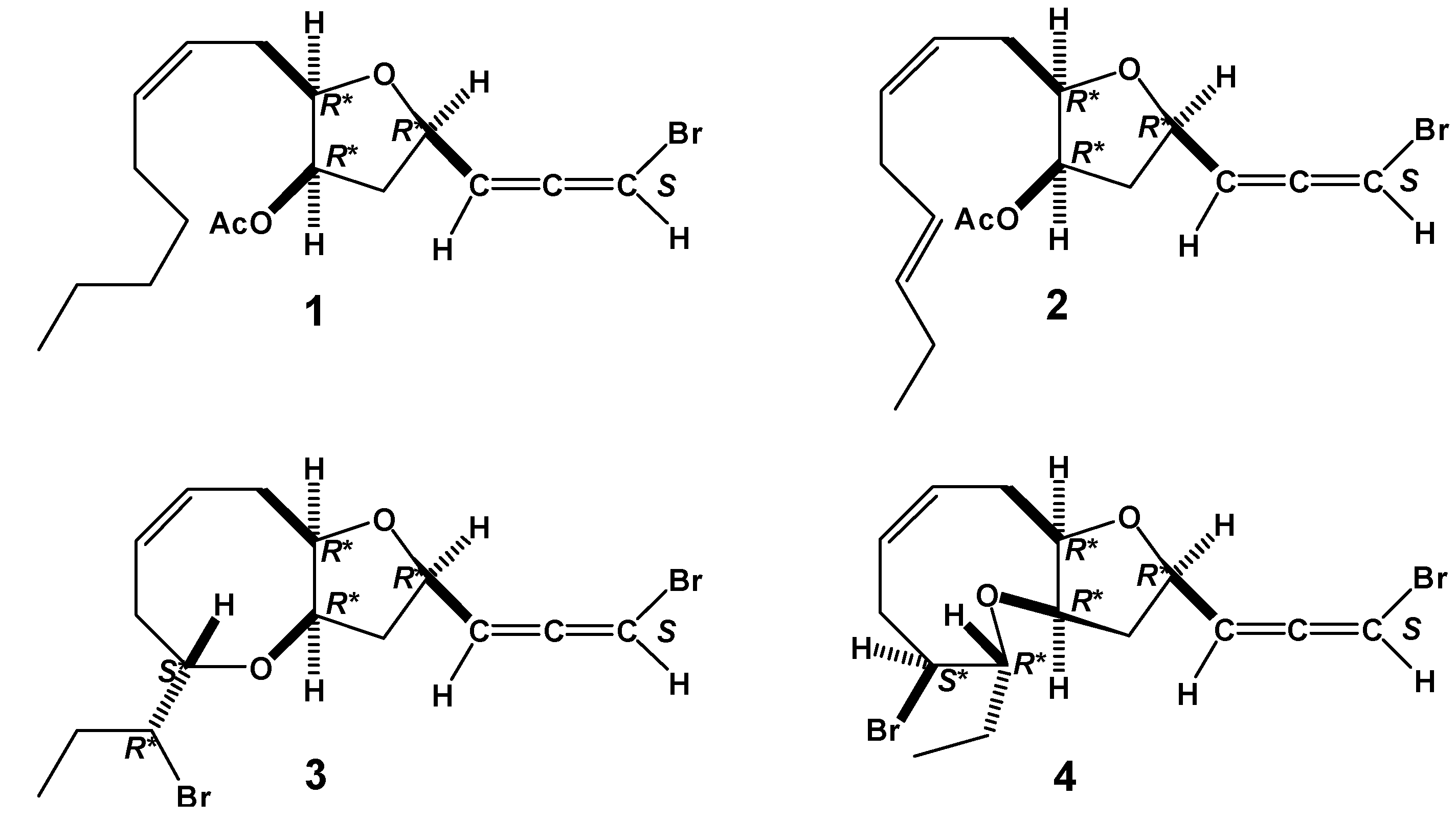
2. Results and Discussion


3. Experimental
3.1. General
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Dihydroitomanallene B (1)
| C | 1 | 2 | ||
|---|---|---|---|---|
| 13C | 1H ( J in Hz) | 13C | 1H ( J in Hz) | |
| 1 | 73.5 | 5.66 (dd, J = 5.8, 2.0 Hz, 1H) | 73.6 | 5.67 (dd, J = 5.8, 2.0 Hz, 1H) |
| 2 | 201.5 | - | 202.5 | - |
| 3 | 102.6 | 5.32 (dd, J = 5.8, 5.8 Hz, 1H) | 102.6 | 5.31 (dd, J = 5.8, 5.8 Hz, 1H) |
| 4 | 74.0 | 4.16 (dddd, J = 7.3, 5.8, 5.4, 2.0, 1H) | 74.0 | 4.15 (dddd, J = 7.3, 5.8, 5.4, 2.0, 1H) |
| 5 | 38.9 | 1.92 (ddd, J = 13.7, 5.4, 1.5 Hz, 1H) | 38.9 | 1.90 (ddd, J = 13.7, 5.4, 1.5 Hz, 1H) |
| 1.67 (m, 1H) | 1.65 (m, 1H) | |||
| 6 | 73.9 | 5.08 (m, 1H) | 73.9 | 5.04 (m, 1H) |
| 7 | 81.8 | 3.47 (ddd, J = 7.3, 7.3, 3.4 Hz, 1H) | 81.6 | 3.44 (ddd, J = 7.3, 7.3, 3.4 Hz, 1H) |
| 8 | 27.5 | 2.54 (ddd, J = 13.7, 7.3, 3.4 Hz, 1H) | 27.3 | 2.51 (ddd, J = 13.7, 7.3, 3.4 Hz, 1H) |
| 2.46 (ddd, J = 13.7, 7.3, 3.4 Hz, 1H) | 2.43 (ddd, J = 13.7, 7.3, 3.4 Hz, 1H) | |||
| 9 | 124.9 | 5.49 (m, 1H) | 125.5 | 5.50 (m, 1H) |
| 10 | 132.1 | 5.49 (m, 1H) | 130.0 | 5.54 (m, 1H) |
| 11 | 27.4 | 2.02 (m, 2H) | 30.5 | 2.75 (br t, J = 6.8 Hz, 2H) |
| 12 | 29.4 | 1.30 (m, 2H) | 127.0 | 5.39 (m, 1H) |
| 13 | 31.5 | 1.22 (m, 2H) | 132.4 | 5.45 (m, 1H) |
| 14 | 22.7 | 1.24 (m, 2H) | 25.6 | 1.94 (m, 2H) |
| 15 | 13.9 | 0.87 (t, J = 7.3 Hz, 3H) | 13.7 | 0.92 (t, J = 7.3 Hz, 3H) |
| OAc | 169.5 | - | 170.3 | - |
| 20.4 | 1.67 (s, 1H) | 20.4 | 1.66 (s, 1H) | |
4. Conclusions
Supplementary Materials
Acknowledgements
- Sample Availability: Not available.
References and Notes
- Suzuki, M.; Vairappan, C.S. Halogenated secondary metabolites from Japanese species of the red algal genus Laurencia (Rhodomelaceae, Ceramiales). Curr. Top. Phytochem. 2005, 1–34. [Google Scholar]
- Masuda, M.; Abe, T.; Suzuki, T.; Suzuki, M. Morphological and chemotaxanomic studies on Laurencia composita and L. okamurae (Ceramiales, Rhodophyta). Phycologia 1996, 35, 550–562. [Google Scholar]
- Masuda, M.; Abe, T.; Sato, S.; Suzuki, T.; Suzuki, M. Diversity of halogenated secondary metabolites in the red alga Laurencia nipponica (Rhodomelaceae, Ceramiales). J. Phycol. 1997, 33, 196–208. [Google Scholar]
- Abe, T.; Masuda, M.; Suzuki, T.; Suzuki, M. Chemical races in the red alga Laurencia nipponica (Rhodomelaceae, Ceramiales). Phycol. Res. 1999, 47, 87–95. [Google Scholar] [CrossRef]
- Tan, K.L.; Matsunaga, S.; Vairappan, C.S. Halogenated chamigranes of red alga Laurencia snackeyi (Weber-van Bosse) Masuda from Sulu-Sulawesi Sea. Biochem. Syst. Ecol. 2011, 39, 213–215. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Ang, M.Y.; Ong, C.Y.; Phang, S.M. Biologically active polybrominated indoles in red algae, Laurencia similis from the coastal waters of Sabah (Rhodomelaceae, Ceramiales). Malays. J. Sci. 2004, 23, 119–126. [Google Scholar]
- Vairappan, C.S.; Tan, K.L. C-15 Halogenated acetogenin with antibacterial activity against food pathogens. Malays. J. Sci. 2009, 28, 263–268. [Google Scholar]
- Vairappan, C.S.; Daitoh, M.; Suzuki, M.; Abe, T.; Masuda, M. Antibacterial halogenated metabolites from the Malaysian Laurencia sp. Phytochemistry 2001, 58, 291–297. [Google Scholar]
- Vairappan, C.S. Potent antibacterial activity of Malaysian red algal halogenated metabolites against human pathogenic bacteria. Biomol. Eng. 2003, 20, 255–259. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Phang, S.M. Morphology and halochamigrene metabolites in Spratly Island species of Laurencia majuscula (Rhodomelaceae, Ceramiales). Malays. J. Sci. 2005, 24, 21–27. [Google Scholar]
- Vairappan, C.S.; Sangeetha, P.A.; Tan, K.L.; Matsunaga, S. Role of secondary metabolites as defense chemicals against ice-ice disease bacteria in biofoulers at carrageenophyte farm. J. Appl. Phycol. 2010, 22, 305–311. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Ishii, T.; Tan, K.L.; Suzuki, M.; Zhan, Z. Antibacterial activities of halogenated secondary metabolites from Borneon Laurencia spp. Marine Drug. 2010, 8, 1743–1749. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Okino, T.; Ishii, T.; Abe, T.; Masuda, M. Antibacterial secondary metabolites from Malaysian Laurencia sp. Phytochemistry 2008, 69, 2490–2494. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, Y.; Mitome, Y.; Itoh, T.; Abe, T.; Masuda, M. Brominated metabolites from an Okinawan Laurencia intricate. Phytochemistry 2002, 60, 861–867. [Google Scholar]
- Suzuki, M.; Takahasi, Y.; Matsuo, Y.; Masuda, M. Pannosallene, a brominated C15 nonterpenoid from Laurencia pannosa. Phytochemistry 1996, 41, 1101–1103. [Google Scholar]
- Lowe, G. The absolute configuration of allenes. Chem. Commun. 1965, 411–413. [Google Scholar]
- Kurosawa, E.; Fukuzawa, A.; Irie, T. trans- and cis-Laurediol, unsaturated glycols from Laurencia nipponica Yamada. Tetrahedron Lett. 1972, 13, 2121–2124. [Google Scholar] [CrossRef]
- Fukuzawa, A.; Honma, T.; Takasugi, Y.; Murai, A. Biogenetic intermediates, (3E and 3Z,12Z)-laurediols and (3E and 3Z)-12,13-dihydrolaurediols isolated from Laurencia nipponica. Phytochemistry 1993, 32, 1435–1438. [Google Scholar]
- Kikuchi, H.; Suzuki, T.; Kurosawa, E.; Suzuki, M. The structure of notoryne, a halogenated C15 nonterpenoid with a novel carbon skeleton from the red alga Laurencia nipponica Yamada. Bull. Chem. Soc. Jpn. 1991, 64, 1763–1775. [Google Scholar] [CrossRef]
- Masuda, M.; Abe, T.; Kogame, K.; Kawaguchi, S.; Phang, S.M.; Daitoh, M.; Sakai, T.; Takahashi, Y.; Suzuki, M. Taxonomic notes on marine algae from Malaysia. VIII. Three species of Laurencia (Rhodophyceae). Bot. Mar. 2002, 45, 571–579. [Google Scholar]
- Masuda, M. A taxonomic study of the genus Laurencia (Ceramiales, Rhodophyta) from Vietnam. IV. Laurencia nangii sp. nov. Cryptogamie Algol. 1997, 18, 309–318. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kamada, T.; Vairappan, C.S. A New Bromoallene-Producing Chemical Type of the Red Alga Laurencia nangii Masuda. Molecules 2012, 17, 2119-2125. https://doi.org/10.3390/molecules17022119
Kamada T, Vairappan CS. A New Bromoallene-Producing Chemical Type of the Red Alga Laurencia nangii Masuda. Molecules. 2012; 17(2):2119-2125. https://doi.org/10.3390/molecules17022119
Chicago/Turabian StyleKamada, Takashi, and Charles Santhanaraju Vairappan. 2012. "A New Bromoallene-Producing Chemical Type of the Red Alga Laurencia nangii Masuda" Molecules 17, no. 2: 2119-2125. https://doi.org/10.3390/molecules17022119





