Enhancement of Mechanical and Thermal Properties of Oil Palm Empty Fruit Bunch Fiber Poly(butylene adipate-co-terephtalate) Biocomposites by Matrix Esterification Using Succinic Anhydride
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Spectroscopy
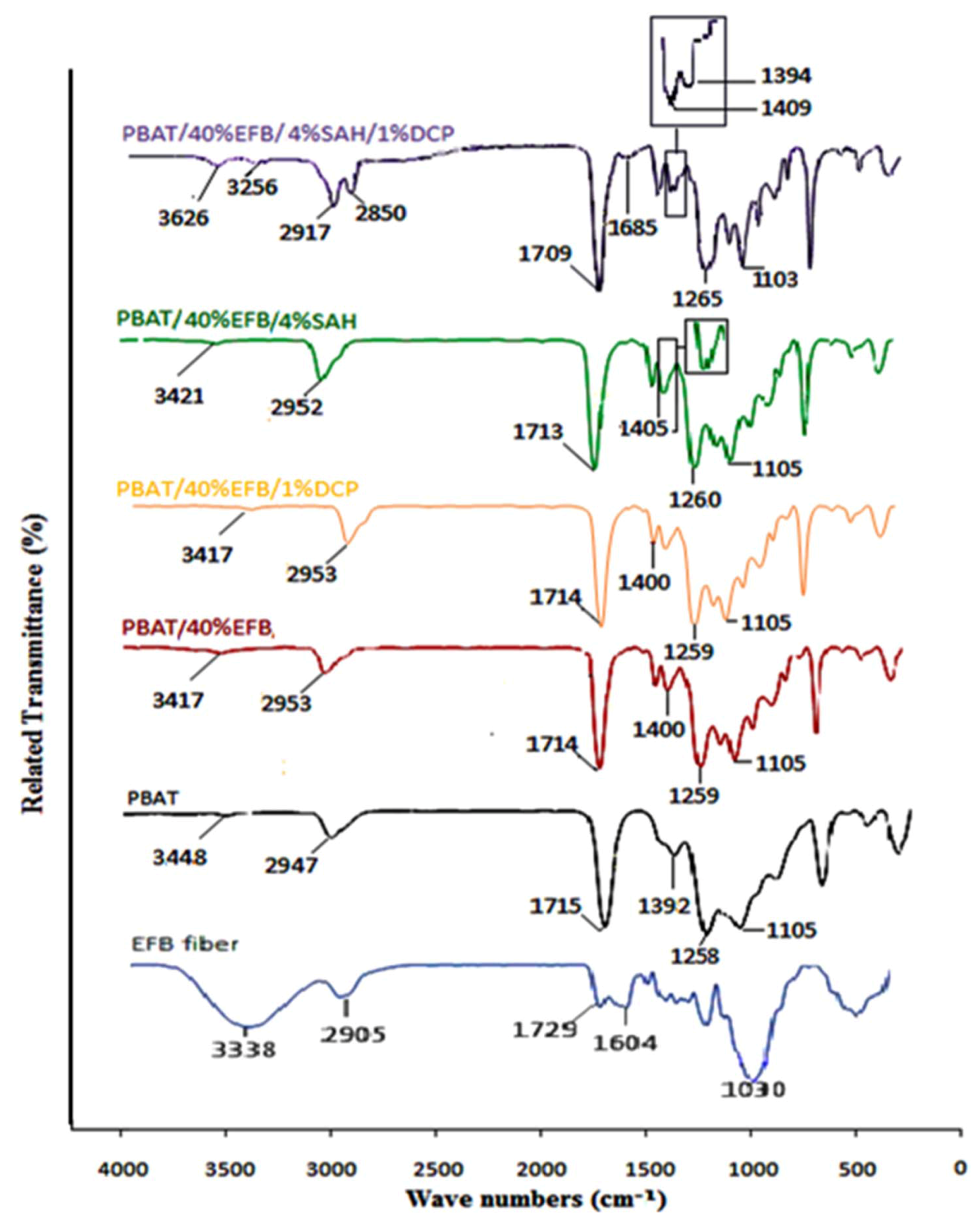
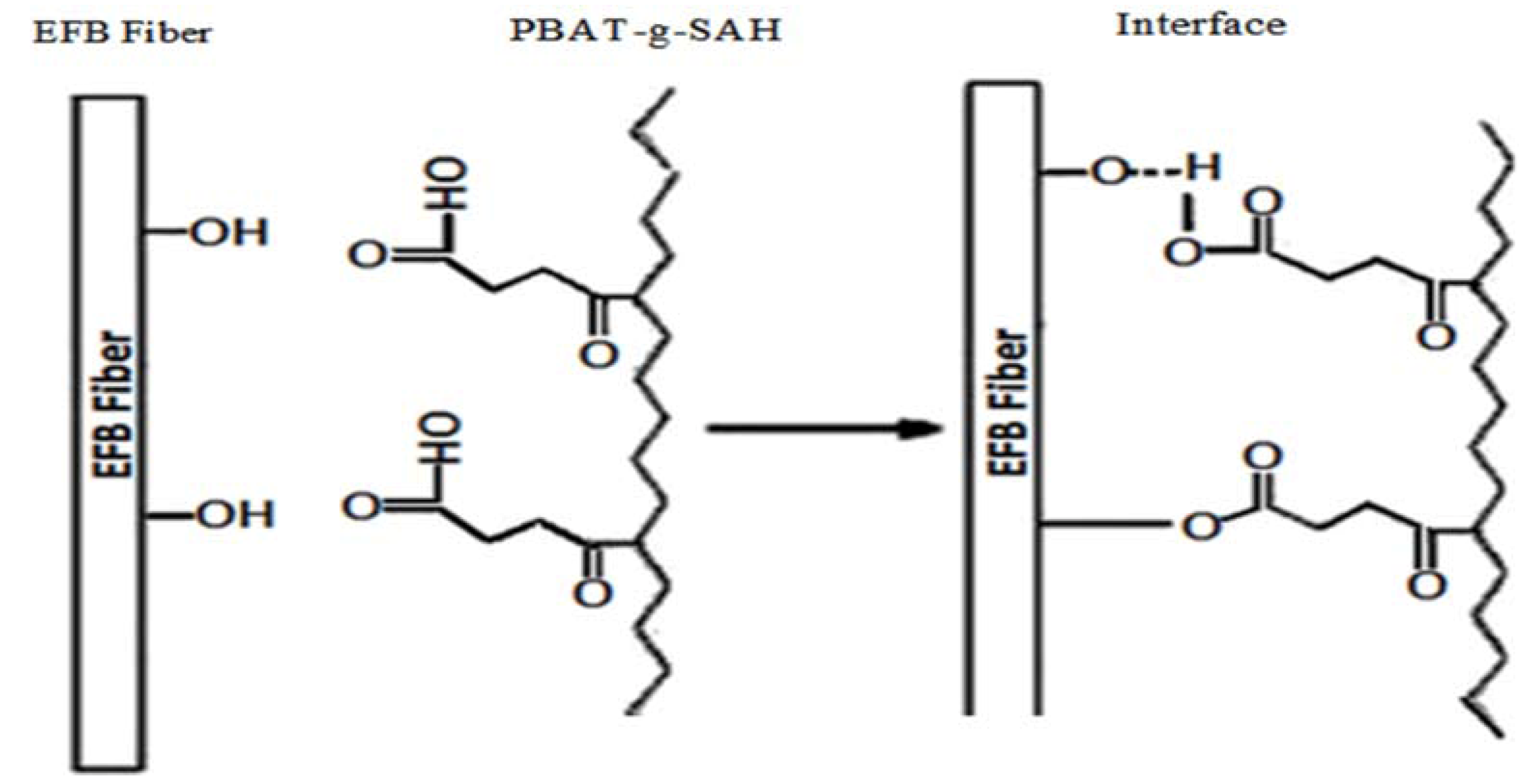
2.2. Effect of Fiber Loading on Tensile Properties of Biocomposites

2.3. Effects of Chemical Modification on Tensile Properties of PBAT/EFB Biocomposites
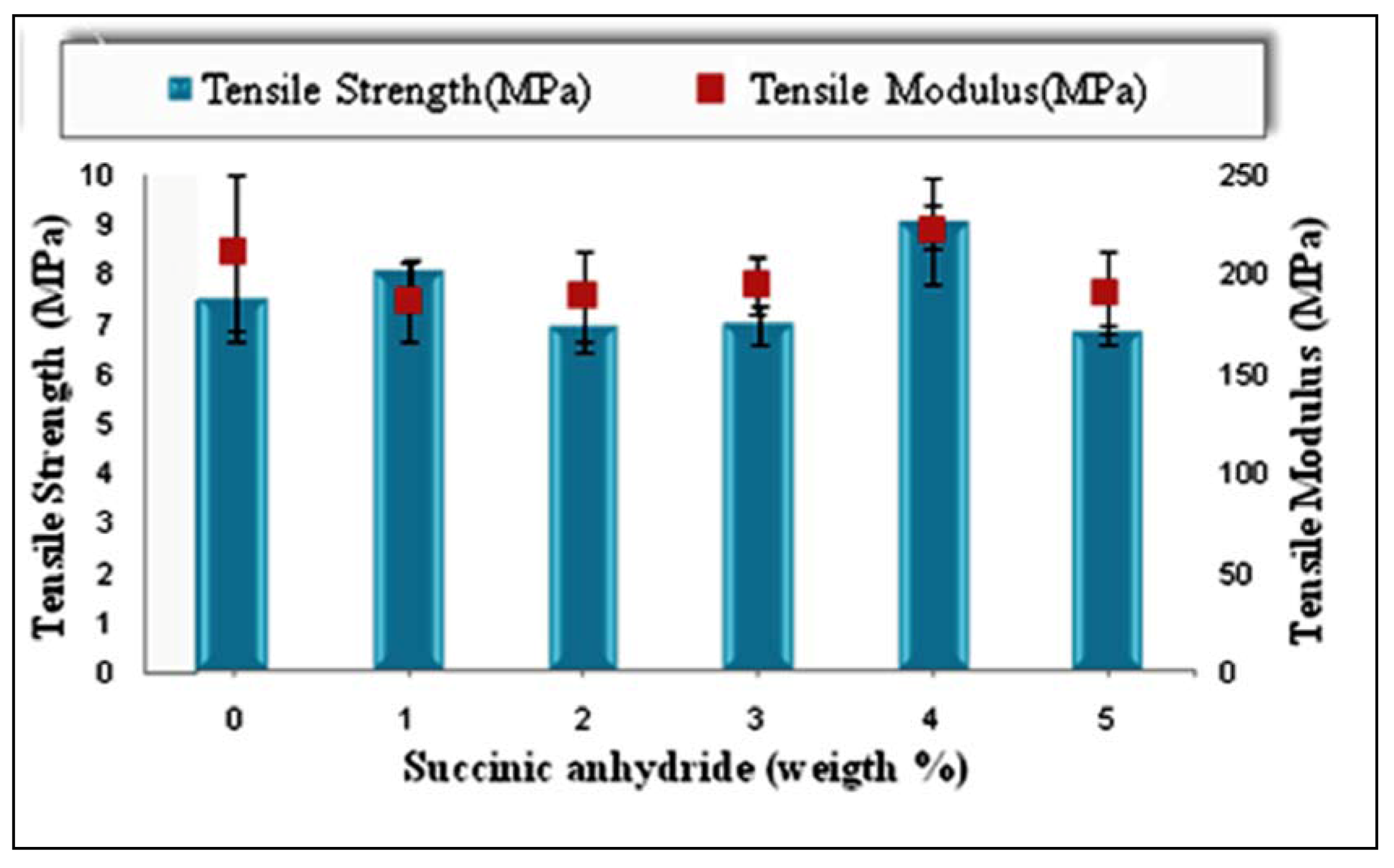
2.3.1. Effect of Type and Amount of Initiator on Tensile Properties of PBAT/EFB Biocomposites
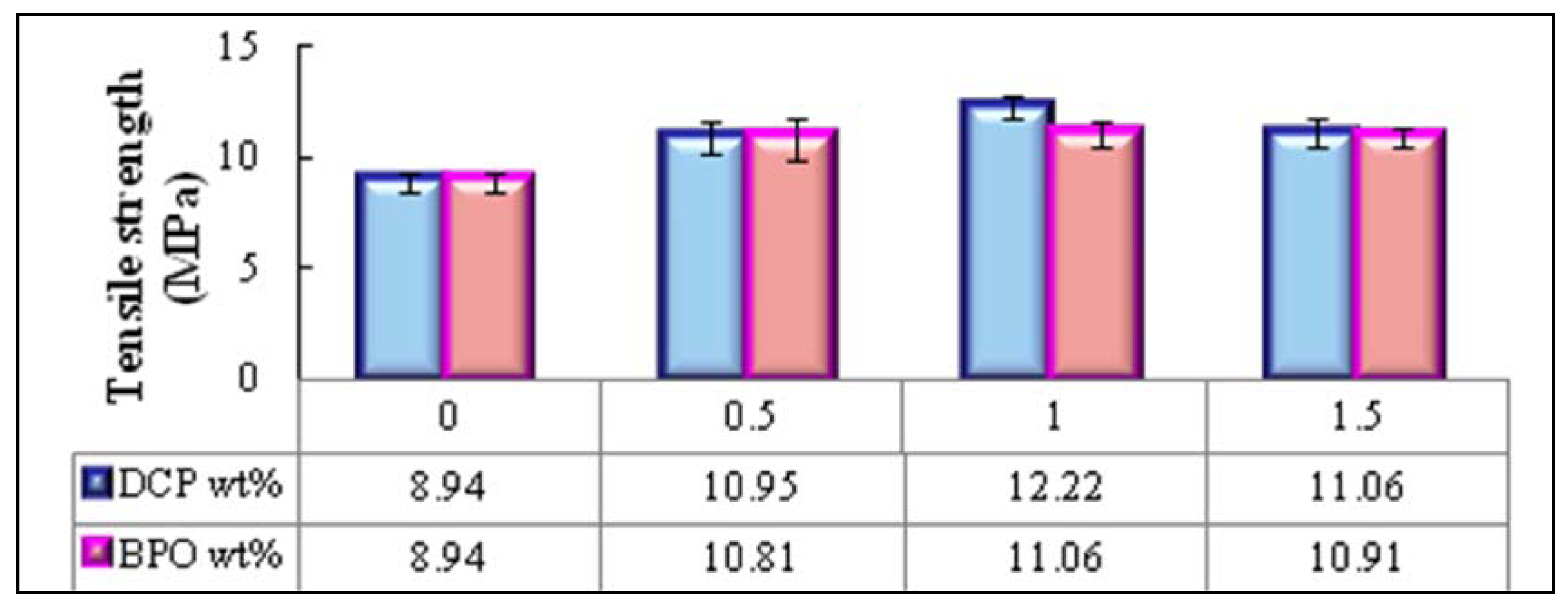
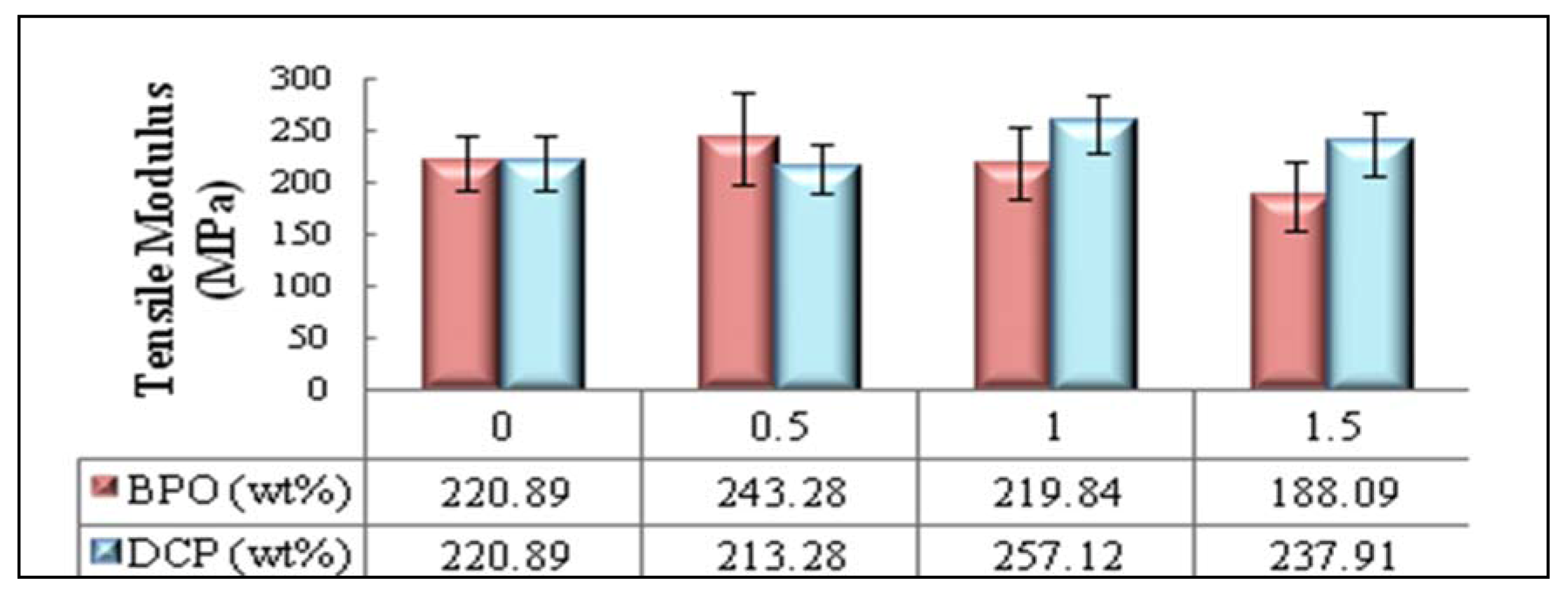
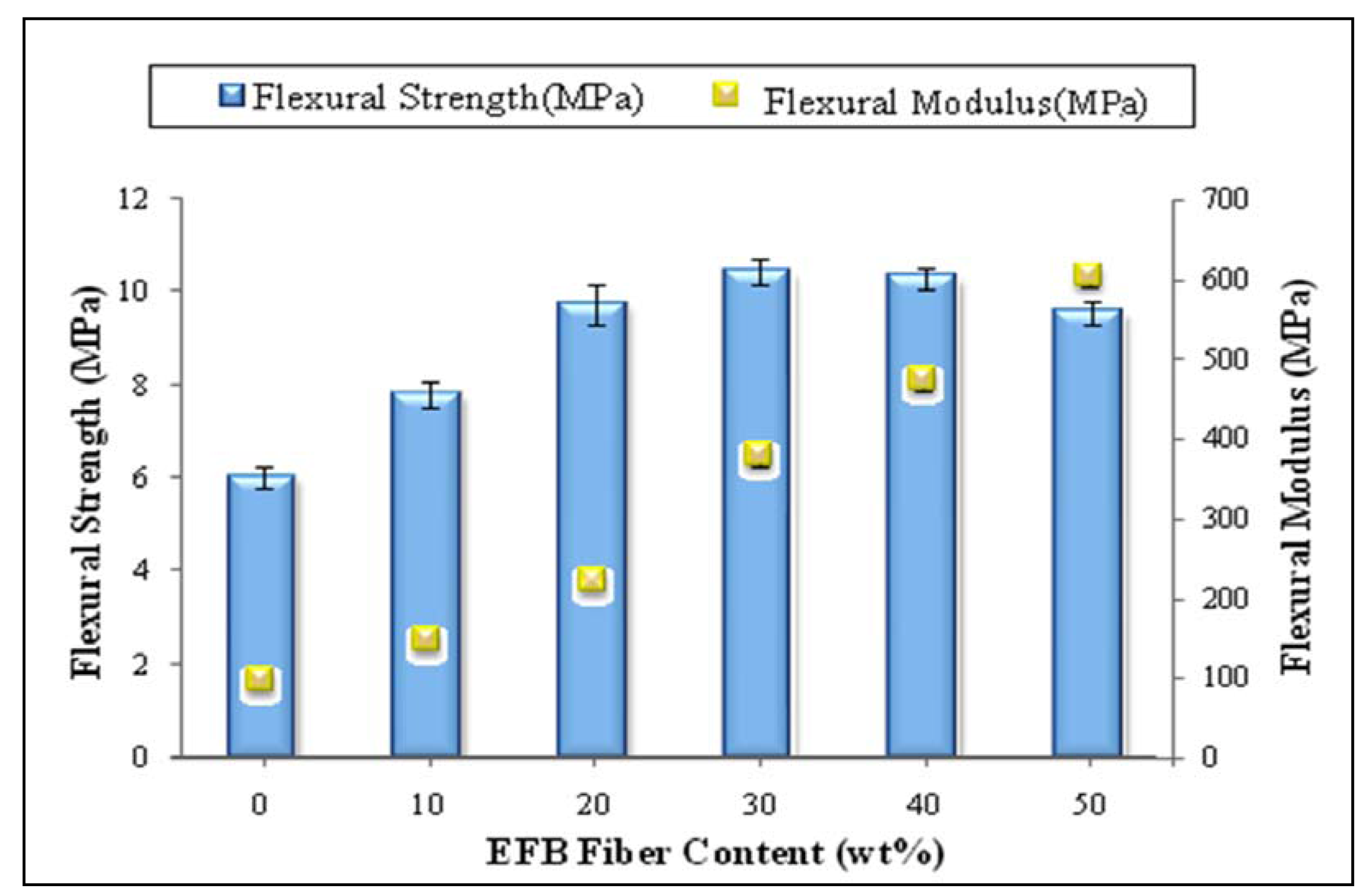
2.4. Effect of Chemical Modification Using SAH and Initiator on Flexural Properties of PBAT/EFB Biocomposites
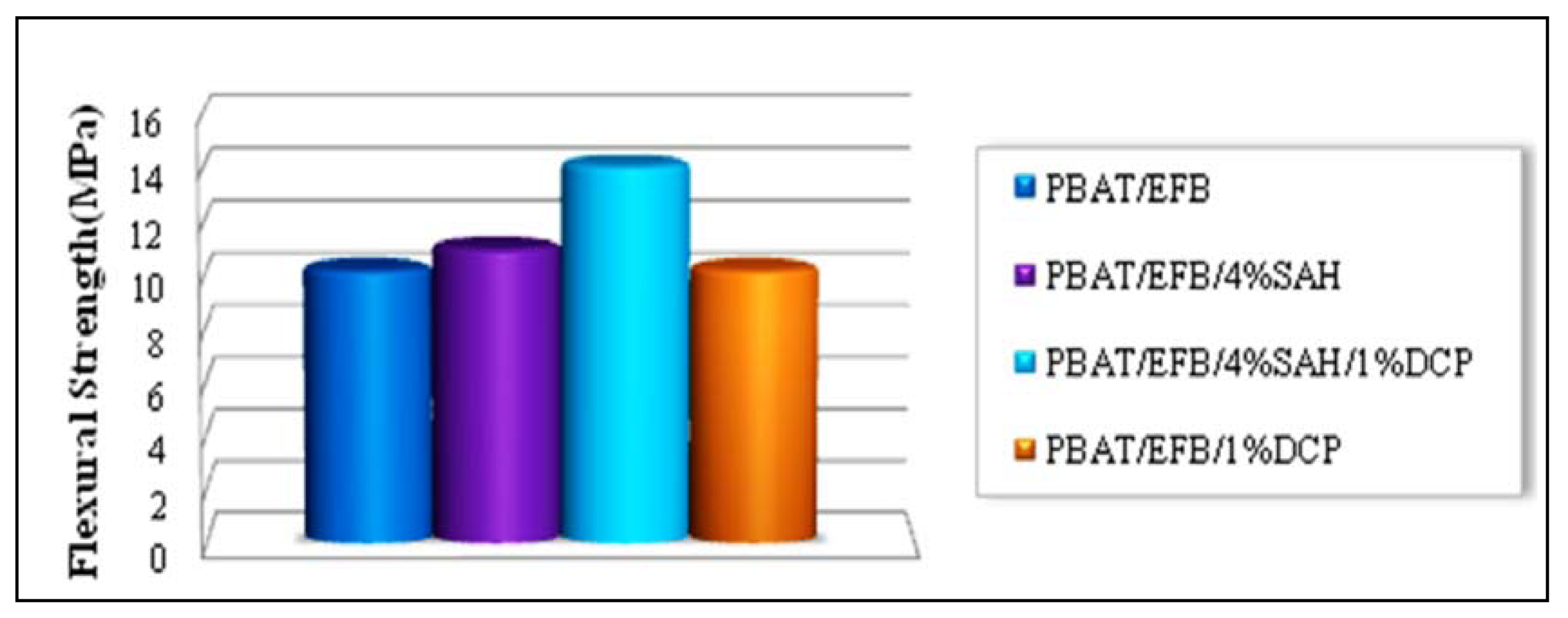
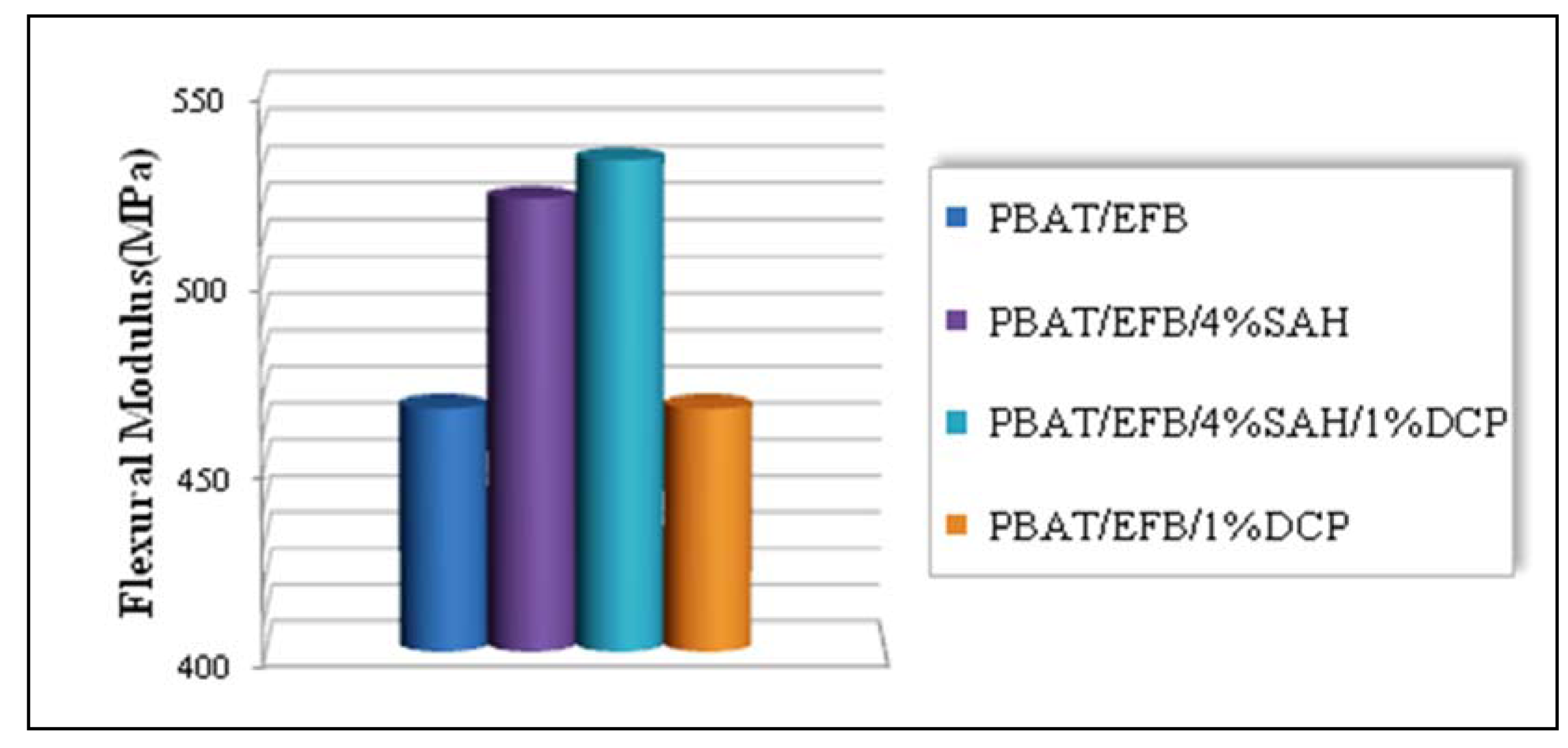
2.5. Effect of Fiber Loading and Chemical Modification on Thermal Properties of PBAT/EFB Biocomposites
2.5.1. Effect of Fiber Loading on the Thermal Properties of PBAT/EFB Fiber Composites
| Specimens | First peak (°C) | Second peak (°C) | Third peak (°C) |
|---|---|---|---|
| PBAT | □ | □ | 379.6 |
| PBAT/EFB10% | □ | □ | 386.1 |
| PBAT/EFB20% | ‒ | 305.5 | 383.3 |
| PBAT/EFB30% | 88.0 | 307.5 | 382.2 |
| PBAT/EFB40% | 88.8 | 307.0 | 380.9 |
| PBAT/EFB50% | 79.5 | 307.2 | 379.4 |
| Sample | Water content (%) | Initial degradation temperature (°C) * | T10% | T50% | T80% | Final degradation temperature (°C) | Ash content (%) ** |
|---|---|---|---|---|---|---|---|
| PBAT | □ | 311.5 | 352.8 | 381.3 | 398.4 | 413.3 | 4.4 |
| PBAT/EFB10% | □ | 280.4 | 339.7 | 382.8 | 399.2 | 431.7 | 6.7 |
| PBAT/EFB20% | 1.3 | 248.8 | 302.3 | 378.0 | 398.6 | 432.5 | 9.1 |
| PBAT/EFB30% | 2 | 210.2 | 276.7 | 373.6 | 401.5 | 440.2 | 11.2 |
| PBAT/EFB40% | 3.9 | 153.3 | 275.2 | 371.1 | 400.2 | 443.6 | 12.2 |
| PBAT/EFB50% | 3.4 | 148.2 | 233.3 | 360.2 | 398.8 | 447.7 | 17.7 |
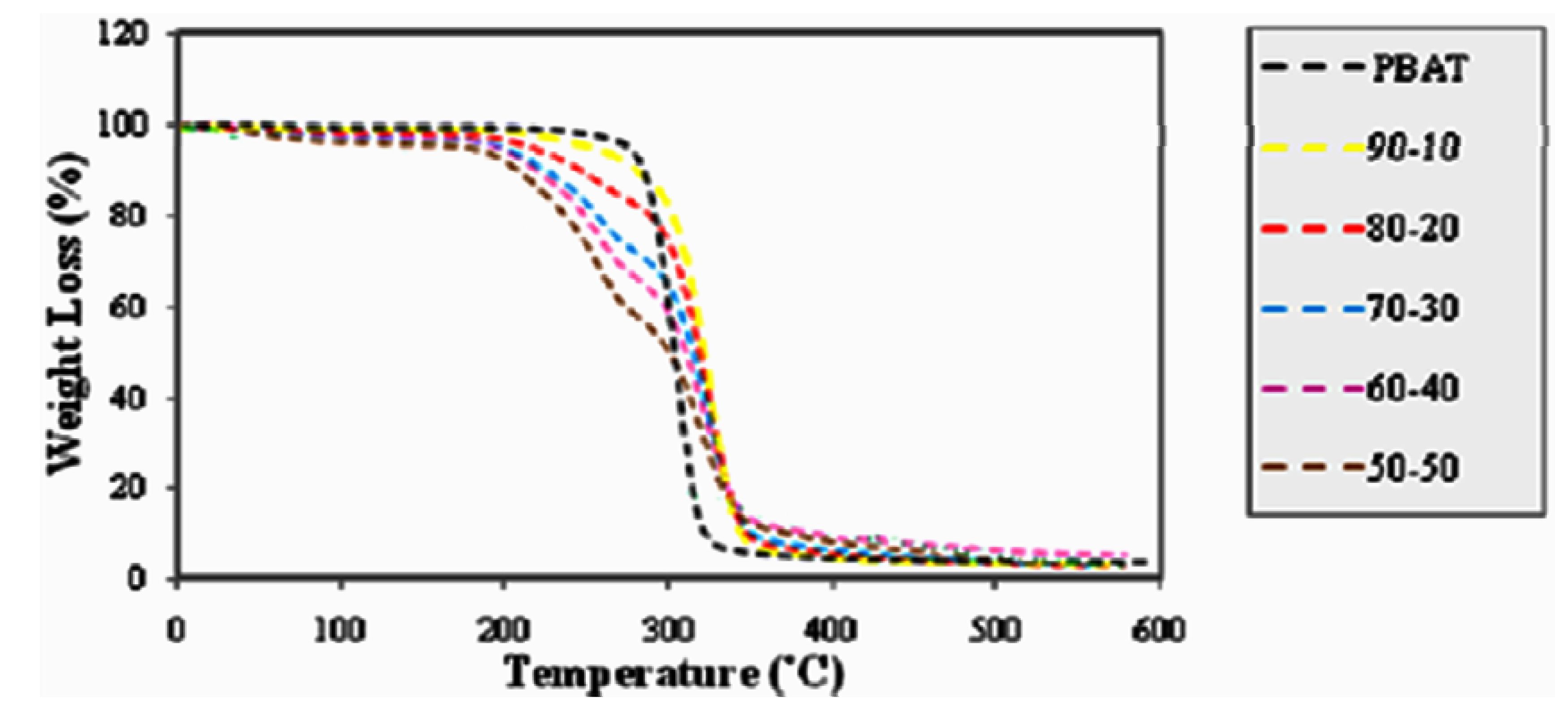
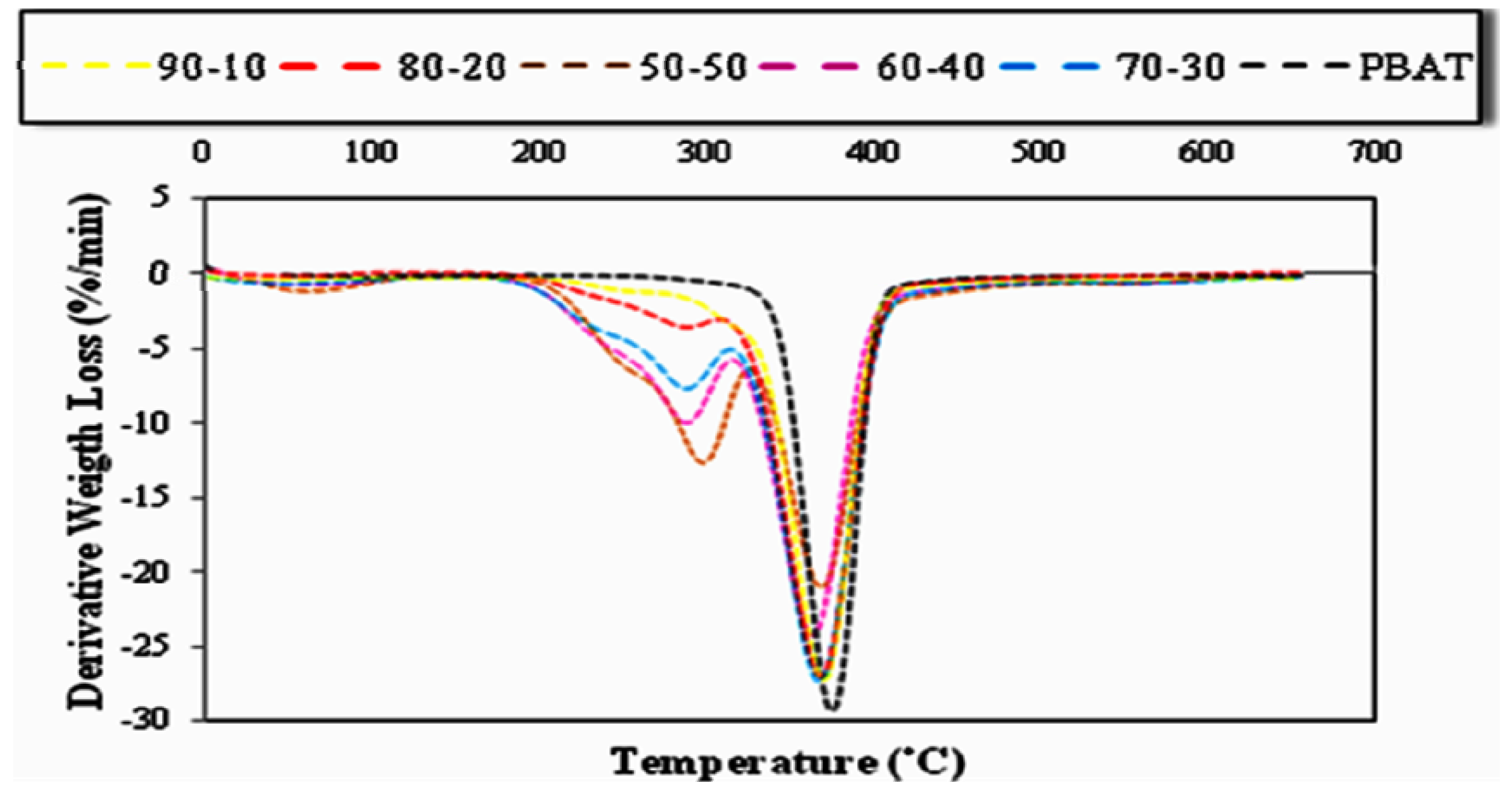
2.5.2. Effect of Chemical Modification on the Thermal Properties of PBAT/EFB Fiber Composites
| Sample | Water content (%) | Initial degradation temperature (°C) * | T10% | T50% | T80% | Final degradation temperature (°C) | Ash content (%) ** |
|---|---|---|---|---|---|---|---|
| Neat PBAT | □ | 311.5 | 352.8 | 381.3 | 398.4 | 413.3 | 4.4 |
| PBAT/40%EFB | 3.9 | 153.3 | 275.2 | 371.1 | 400.2 | 443.6 | 12.2 |
| PBAT/40%EFB/4%SAH | 1.9 | 114.7 | 268.7 | 373.7 | 445.9 | 445.5 | 13.4 |
| PBAT/40%EFB/4%SAH/1%DCP | 1.8 | 127.4 | 284.2 | 378.6 | 419.8 | 449.2 | 14.1 |
| Specimens | First peak (°C) | Second peak (°C) | Third peak (°C) | Fourth peak (°C) |
|---|---|---|---|---|
| PBAT | □ | □ | □ | 379.6 |
| PBAT/40%EFB | 88.8 | □ | 307.0 | 380.9 |
| PBAT/40%EFB/4%SAH | 76.7 | 247.5 | 309.7 | 380.4 |
| PBAT40%EFB/4%SAH1%/DCP | 76.2 | 272.0 | 333.6 | 390.0 |
2.6. Morphological Study of PBAT/EFB Biocomposite at 40% Fiber Loading

2.7. Morphological Study of PBAT/EFB Biocomposite after Cemical Modification
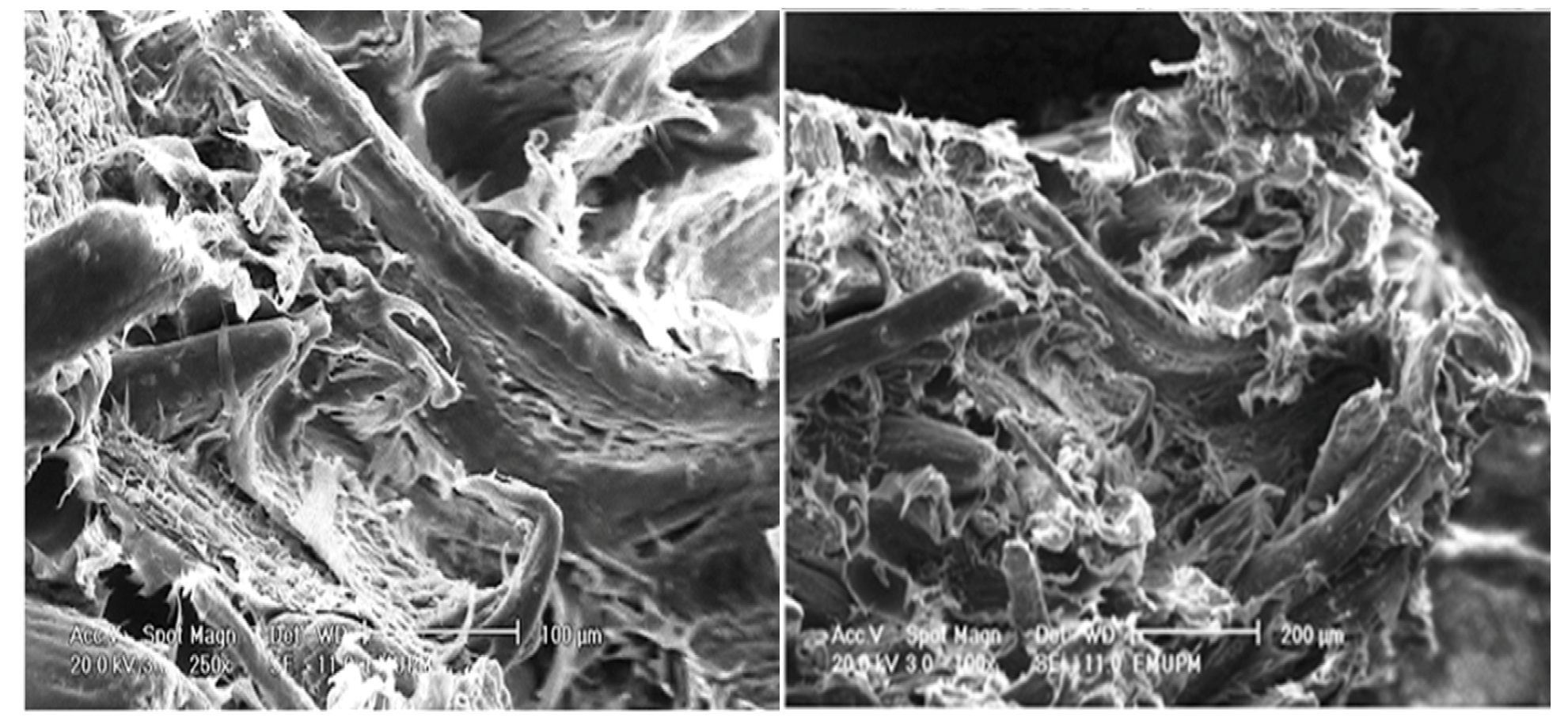
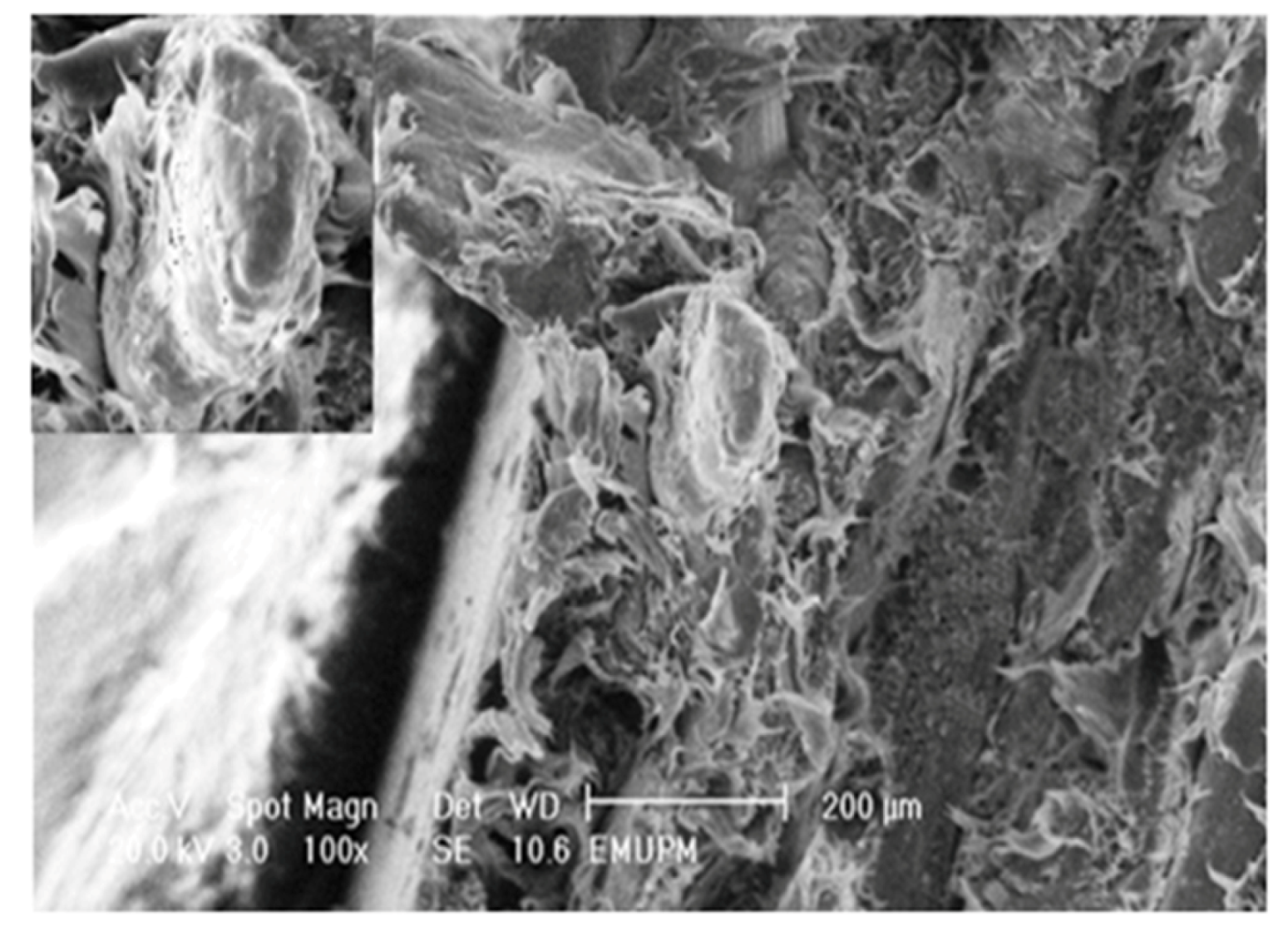
3. Experimental
3.1. Materials
3.2. Preparation of the Composite
3.3. FTIR Spectroscopy
3.4. Biocomposite Characterization
3.4.1. Mechanical Testing of Biocomposites
3.4.2. Thermal Behavior (Thermogravimetric Analysis, TGA)
3.4.3. Morphological Features
4. Conclusions
Acknowledgements
- Sample Availability: Samples of all compounds are available from the authors.
References and Notes
- Jean-Marie, R.; Ramani, N.; Philippe, D. Recent Advances in Reactive Extrusion Processing of Biodegradable Polymer-Based Compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Misra, M.; Drzal, L.T.; Robledo, D.; Freile-Pelegrin, Y. Preparation and characterization of biodegradable agar/poly (butylene adipate-co-terephthalate) composites. Polym. Eng. Sci. 2009, 49, 1117–1126. [Google Scholar] [CrossRef]
- Malkapuram, R.; Kumar, V.; Negi, Y.S. Recent Development in Natural Fiber Reinforced Polypropylene Composites. J. Reinf. Plast. Compos. 2009, 28, 1169–1189. [Google Scholar] [CrossRef]
- Kalam, A.; Berhan, M.N.; Ismail, H. Physical and mechanical characterizations of oil palm fruit bunch fiber filled polypropylene composites. J. Reinf. Plast. Compos. 2010, 29, 3173–3184. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Ahmad, S.N.A.; Yunus, W.M.Z.W.; Dahlan, K.Z.M. Effect of electron beam irradiation and poly(vinyl pyrrolidone) addition on mechanical properties of polycaprolactone with empty fruit bunch fibre (OPEFB) composite. EXPRESS Polym. Lett. 2009, 3, 226–234. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.T.; Uyama, U.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Chin, L.-S. Characterization of Natural Fibre Polymer Composites for Structural Application. Master Thesis, Universiti Teknologi Malaysia (UTM), Johor, Malaysia, 2008. [Google Scholar]
- Atikler, U. Preparation and Characterization of Polypropylene-Cellulose Composites. Master Thesis, Izmir Institute of Technology, Izmir, Turkey, 2004. [Google Scholar]
- Jayamol, G.; Sreekala, M.S.; Sabu, T. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Lu, J.Z.; Wu, Q.L.; McNabb, H.S. Chemical Coupling in Wood Fiber and Polymer Composites: A Review of Coupling Agents and Treatments. Wood Fiber Sci. 2000, 32, 88–104. [Google Scholar]
- Rozman, H.D.; Musa, L.; Abubakar, A. Rice husk–polyester composites: the effect of chemical modification of rice husk on the mechanical and dimensional stability properties. J. Appl. Polym. Sci. 2005, 97, 1237–1247. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J.; Wang, N. Preparation and properties of biodegradable poly(propylene carbonate)/thermoplastic dried starch composites. Carbohydr. Polym. 2008, 71, 229–234. [Google Scholar] [CrossRef]
- Lu, J.Z.; Wu, Q.; Negulescu, I.I. Wood-Fiber/High-Density-Polyethylene Composites: Coupling Agent Performance. J. Appl. Polym. Sci. 2005, 96, 93–102. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Zhao, A. Properties of biodegradable poly(propylene carbonate)/starch composites with succinic anhydride. Compos. Sci. Technol. 2006, 66, 2360–2366. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Yunus, W.M.Z.W.; Abu-Ilaiwi, F.A.F.; Rahman, M.Z.A.; Ahmad, M.B.; Dahlan, K.Z.M. Optimized condition for grafting reaction of poly(butyl acrylate) onto oil palm empty fruit bunch fibre. Polym. Int. 2003, 52, 1119–1124. [Google Scholar] [CrossRef]
- Li, J.; Li, H.-M. Functionalization of syndiotactic polystyrene with succinic anhydride in the presence of aluminum chloride. Eur. Polym. J. 2005, 41, 823–829. [Google Scholar] [CrossRef]
- Pandey, J.K. Degradability of Polymer Composites from Renewable Resources. Ph.D. Thesis, National Chemical Laboratory, Pune, India, 2004. [Google Scholar]
- Wu, C.-S. Antibacterial and static dissipating composites of poly(butylene adipate-co-terephthalate) and multi-walled carbon nanotubes. Carbon 2009, 47, 3091–3098. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Hadithon, K.A.; Abdan, K. Effect of Fiber Treatment on Mechanical Properties of Kenaf Fiber-Ecoflex Composites. J. Reinf. Plast. Compos. 2009, 30, 1029–1037. [Google Scholar]
- Mohanty, S.; Verma, K.; Nayak, S.; Sudhansu, S.K.; Tripathy, S. Influence of fiber treatment on the performance of sisal-polypropylene composites. J. Appl. Polym. Sci. 2004, 94, 1336–1345. [Google Scholar] [CrossRef]
- Rozman, H.D.; Lim, P.P.; Abusamah, A.; Kumar, R.N.; Ismail, H.; Ishak, Z.M. The Physical Properties of Oil Palm Empty Fruit Bunch (EFB) Composites Made from Various Thermoplastics. Int. J. Polym. Mater. 1999, 44, 179–195. [Google Scholar] [CrossRef]
- Rozman, H.D.; Ismail, H.; Jaffri, R.M.; Aminullah, A.; Mohd Ishak, Z.A. Mechanical Properties of Polyethylene-Oil Palm Empty Fruit Bunch Composites. Polym.-Plast. Technol. Eng. 1998, 37, 495–507. [Google Scholar]
- Abdul Khalil, H.P.S.; Poh, B.T.; Jawaid, M. The Effect of Soil Burial Degradation of Oil Palm Trunk Fiber-filled Recycled Polypropylene Composites. J. Reinf. Plast. Compos. 2010, 29, 1653–1663. [Google Scholar] [CrossRef]
- Sykacek, E.; Hrabalova, M.; Frech, H.; Mundigler, N. Extrusion of five biopolymers reinforced with increasing wood flour concentration on a production machine, injection moulding and mechanical performance. Compos. Part A-Appl. S. 2009, 40, 1272–1282. [Google Scholar]
- Kim, H.S.; Lee, B.H.; Choi, S.W.; Kim, S.; Kim, H.J. The effect of types of maleic anhydride-grafted polypropylene (MAPP) on the interfacial adhesion properties of bio-flour-filled polypropylene composites. Compos. Part A-Appl. S. 2007, 38, 1473–1482. [Google Scholar]
- Khalid, M.; Ali, S.; Abdullah, L.C.; Ratnam, C.T.; Thomas Choong, S.Y. Effect of MAPP as Coupling Agent on the Mechanical Properties of Palm Fiber Fruit Bunch and Cellulose Polypropylene Biocomposites. Int. J. Eng. Technol. 2006, 3, 79–84. [Google Scholar]
- Razavi Aghjeh, M.K.; Nazockdast, H.; Assempour, H. Parameters affecting the free-radical melt grafting of maleic anhydride onto linear low-density polyethylene in an internal mixer. J. Appl. Polym. Sci. 2006, 99, 141–149. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Nabar, Y.; Narayan, R.; Dubois, P. Novel High-Performance Talc/Poly[(butylene adipate)-co-terephthalate] Hybrid Materials. Macromol. Mater. Eng. 2008, 293, 310–320. [Google Scholar] [CrossRef]
- Khalid, M.; Ratnam, C.T.; Chuah, T.G.; Ali, S.; Choong, T.S.Y. Comparative study of polypropylene composites reinforced with oil palm empty fruit bunch fiber and oil palm derived cellulose. Mater. Design 2008, 29, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Verma, S.K.; Nayak, S.K. Dynamic mechanical and thermal properties of MAPE treated jute/HDPE composites. Compos. Sci. Technol. 2006, 66, 538–547. [Google Scholar]
- Abu Bakar, A. Mechanical, Thermal and Processing Properties of Oil Palm Empty Fruit Bunch-Filled Impact Modified Unplasticised Poly (Vinyl Chloride) Composites. Ph.D. Thesis, Universiti Teknologi Malaysia (UTM), Johor, Malaysia, 2006. [Google Scholar]
- Mohanty, S.; Nayak, S.K. Aromatic-Aliphatic Poly(butylene adipate-coterephthalate) Bionanocomposite: Influence of Organic Modification on Structure and Properties. Polym. Compos. 2010, 31, 1194–1204. [Google Scholar]
- Araújo, J.R.; Waldman, W.R.; De Paoli, M.A. Thermal properties of high density polyethylene composites with natural fibers: Coupling agent effect. Polym. Degrad. Stab. 2008, 93, 1770–1775. [Google Scholar] [CrossRef]
- Rahman, W.A.; Adenan, N.; Rasti, A.R.; Sulaiman, H. Effect of silane crosslinker on the thermal properties of rice straw/HDPE biocomposite. J. Appl. Polym. Sci. 2009, 9, 3041–3047. [Google Scholar]
- Ibrahim, N.A.; Yunus, W.M.Z.W.; Abu-Ilaiwi, F.A.F.; Rahman, M.Z.A.; Ahmad, M.B.; Dahlan, K.Z.M. Graft copolymerization of methyl methacrylate onto oil palm empty fruit bunch fiber using H2O2/Fe2 as an initiator. J. Appl. Polym. Sci. 2003, 89, 2233–2238. [Google Scholar] [CrossRef]
- Standard Test Method for Tensile Properties of Plastic; ASTM Standard D638-99; American Society for Testing and Materials: New York, NY, USA, 1 July 1999.
- Standard Test Method for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulation Materials; ASTM Standard D790-07; American Society for Testing and Materials: Philadelphia, PA, USA, 1997.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siyamak, S.; Ibrahim, N.A.; Abdolmohammadi, S.; Yunus, W.M.Z.B.W.; Rahman, M.Z.A. Enhancement of Mechanical and Thermal Properties of Oil Palm Empty Fruit Bunch Fiber Poly(butylene adipate-co-terephtalate) Biocomposites by Matrix Esterification Using Succinic Anhydride. Molecules 2012, 17, 1969-1991. https://doi.org/10.3390/molecules17021969
Siyamak S, Ibrahim NA, Abdolmohammadi S, Yunus WMZBW, Rahman MZA. Enhancement of Mechanical and Thermal Properties of Oil Palm Empty Fruit Bunch Fiber Poly(butylene adipate-co-terephtalate) Biocomposites by Matrix Esterification Using Succinic Anhydride. Molecules. 2012; 17(2):1969-1991. https://doi.org/10.3390/molecules17021969
Chicago/Turabian StyleSiyamak, Samira, Nor Azowa Ibrahim, Sanaz Abdolmohammadi, Wan Md Zin Bin Wan Yunus, and Mohamad Zaki AB Rahman. 2012. "Enhancement of Mechanical and Thermal Properties of Oil Palm Empty Fruit Bunch Fiber Poly(butylene adipate-co-terephtalate) Biocomposites by Matrix Esterification Using Succinic Anhydride" Molecules 17, no. 2: 1969-1991. https://doi.org/10.3390/molecules17021969




