The Use of Stable and Radioactive Sterol Tracers as a Tool to Investigate Cholesterol Degradation to Bile Acids in Humans in Vivo
Abstract
:Abbreviations
| CA | cholic acid |
| CDCA | chenodeoxycholic acid |
| CYP7A1 | cholesterol 7α-hydroxylase |
| CYP27A1 | sterol 27-hydroxylase |
| CTX | cerebrotendinous xanthomatosis |
| DCA | deoxycholic acid |
| FGF | fibroblast growth factor |
| FXR | farnesoid X receptor |
| LXR | liver X receptor |
| PPAR | peroxysomal proliferator-activated receptor |
| SHP | small heterodimer partner |
| SREBP | sterol regulatory element binding protein |
| UDCA | ursodeoxycholic acid |
1. Introduction
2. Biochemistry of Bile Acid Synthesis
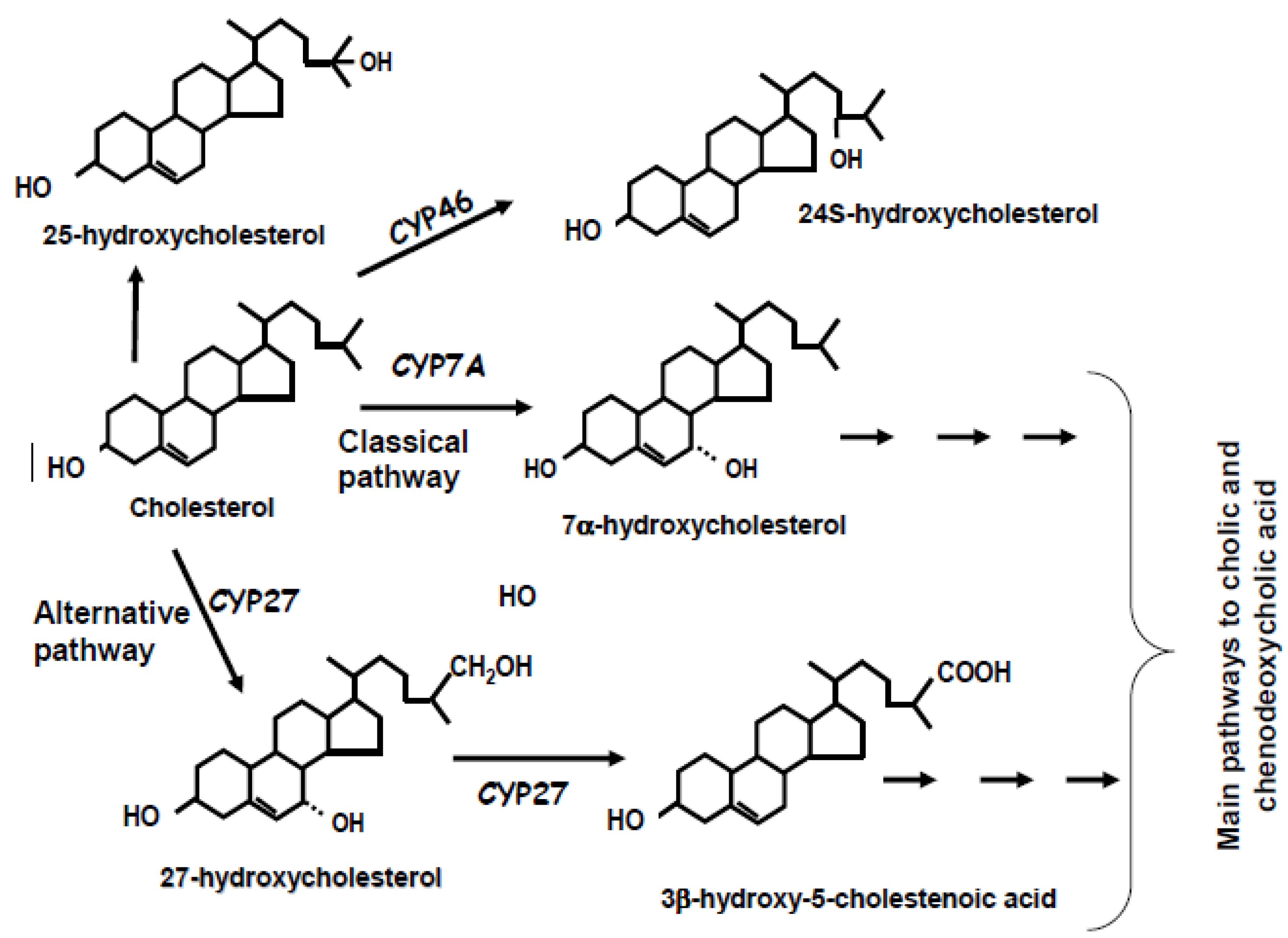
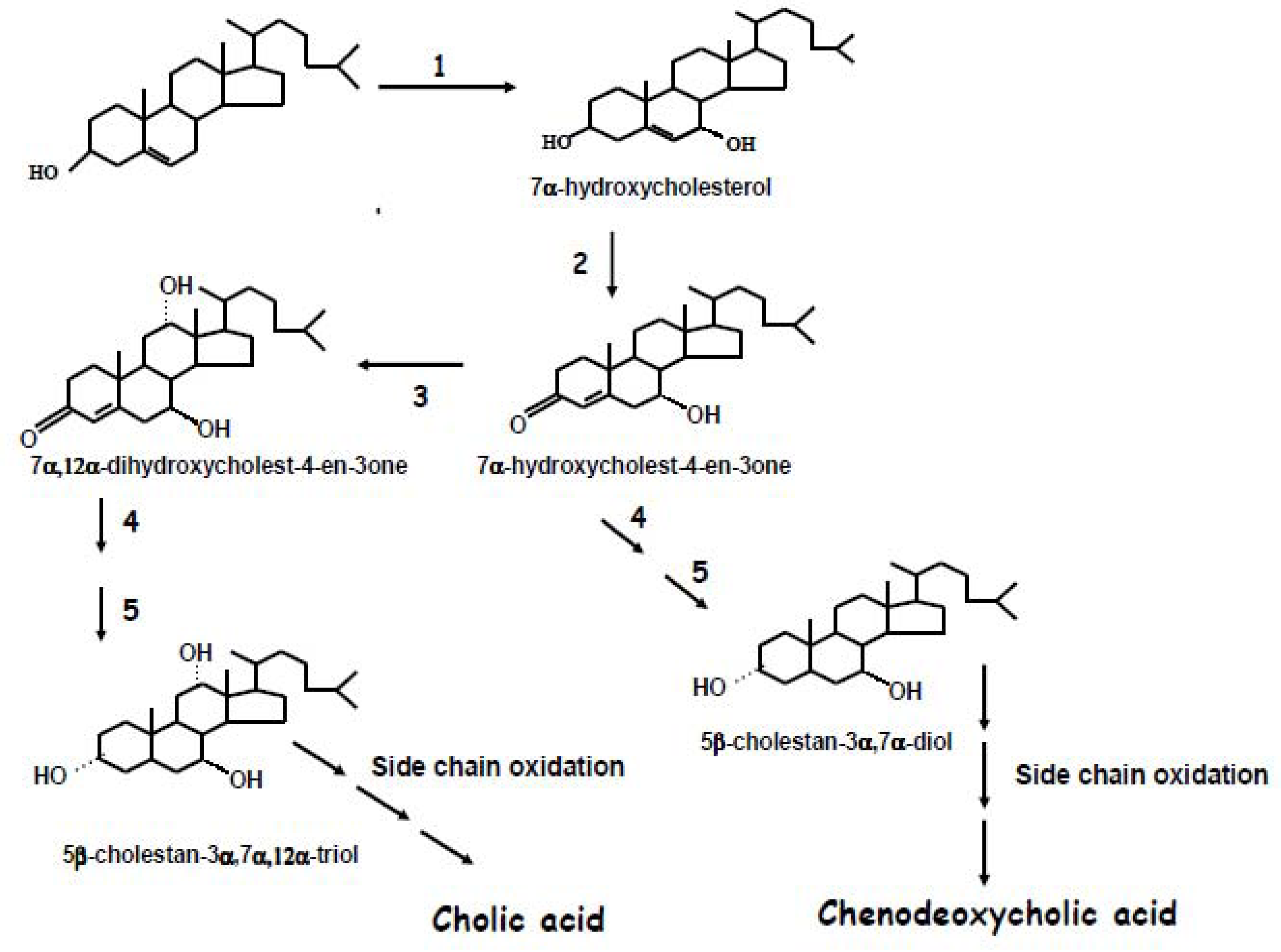
3. Alternative Approaches for the Quantification of Bile Acid Synthesis
4. Use of Radioactive Tracers for the Measurement of Bile Acid Synthesis
5. Use of Stable Isotopes for the Measurement of Bile Acid Synthesis
5.1. Bile Acid Kinetics Studies
5.2. Studies on the Relative Contributions of the Classical and Alternate Pathways
6. Regulation of Bile Acid Synthesis in Different Situations
6.1. Aging
6.2. Liver Disease and Cholestasis
6.3. Nutrition and Obesity
6.4. Metabolic and Endocrine Disorders
6.5. Gallstone Disease
6.6. Bile Acid Feeding
6.7. Drug Treatment
6.7.1. Agents Affecting Lipid Metabolism
6.7.2. Other Drugs
7. Concluding Remarks
Acknowledgements and Grant Support
Conflict of Interest
References
- Tabas, I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. J. Clin. Invest. 2002, 110, 905–911. [Google Scholar]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993, 34, 1637–1659. [Google Scholar]
- Bertolotti, M.; Gabbi, C.; Anzivino, C.; Carulli, L.; Loria, P.; Carulli, N. Nuclear receptors as potential molecular targets in cholesterol accumulation conditions: Insights from evidence on hepatic cholesterol degradation and gallstone disease in humans. Curr. Med. Chem. 2008, 15, 2271–2284. [Google Scholar] [CrossRef]
- Bertolotti, M.; Abate, N.; Loria, P.; Dilengite, M.; Carubbi, F.; Pinetti, A.; Digrisolo, A.; Carulli, N. Regulation of bile acid synthesis in humans: Effect of treatment with bile acids. Hepatology 1991, 14, 830–837. [Google Scholar] [CrossRef]
- Goodwin, B.; Kliewer, S.A. I. Nuclear receptors and bile acid homeostasis. Am. J. Physiol. Gastrointest. Liver. Physiol. 2002, 282, G926–G931. [Google Scholar]
- de Fabiani, E.; Mitro, N.; Godio, C.; Gilardi, F.; Caruso, D.; Crestani, M. Bile acid signaling to the nucleus: Finding new connections in the transcriptional regulation of metabolic pathways. Biochimie 2004, 86, 771–778. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J. Lipid Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Björkhem, I. Mechanism of degradation of the steroid side chain in the formation of bile acids. J. Lipid Res. 1992, 33, 455–472. [Google Scholar]
- Javitt, N.B. Bile acid synthesis from cholesterol: Regulatory and auxiliary pathways. FASEB J. 1994, 8, 1308–1311. [Google Scholar]
- Crosignani, A.; Zuin, M.; Allocca, M.; Del Puppo, M. Oxysterols in bile acid metabolism. Clin. Chim. Acta 2011, 412, 2037–2045. [Google Scholar] [CrossRef]
- Russell, D.W.; Setchell, K.D.R. Bile acid biosynthesis. Biochemistry 1992, 31, 4737–4349. [Google Scholar] [CrossRef]
- Ogishima, T.; Deguchi, S.; Okuda, K. Purification and characterization of cholesterol 7alpha-hydroxylase from rat liver microsomes. J. Biol. Chem. 1987, 262, 7646–7650. [Google Scholar]
- Norlin, M.; Andersson, U.; Björkhem, I.; Wikvall, K. Oxysterol 7alpha-hydroxylase activity by cholesterol 7alpha-hydroxylase (CYP7A). J. Biol. Chem. 2000, 275, 34046–34053. [Google Scholar]
- Vlahcevic, Z.R.; Heuman, D.M.; Hylemon, P.B. Regulation of bile acids synthesis. Hepatology 1991, 13, 590–600. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid regulation of gene expression: Roles of nuclear hormone receptors. Endocr. Rev. 2002, 23, 443–463. [Google Scholar] [CrossRef]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1 and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- de Fabiani, E.; Mitro, N.; Gilardi, F.; Caruso, D.; Galli, G.; Crestani, M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J. Biol. Chem. 2003, 278, 39124–39132. [Google Scholar]
- Chiang, J.Y.L. Regulation of bile acid synthesis. Front. Biosci. 1998, 3, d176–d193. [Google Scholar]
- Kondo, K.H.; Kai, M.H.; Setoguchi, Y.; Eggertsen, G.; Sjöblom, P.; Setoguchi, T.; Okuda, K.I.; Björkhem, I. Cloning and expression of cDNA of human delta 4-3-oxosteroid 5 beta-reductase and substrate specificity of the expressed enzyme. Eur. J. Biochem. 1994, 219, 357–363. [Google Scholar] [CrossRef]
- Usui, E.; Okuda, K.; Kato, Y.; Noshiro, M. Rat hepatic 3alpha-hydroxysteroid dehydrogenase: Expression of cDNA and physiological function in bile acid biosynthetic pathway. J. Biochem. 1994, 115, 230–237. [Google Scholar]
- Björkhem, I.; Andersson, O.; Diczfalusy, U.; Sevastik, B.; Xiu, R.J.; Duan, C.; Lund, E. Atherosclerosis and sterol 27-hydroxylase: Evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc. Natl. Acad. Sci. USA 1994, 91, 8592–8596. [Google Scholar]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Babiker, A.; Andersson, O.; Lund, E.; Xiu, R.J.; Deeb, S.; Reshef, A.; Leitersdorf, E.; Diczfalusy, U.; Björkhem, I. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J. Biol. Chem. 1997, 272, 26253–26261. [Google Scholar]
- Schwarz, M.; Lund, E.G.; Russell, D.W. Two 7alpha-hydroxylase enzymes in bile acid biosynthesis. Curr. Opin. Lipidol. 1998, 9, 113–118. [Google Scholar] [CrossRef]
- Duane, W.C.; Javitt, N.B. 27-hydroxycholesterol: Production rates in normal human subjects. J. Lipid Res. 1999, 40, 1194–1199. [Google Scholar]
- Lund, E.; Andersson, O.; Zhang, J.; Babiker, A.; Ahlborg, G.; Diczfalusy, U.; Einarsson, K.; Sjövall, J.; Björkhem, I. Importance of a novel oxidative mechanism for elimination of intracellular cholesterol in humans. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 208–212. [Google Scholar] [CrossRef]
- Lund, E.G.; Guileyardo, J.M.; Russell, D.W. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7238–7243. [Google Scholar]
- Bertolotti, M.; Loria, P.; Carubbi, F.; Bozzoli, M.; Dilengite, M.A.; Carulli, N. Biochemical methods to study hepatic cholesterol metabolism. In Methods in Biliary Research; Muraca, M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 81–98. [Google Scholar]
- Myant, N.B.; Mitropoulos, K.A. Cholesterol 7α-hydroxylase. J. Lipid Res. 1977, 18, 135–153. [Google Scholar]
- Crosignani, A.; Del Puppo, M.; Longo, M.; de Fabiani, E.; Caruso, D.; Zuin, M.; Podda, M.; Javitt, N.B.; Kienle, M.G. Changes in classic and alternative pathways of bile acid synthesis in chronic liver disease. Clin. Chim. Acta 2007, 382, 82–88. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Ahrens, E.H., Jr.; Grundy, S.M. Quantitative isolation and gas-liquid chromatographic analysis of total dietary and fecal neutral steroids. J. Lipid Res. 1965, 6, 411–424. [Google Scholar]
- Miettinen, T.A. Gas-liquid chromatographic determination of fecal neutral sterols using a capillary column. Clin. Chim. Acta 1982, 124, 245–248. [Google Scholar] [CrossRef]
- Duane, W.C.; Holloway, D.E.; Hutton, S.W.; Corcoran, P.J.; Haas, N.A. Comparison of bile acid synthesis determined by isotope dilution versus fecal acidic sterol output in human subjects. Lipids 1982, 17, 345–348. [Google Scholar] [CrossRef]
- Duane, W.C. Measurement of bile acid synthesis by three different methods in hypertriglyceridemic and control subjects. J. Lipid Res. 1997, 38, 183–188. [Google Scholar]
- Hahn, C.; von Bergmann, K. Relationship between the serum concentration of 7α-hydroxycholesterol and fecal bile acid excretion in humans. Scand. J. Gastroenterol. 1996, 31, 804–808. [Google Scholar] [CrossRef]
- Björkhem, I.; Reihnér, E.; Angelin, B.; Ewerth, S.; Åkerlund, J.E.; Einarsson, K. On the possible use of the serum level of 7α-hydroxycholesterol as a marker for increased activity of the cholesterol 7alpha-hydroxylase in humans. J. Lipid Res. 1987, 28, 889–894. [Google Scholar]
- Hahn, C.; Reichel, C.; von Bergmann, K. Serum concentration of 7α-hydroxycholesterol as an indicator of bile acid synthesis in humans. J. Lipid Res. 1995, 36, 2059–2066. [Google Scholar]
- Axelson, M.; Aly, A.; Sjövall, J. Levels of 7alpha-hydroxy-4-cholesten-3-one in plasma reflect rates of bile acid synthesis in man. FEBS Lett. 1988, 239, 324–328. [Google Scholar] [CrossRef]
- Axelson, M.; Björkhem, I.; Reihnér, E.; Einarsson, K. The plasma level of 7alpha-hydroxy-4-cholesten-3-one reflects the activity of hepatic cholesterol 7alpha-hydroxylase in man. FEBS Lett. 1991, 284, 216–218. [Google Scholar] [CrossRef]
- Sauter, G.; Berr, F.; Beuers, U.; Fischer, S.; Paumgartner, G. Serum concentrations of 7α-hydroxy-4-cholesten-3-one reflect bile acid synthesis in humans. Hepatology 1996, 24, 123–126. [Google Scholar]
- Bertolotti, M.; Del Puppo, M.; Gabbi, C.; Corna, F.; Carulli, L.; Pellegrini, E.; Zambianchi, L.; Anzivino, C.; Ricchi, M.; Loria, P.; et al. Correlation between plasma levels of 7alpha-hydroxy-4-cholesten-3-one and cholesterol 7alpha-hydroxylation rates in vivo in hyperlipidemic patients. Steroids 2008, 73, 1197–1202. [Google Scholar] [CrossRef]
- Del Puppo, M.; Galli Kienle, M.; Petroni, M.L.; Crosignani, A.; Podda, M. Serum 27-hydroxycholesterol in patients with primary biliary cirrhosis suggests alteration of cholesterol catabolism to bile acids via the acidic pathway. J. Lipid Res. 1998, 39, 2477–2482. [Google Scholar]
- Javitt, N.B. Cholesterol, hydroxycholesterols, and bile acids. Biochem. Biophys. Res. Commun. 2002, 292, 1147–1153. [Google Scholar] [CrossRef]
- Harik-Khan, R.; Holmes, R.P. Estimation of 26-hydroxycholesterol in serum by high-performance liquid chromatography and its measurement in patients with atherosclerosis. J. Steroid Biochem. 1990, 36, 351–355. [Google Scholar] [CrossRef]
- Lindstedt, S. The turnover of cholic acid in man: Bile acids and steroids. Acta Physiol. Scand. 1957, 40, 1–9. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hoffman, N. Measurement of bile and acid kinetics by isotope dilution in man. Gastroenterology 1974, 67, 314–323. [Google Scholar]
- Vantrappen, G.; Rutgeerts, P.; Ghoos, Y. A new method for the measurement of bile acid turnover and pool size by a double label, single intubation technique. J. Lipid Res. 1981, 22, 528–531. [Google Scholar]
- Quarfordt, S.H.; Greenfield, M.F. Estimation of cholesterol and bile acid turnover in man by kinetic analysis. J. Clin. Invest. 1973, 52, 1937–1945. [Google Scholar] [CrossRef]
- Duane, W.C.; Javitt, N.B. Conversion of 7alpha-hydroxycholesterol to bile acid in human subjects: Is there an alternate pathway favoring cholic acid synthesis? J. Lab. Clin. Med. 2002, 139, 109–115. [Google Scholar] [CrossRef]
- Rosenfeld, R.S.; Bradlow, H.L.; Levin, J.; Zumoff, B. Preparation of [24,25-3H] cholesterol. Oxidation in man as a measure of bile acid formation. J. Lipid Res. 1978, 19, 850–855. [Google Scholar]
- Davidson, N.O.; Bradlow, H.L.; Ahrens, E.H.Jr.; Rosenfeld, R.S.; Schwartz, C.C. Bile acid production in human subjects: Rate of oxidation of [24,25-3H]cholesterol compared to fecal bile acid excretion. J. Lipid Res. 1986, 27, 183–195. [Google Scholar]
- Duane, W.C.; Levitt, D.G.; Mueller, S.M.; Behrens, J.C. Regulation of bile acid synthesis in man: Presence of a diurnal rhythm. J. Clin. Invest. 1983, 72, 1930–1936. [Google Scholar] [CrossRef]
- Mitchell, J.C.; Stone, B.G.; Logan, G.M.; Duane, W.C. Role of cholesterol synthesis in regulation of bile acid synthesis and biliary cholesterol secretion in humans. J. Lipid Res. 1991, 31, 1143–1149. [Google Scholar]
- Bertolotti, M.; Carulli, N.; Menozzi, D.; Zironi, F.; Digrisolo, A.; Pinetti, A.; Baldini, M.G. In vivo evaluation of cholesterol 7α-hydroxylase in humans: Effect of disease and drug treatment. J. Lipid Res. 1986, 27, 1278–1286. [Google Scholar]
- Watkins, J.B.; Ingall, D.; Klein, P.D.; Lester, R.; Szczepanik, P.A. Bile salt metabolism in the new-born. Measurement of pool size and synthesis by stable isotope technique. N. Engl. J. Med. 1973, 288, 431–434. [Google Scholar] [CrossRef]
- Stellaard, F.; Sackmann, M.; Sauerbruch, T.; Paumgartner, G. Simultaneous determination of cholic acid and chenodeoxycholic acid pool sizes and fractional turnover rates in human serum using 13C-labeled bile acids. J. Lipid Res. 1984, 25, 1313–1319. [Google Scholar]
- Everson, G.T. Steady-state kinetics of serum bile acids in healthy human subjects: Single and dual isotope techniques using stable isotopes and mass spectrometry. J. Lipid Res. 1987, 28, 238–252. [Google Scholar]
- DeMark, B.R.; Everson, G.T.; Klein, P.D.; Showalter, R.B.; Kern, F.Jr. A method for the accurate measurement of isotope ratios of chenodeoxycholic and cholic acids in serum. J. Lipid Res. 1982, 23, 204–210. [Google Scholar]
- Stellaard, F.; Schubert, R.; Paumgartner, G. Measurement of bile acid kinetics in human serum using stable isotope labelled chenodeoxycholic acid and capillary gas chromatography electron impact mass spectrometry. Biomed. Mass Spectrom. 1983, 10, 187–191. [Google Scholar] [CrossRef]
- Stellaard, F.; Paumgartner, G.; van Berge Henegouwen, G.P.; van der Werf, S.D.J. Determination of deoxycholic acid pool size and input rate using [24-13C] deoxycholic acid and serum sampling. J. Lipid Res. 1986, 27, 1222–1225. [Google Scholar]
- Stellaard, F.; Sackmann, M.; Berr, F.; Paumgartner, G. Simultaneous determination of pool sizes and fractional turnover rates, of deoxycholic acid, cholic acid and chenodeoxycholic acid in man by isotope dilution with 2H and 13C labels and serum sampling. Biomed. Environ. Mass Spectrom. 1987, 14, 609–611. [Google Scholar] [CrossRef]
- Koopman, B.J.; Kuipers, F.; Bijleveld, C.M.; van der Molen, J.C.; Nagel, G.T.; Vonk, R.J.; Wolthers, B.G. Determination of cholic acid and chenodeoxycholic acid pool sizes and fractional turnover rates by means of stable isotope dilution technique, making use of deuterated cholic acid and chenodeoxycholic acid. Clin. Chim. Acta 1988, 175, 143–155. [Google Scholar] [CrossRef]
- Hellerstein, M.K.; Neese, R.A. Mass isotopomer distribution analysis at eight years: Theoretical, analytic, and experimental considerations. Am. J. Physiol. 1999, 276, E1146–E1170. [Google Scholar]
- Wolfe, R.R. Radioactive and stable isotope tracers in biomedicine. In Principles and Practice of Kinetic Analysis; Wolfe, R.R., Ed.; Wiley-Liss Inc.: New York, NY, USA, 1992. [Google Scholar]
- Del Puppo, M.; Crosignani, A.; Longo, M.; Zuin, M.; Podda, M.; Galli, G.; de Fabiani, E.; Ciuffreda, P.; Santaniello, E.; Javitt, N.B.; et al. A minimally invasive technique for the evaluation of the regulatory steps of the two major pathways of bile acid synthesis. Clin. Chim. Acta 2005, 355, 23–31. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Kimmel, R.; Stroup, D. Regulation of cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRalpha). Gene 2001, 262, 257–265. [Google Scholar] [CrossRef]
- Reihnér, E.; Björkhem, I.; Angelin, B.; Ewerth, S.; Einarsson, K. Bile acid synthesis in humans: Regulation of hepatic microsomal cholesterol 7alpha-hydroxylase activity. Gastroenterology 1989, 97, 1498–1505. [Google Scholar]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef]
- Lundåsen, T.; Gälman, C.; Angelin, B.; Rudling, M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006, 260, 530–536. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA 1999, 96, 11041–11048. [Google Scholar] [CrossRef]
- Costet, P.; Luo, Y.; Wang, N.; Tall, A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar]
- Venkateswaran, A.; Laffitte, B.A.; Joseph, S.B.; Mak, P.A.; Wilpitz, D.C.; Edwards, P.A.; Tontonoz, P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRa. Proc. Natl. Acad. Sci. USA 2000, 97, 12097–12102. [Google Scholar]
- Szanto, A.; Benko, S.; Szatmari, I.; Balint, B.L.; Furtos, I.; Rühl, R.; Molnar, S.; Csiba, L.; Garuti, R.; Calandra, S.; et al. Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signaling in macrophages. Mol. Cell Biol. 2004, 24, 8154–8166. [Google Scholar] [CrossRef]
- Quinn, C.M.; Jessup, W.; Wong, J.; Kritharides, L.; Brown, A.J. Expression and regulation of sterol 27-hydroxylase (CYP27A1) in human macrophages: A role for RXR and PPARgamma ligands. Biochem. J. 2005, 385, 823–830. [Google Scholar] [CrossRef]
- Crosignani, A.; Del Puppo, M.; de Fabiani, E.; Caruso, D.; Gallisai, D.; Mela, M.G.; Melzi, M.L.; Galli Kienle, M.; Colombo, C. Plasma oxysterols in normal and cholestatic children as indicators of the two pathways of bile acid synthesis. Clin. Chim. Acta 2008, 395, 84–88. [Google Scholar] [CrossRef]
- Setchell, K.D.; Schwarz, M.; O’Connell, N.C.; Lund, E.G.; Davis, D.L.; Lathe, R.; Thompson, H.R.; Weslie Tyson, R.; Sokol, R.J.; Russell, D.W. Identification of a new inborn error in bile acid synthesis: Mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J. Clin. Invest. 1998, 102, 1690–1703. [Google Scholar] [CrossRef]
- Einarsson, K.; Nilsell, K.; Leijd, B.; Angelin, B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N. Engl. J. Med. 1985, 313, 277–282. [Google Scholar] [CrossRef]
- Bertolotti, M.; Abate, N.; Bertolotti, S.; Loria, P.; Concari, M.; Messora, R.; Carubbi, F.; Pinetti, A.; Carulli, N. Effect of aging on cholesterol 7α-hydroxylation in humans. J. Lipid Res. 1993, 34, 1001–1007. [Google Scholar]
- Bertolotti, M.; Gabbi, C.; Anzivino, C.; Crestani, M.; Mitro, N.; Del Puppo, M.; Godio, C.; de Fabiani, E.; Macchioni, D.; Carulli, L.; et al. Age-related changes in bile acid synthesis and hepatic nuclear receptor expression. Eur. J. Clin. Invest. 2007, 37, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Heiss, G.; Tamir, I.; Davis, C.E.; Tyroler, H.A.; Rifkind, B.M.; Schonfeld, G.; Jacobs, D.; Frantz, I.D.Jr. Lipoprotein-cholesterol distributions in selected North American populations: The lipid research clinics program prevalence study. Circulation 1980, 61, 302–315. [Google Scholar] [CrossRef]
- Longo, M.; Crosignani, A.; Battezzati, P.M.; Squarcia Giussani, C.; Invernizzi, P.; Zuin, M.; Podda, M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 2002, 51, 265–269. [Google Scholar] [CrossRef]
- Crosignani, A.; Podda, M.; Battezzati, P.M.; Bertolini, E.; Zuin, M.; Watson, D.; Setchell, K.D. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology 1991, 14, 1000–1007. [Google Scholar] [CrossRef]
- Mc Cormick, W.C., III; Bell, C.C.Jr.; Swell, L.; Vlahcevic, Z.R. Cholic acid synthesis as an index of the severity of liver disease in man. Gut 1973, 14, 895–902. [Google Scholar] [CrossRef]
- Vlahcevic, Z.R.; Juttijudata, P.; Bell, C.C.; Swell, L. Bile acid metabolism in patients with cirrhosis. II. Cholic and chenodeoxycholic acid metabolism. Gastroenterology 1972, 62, 1174–1181. [Google Scholar]
- Sauer, P.; Rudolph, G.; Endele, R.; Senn, M.; Theilmann, L.; Otto, G.; Stremmel, W.; Stiehl, A. Kinetics of primary bile acids in patients after orthotopic liver transplantation. Eur. J. Clin. Invest. 1996, 26, 979–982. [Google Scholar]
- Dueland, S.; Reichen, J.; Everson, G.T.; Davis, R.A. Regulation of cholesterol and bile acid homeostasis in bile-obstructed rats. Biochem. J. 1991, 280, 373–377. [Google Scholar]
- Okamoto, S.; Fukushima, K.; Higashijima, H.; Makino, I.; Kishinaka, M.; Oda, H.; Yamashita, H.; Ichimiya, H.; Chijiiwa, K.; Kuroki, S. Serum 7α-hydroxycholesterol reflects hepatic bile acid synthesis in patients with obstructive jaundice after external biliary drainage. Hepatology 1994, 20, 95–100. [Google Scholar]
- Bertolotti, M.; Carulli, L.; Concari, M.; Martella, P.; Loria, P.; Tagliafico, E.; Ferrari, S.; Del Puppo, M.; Amati, B.; de Fabiani, E.; et al. Suppression of bile acid synthesis, but not of hepatic cholesterol 7alpha-hydroxylase expression, by obstructive cholestasis in humans. Hepatology 2001, 34, 234–242. [Google Scholar]
- Schaap, F.G.; van der Gaag, N.A.; Gouma, D.J.; Jansen, P.L. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009, 49, 1228–1235. [Google Scholar] [CrossRef]
- Breuer, O.; Sudjana-Sugiaman, E.; Eggertsen, G.; Chiang, J.Y.L.; Björkhem, I. Cholesterol 7α-hydroxylase is up-regulated by the competitive inhibitor 7-oxocholesterol in rat liver. Eur. J. Biochem. 1993, 215, 705–710. [Google Scholar] [CrossRef]
- Beuers, U.; Spengler, U.; Zwiebel, F.M.; Pauletzki, J.; Fischer, S.; Paumgartner, G. Effect of ursodeoxycholic acid on the kinetics of the major hydrophobic bile acids in health and in chronic cholestatic liver disease. Hepatology 1992, 15, 603–608. [Google Scholar] [CrossRef]
- Rudolph, G.; Endele, R.; Senn, M.; Stiehl, A. Effect of ursodeoxycholic acid on the kinetics of cholic acid and chenodeoxycholic acid in patients with primary sclerosing cholangitis. Hepatology 1993, 17, 1028–1232. [Google Scholar] [CrossRef]
- Allocca, M.; Crosignani, A.; Gritti, A.; Ghilardi, G.; Gobatti, D.; Caruso, D.; Zuin, M.; Podda, M.; Battezzati, P.M. Hypercholesterolaemia is not associated with early atherosclerotic lesions in primary biliary cirrhosis. Gut 2006, 55, 1795–1800. [Google Scholar] [CrossRef]
- Einarsson, K.; Hellström, K.; Kallner, M. Bile acid kinetics in relation to sex, serum lipids, body weights, and gallbladder disease in patients with various types of hyperlipoproteinemia. J. Clin. Invest. 1974, 54, 1301–1311. [Google Scholar] [CrossRef]
- Bennion, L.J.; Grundy, S.M. Effects of obesity and caloric intake on biliary lipid metabolism in man. J. Clin. Invest. 1975, 56, 996–1011. [Google Scholar] [CrossRef]
- Shaffer, E.A.; Small, D.M. Biliary lipid secretion in cholesterol gallstone disease. The effect of choleystectomy and obesity. J. Clin. Invest. 1977, 59, 828–840. [Google Scholar] [CrossRef]
- Ståhlberg, D.; Rudling, M.; Angelin, B.; Björkhem, I.; Forsell, P.; Nilsell, K.; Einarsson, K. Hepatic cholesterol metabolism in human obesity. Hepatology 1997, 25, 1447–1450. [Google Scholar] [CrossRef]
- Mazzella, G.; Cipolla, A.; Villanova, N.; Polimeni, C.; Sipahi, A.; Parini, P.; Fusaroli, P.; Festi, D.; Roda, E. Changes in biliary lipid secretion and cholic acid kinetics induced by diet, diet plus simvastatin and diet plus ursodeoxycholic acid in obese subjects. Ital. J. Gastroenterol. 1995, 27, 441–445. [Google Scholar]
- Duane, W.C. Effects of lovastatin and dietary cholesterol on sterol homeostasis in healthy human subjects. J. Clin. Invest. 1993, 92, 911–918. [Google Scholar] [CrossRef]
- Duane, W.C. Effects of lovastatin and dietary cholesterol on bile acid kinetics and bile lipid composition in healthy male subjects. J. Lipid Res. 1994, 35, 501–509. [Google Scholar]
- Andersén, E.; Hellström, K. The effect of cholesterol feeding on bile acid kinetics and biliary lipids in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 1979, 20, 1020–1027. [Google Scholar]
- Kern, F., Jr. Effects of dietary cholesterol on cholesterol and bile acid homeostasis in patients with cholesterol gallstones. J. Clin. Invest. 1994, 93, 1186–1194. [Google Scholar] [CrossRef]
- Duane, W.C.; Ginsberg, R.L.; Bennion, L.J. Effects of fasting on bile acid metabolism and and biliary lipid composition in man. J. Lipid Res. 1976, 17, 211–219. [Google Scholar]
- Bertolotti, M.; Spady, D.K.; Dietschy, J.M. Regulation of hepatic cholesterol metabolism in the rat in vivo: Effect of a synthetic fat-free diet on sterol synthesis and low density lipoprotein transport. Biochim. Biophys. Acta 1995, 1255, 293–300. [Google Scholar] [CrossRef]
- Dawes, L.G.; Muldoon, J.P.; Greiner, M.A.; Bertolotti, M. Cholecystokinin increases bile acid synthesis with total parenteral nutrition but does not prevent stone formation. J. Surg. Res. 1997, 67, 84–89. [Google Scholar] [CrossRef]
- Bisschop, P.H.; Bandsma, R.H.J.; Stellaard, F.; Harmsel, A.; Meijer, A.J.; Sauerwein, H.P.; Kuipers, F.; Romijn, J.A. Low-fat, high-carbohydrate and high-fat, low-carbohydrate diets decrease primary bile acid synthesis in humans. Am. J. Clin. Nutr. 2004, 79, 570–576. [Google Scholar]
- Huijbregts, A.W.; van Schaik, A.; van Berge-Henegouwen, G.P.; van der Werf, S.D. Serum lipids, biliary lipid composition, and bile acid metabolism in vegetarians as compared to normal controls. Eur. J. Clin. Invest. 1980, 10, 443–449. [Google Scholar] [CrossRef]
- Bennion, L.J.; Grundy, S.M. Effects of diabetes mellitus on cholesterol metabolism in man. N. Engl. J. Med. 1977, 296, 1365–1371. [Google Scholar] [CrossRef]
- Ponz de Leon, M.; Ferenderes, R.; Carulli, N. Bile lipid composition and bile acid pool size in diabetes. Am. J. Dig. Dis. 1978, 23, 710–716. [Google Scholar] [CrossRef]
- Brufau, G.; Stellaard, F.; Prado, K.; Bloks, V.W.; Jonkers, E.; Boverhof, R.; Kuipers, F.; Murphy, E.J. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology 2010, 52, 1455–1464. [Google Scholar] [CrossRef]
- Angelin, B. Bile acid metabolism in heterozygous familial hypercholesterolemia: A study comparing affected and unaffected siblings of four kindreds. Eur. J. Clin. Invest. 1988, 18, 153–161. [Google Scholar]
- Bertolotti, M.; Concari, M.; Loria, P.; Abate, N.; Pinetti, A.; Guicciardi, M.E.; Carulli, N. Effects of different phenotypes of hyperlipoproteinemia and of treatment with fibric acid derivatives on the rates of cholesterol 7α-hydroxylation in humans. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1064–1069. [Google Scholar] [CrossRef]
- Angelin, B.; Hershon, K.S.; Brunzell, J.D. Bile acid metabolism in hereditary forms of hypertriglyceridemia: Evidence for an increased synthesis rate in monogenic familial hypertriglyceridemia. Proc. Natl. Acad. Sci. USA 1987, 84, 5434–5438. [Google Scholar] [CrossRef]
- Bertolotti, M.; Del Puppo, M.; Corna, F.; Anzivino, C.; Gabbi, C.; Baldelli, E.; Carulli, L.; Loria, P.; Galli Kienle, M.; Carulli, N. Increased appearance rate of 27-hydroxycholesterol in vivo in hypercholesterolemia: A possible compensatory mechanism. Nutr. Metab. Cardiovasc. Dis. 2011, in press. [Google Scholar] [CrossRef]
- Weingärtner, O.; Laufs, U.; Böhm, M.; Lütjohann, D. An alternative pathway of reverse cholesterol transport: The oxysterol 27-hydroxycholesterol. Atherosclerosis 2010, 209, 39–41. [Google Scholar] [CrossRef]
- Angelin, B.; Einarsson, K.; Leijd, B. Bile acid metabolism in hypothyroid subjects: Response to substitution therapy. Eur. J. Clin. Invest. 1983, 13, 99–106. [Google Scholar] [CrossRef]
- Pauletzki, J.; Stellaard, F.; Paumgartner, G. Bile acid metabolism in human hyperthyroidism. Hepatology 1989, 9, 852–855. [Google Scholar] [CrossRef]
- Loria, P.; Carulli, L.; Bertolotti, M.; Lonardo, A. Endocrine and liver interaction: The role of endocrine pathways in NASH. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 236–247. [Google Scholar] [CrossRef]
- Fujiyama, J.; Kuriyama, M.; Arima, S.; Shibata, Y.; Nagata, K.; Takenaga, S.; Tanaka, H.; Osame, M. Atherogenic risk factors in cerebrotendinous xanthomatosis. Clin. Chim. Acta 1991, 200, 1–11. [Google Scholar] [CrossRef]
- Leitersdorf, E.; Reshef, A.; Meiner, V.; Levitzki, R.; Schwartz, S.P.; Dann, E.J.; Berkman, N.; Cali, J.J.; Klapholz, L.; Berginer, V.M. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin. J. Clin. Invest. 1993, 91, 2488–2496. [Google Scholar] [CrossRef]
- Panzenboeck, U.; Andersson, U.; Hansson, M.; Sattler, W.; Meaney, S.; Björkhem, I. On the mechanism of cerebral accumulation of cholestanol in patients with cerebrotendinous xanthomatosis. J. Lipid Res. 2007, 48, 1167–1174. [Google Scholar] [CrossRef]
- Del Puppo, M.; Corna, F.; Dotti, M.T.; de Fabiani, E.; Galli Kienle, M. In vivo evaluation of bile acid synthesis in patients with cerebrotendinous xanthomatosis. Chem. Phys. Lipids 2007, 149, S75–S76. [Google Scholar]
- Pullinger, C.R.; Eng, C.; Salen, G.; Shefer, S.; Batta, A.K.; Erickson, S.K.; Verhagen, A.; Rivera, C.R.; Mulvihill, S.J.; Malloy, M.J.; et al. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest. 2002, 110, 109–117. [Google Scholar]
- Peake, K.B.; Vance, J.E. Defective cholesterol trafficking in Niemann-Pick C-deficient cells. FEBS Lett. 2010, 584, 2731–2739. [Google Scholar] [CrossRef]
- Xie, C.; Turley, S.D.; Pentchev, P.G.; Dietschy, J.M. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E336–E344. [Google Scholar]
- Bertolotti, M.; Gabbi, C.; Anzivino, C.; Mitro, N.; Godio, C.; de Fabiani, E.; Crestani, M.; Del Puppo, M.; Ricchi, M.; Carulli, L.; et al. Decreased hepatic expression of peroxysome proliferator-activated receptor-γ coactivator 1 in cholesterol cholelithiasis. Eur. J. Clin. Invest. 2006, 36, 170–175. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Parini, P.; Eggertsen, G.; Davis, M.A.; Hu, H.; Suo, G.-J.; Zhang, S.-D.; Rudel, L.L.; Han, T.-Q.; Einarsson, C. Increased expression of LXR alpha, ABCG5, ABCG8, and SR-BI in the liver from normolipidemic, nonobese Chinese gallstone patiets. J. Lipid Res. 2008, 49, 464–472. [Google Scholar]
- Hepner, G.W.; Quarfordt, S.H. Kinetics of cholesterol and bile acids in patients with cholesterol cholelithiasis. Gastroenterology 1975, 69, 318–325. [Google Scholar]
- Carulli, N.; Ponz de Leon, M.; Zironi, F.; Pinetti, A.; Smerieri, A.; Iori, R.; Loria, P. Hepatic cholesterol and bile acid metabolism in subjects with gallstones: Comparative effects of short-term feeding of chenodeoxycholic and ursodeoxycholic acid. J. Lipid Res. 1980, 21, 35–43. [Google Scholar]
- Einarsson, K.; Hellström, K.; Kallner, M. The effect of clofibrate on the elimination of cholesterol as bile acids in patients with hyperlipoproteinaemia type II and IV. Eur. J. Clin. Invest. 1973, 3, 345–351. [Google Scholar] [CrossRef]
- Pertsemlidis, D.; Panveliwalla, D.; Ahrens, E.H., Jr. Effects of clofibrate and of an estrogen-progestin combination on fasting biliary lipids and cholic acid kinetics in man. Gastroenterology 1974, 66, 565–573. [Google Scholar]
- Gälman, C.; Miquel, J.F.; Pérez, R.M.; Einarsson, C.; Ståhle, L.; Marshall, G.; Nervi, F.; Rudling, M. Bile acid synthesis is increased in Chilean Hispanics with gallstones and in gallstone high-risk Mapuche Indians. Gastroenterology 2004, 126, 741–748. [Google Scholar] [CrossRef]
- Pomare, E.W.; Heaton, K.W. Bile salt metabolism in patients with gallstones in functioning gallbladders. Gut 1973, 14, 885–890. [Google Scholar] [CrossRef]
- Pedersen, L.; Arnfred, T. Kinetics and pool size of chenodeoxycholic acid in cholesterol gallstone patients. Scand. J. Gastroenterol. 1975, 10, 557–560. [Google Scholar]
- Nilsell, K.; Angelin, B.; Liljeqvist, L.; Einarsson, K. Biliary lipid output and bile acid kinetics in cholesterol gallstone disease. Evidence for an increased hepatic secretion of cholesterol in Swedish patients. Gastroenterology 1985, 89, 287–293. [Google Scholar]
- Berr, F.; Pratschke, E.; Fischer, S.; Paumgartner, G. Disorders of bile acid metabolism in cholesterol gallstone disease. J. Clin. Invest. 1992, 90, 859–868. [Google Scholar] [CrossRef]
- Almond, H.R.; Vlahcevic, Z.R.; Bell, C.C.Jr.; Gregory, D.H.; Swell, L. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N. Engl. J. Med. 1973, 289, 1213–1216. [Google Scholar] [CrossRef]
- Berr, F.; Stellaard, F.; Pratschke, E.; Paumgartner, G. Effects of colecystectomy on the kinetics of the primary and secondary bile acids. J. Clin. Invest. 1989, 83, 1541–1550. [Google Scholar] [CrossRef]
- Bertolotti, M.; Gabbi, C.; Anzivino, C.; Carulli, L.; Carulli, N. Changes in bile acid synthesis in gallstone disease: Cause, consequence, or neither? Hepatology 2007, 46, 1664. [Google Scholar]
- Reihnér, E.; Angelin, B.; Björkhem, I.; Einarsson, K. Hepatic cholesterol metabolism in cholesterol gallstone disease. J. Lipid Res. 1991, 32, 469–475. [Google Scholar]
- Loria, P.; Carulli, N.; Medici, G.; Menozzi, D.; Salvioli, G.; Bertolotti, M.; Montanari, M. Effect of ursocholic acid on bile lipid secretion and composition. Gastroenterology 1986, 90, 865–874. [Google Scholar]
- Carulli, N.; Bertolotti, M.; Carubbi, F.; Concari, M.; Martella, P.; Carulli, L.; Loria, P. Review article: Effect of bile salt pool composition on hepatic and biliary functions. Alim. Pharmacol. Ther. 2000, 14, 14–18. [Google Scholar] [CrossRef]
- Danzinger, R.G.; Hofmann, A.F.; Thistle, J.L.; Schoenfield, L.J. Effect of oral chenodeoxycholic acid on bile acid kinetics and biliary lipid composition in women with cholelithiasis. J. Clin. Invest. 1973, 52, 2809–2821. [Google Scholar] [CrossRef]
- LaRusso, N.F.; Szczepanik, P.A.; Hofmann, A.F. Effect of deoxycholic acid ingestion on bile acid metabolism and biliary lipid secretion in normal subjects. Gastroenterology 1977, 72, 132–140. [Google Scholar]
- Nilsell, K.; Angelin, B.; Leijd, B.; Einarsson, K. Comparative effects of ursodeoxycholic acid and chenodeoxycholic acid on bile acid kinetics and biliary lipid secretion in humans. Evidence for different modes of action on bile acid synthesis. Gastroenterology 1983, 85, 1248–1256. [Google Scholar]
- Pooler, P.A.; Duane, W.C. Effects of bile acid administration on bile acid synthesis and its circadian rhythm in man. Hepatology 1988, 8, 1140–1146. [Google Scholar] [CrossRef]
- Tauber, G.; Empen, K.; Scheibner, J.; Fuchs, M.; Stange, E.F. Feedback regulation of bile acid synthesis measured by stable isotope kinetics in humans. Eur. J. Gastroenterol. Hepatol. 1996, 8, 23–31. [Google Scholar] [CrossRef]
- Mazzella, G.; Parini, P.; Bazzoli, F.; Villanova, N.; Festi, D.; Aldini, R.; Roda, A.; Cipolla, A.; Polimeni, C.; Tonelli, D.; et al. Ursodeoxycholic acid administration on bile acid metabolism in patients with early stages of primary biliary cirrhosis. Dig. Dis. Sci. 1993, 38, 896–902. [Google Scholar] [CrossRef]
- Princen, H.M.G.; Post, S.M.; Twisk, J. Regulation of bile acid biosynthesis. Curr. Pharm. Des. 1997, 3, 59–84. [Google Scholar]
- Abrahamsson, A.; Gustafsson, U.; Ellis, E.; Nilsson, L.-M.; Sahlin, S.; Björkhem, I.; Einarsson, C. Feedback regulation of bile acid synthesis in human liver: Importance of HNF-4α for regulation of CYP7A1. Biochem. Biophys. Res. Commun. 2005, 330, 395–399. [Google Scholar] [CrossRef]
- Einarsson, C.; Hillebrant, C.G.; Axelson, M. Effects of treatment with deoxycholic acid and chenodeoxycholic acid on the hepatic synthesis of cholesterol and bile acids in healthy subjects. Hepatology 2001, 33, 1189–1193. [Google Scholar] [CrossRef]
- Hillebrant, C.-G.; Nyberg, B.; Angelin, B.; Axelson, M.; Björkhem, I.; Rudling, M.; Einarsson, C. Deoxycholic acid treatment in patients with cholesterol gallstones: Failure to detect a suppression of cholesterol 7alpha-hydroxylase activity. J. Intern. Med. 1999, 246, 399–407. [Google Scholar] [CrossRef]
- Andersén, E. The effect of cholestyramine on bile acid kinetics in healthy controls. Scand. J. Gastroenterol. 1979, 14, 657–662. [Google Scholar] [CrossRef]
- Bertolotti, M.; Zambianchi, L.; Carulli, L.; Simonini, M.S.; Del Puppo, M.; Galli Kienle, M.; Loria, P.; Pinetti, A.; Carulli, N. Influence of newly synthesized cholesterol on bile acid synthesis during chronic inhibition of bile acid absorption. Hepatology 2003, 38, 939–946. [Google Scholar]
- Beysen, C.; Murphy, E.J.; Deines, K.; Chan, M.; Tsang, E.; Glass, A.; Turner, S.M.; Protasio, J.; Riiff, T.; Hellerstein, M.K. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: A randomised controlled study. Diabetologia 2011, 55, 432–442. [Google Scholar]
- Rudling, M.; Angelin, B.; Ståhle, L.; Reihnér, E.; Sahlin, S.; Olivecrona, H.; Björkhem, I.; Einarsson, C. Regulation of hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl Coenzyme A reductase, and cholesterol 7α-hydroxylase mRNAs in human liver. J. Clin. Endocrinol. Metab. 2002, 87, 4307–4313. [Google Scholar] [CrossRef]
- Mazzella, G.; Parini, P.; Festi, D.; Bazzoli, F.; Aldini, R.; Roda, A.; Tonelli, D.; Cipolla, A.; Salzetta, A.; Roda, E. Effect of simvastatin, ursodeoxycholic acid and simvastatin plus ursodeoxycholic acid on biliary lipid secretion and cholic acid kinetics in nonfamilial hypercholesterolemia. Hepatology 1992, 15, 1072–1078. [Google Scholar] [CrossRef]
- Loria, P.; Bertolotti, M.; Cassinadri, M.T.; Dilengite, M.A.; Bozzoli, M.; Carubbi, F.; Concari, M.; Guicciardi, M.E.; Carulli, N. Short-term effects of simvastatin on bile acid synthesis and bile lipid secretion in human subjects. Hepatology 1994, 19, 882–888. [Google Scholar] [CrossRef]
- Kern, F.Jr.; Everson, G.T.; DeMark, B.; McKinley, C.; Showalter, R.; Braverman, D.Z.; Szczepanik-Van Leeuwen, P.; Klein, P.D. Biliary lipids, bile acids, and gallbladder function in the human female: Effects of contraceptive steroids. J. Lab. Clin. Med. 1982, 99, 798–805. [Google Scholar]
- van der Werf, S.D.; van Berge Henegouwen, G.P.; Ruben, A.T.; Palsma, D.M. Biliary lipids, bile acid metabolism, gallbladder motor function and small intestinal transit during ingestion of a sub-fifty oral contraceptive. J. Hepatol. 1987, 4, 318–326. [Google Scholar] [CrossRef]
- Everson, G.T.; Fennessey, P.; Kern, F., Jr. Contraceptive steroids alter the steady-state kinetics of bile acids. J. Lipid Res. 1988, 29, 68–76. [Google Scholar]
- Everson, G.T.; McKinley, C.; Kern, F.Jr. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J. Clin. Invest. 1991, 87, 237–246. [Google Scholar]
- Mazzella, G.; Fusaroli, P.; Pezzoli, A.; Azzaroli, F.; Mazzeo, C.; Zambonin, L.; Simoni, P.; Festi, D.; Roda, E. Methylprednisolone administration in primary biliary cirrhosis increases cholic acid turnover, synthesis, and deoxycholate concentration in bile. Dig. Dis. Sci. 1999, 44, 2478–2483. [Google Scholar] [CrossRef]
- Veysey, M.J.; Thomas, L.A.; Mallet, A.I.; Jenkins, P.J.; Besser, G.M.; Murphy, G.M.; Dowling, R.H. Colonic transit influences deoxycholic acid kinetics. Gastroenterology 2001, 121, 812–822. [Google Scholar] [CrossRef]
- Olivecrona, H.; Ericsson, S.; Angelin, B. Growth hormone treatment does not alter biliary lipid metabolism in healthy adult men. J. Clin. Endocrinol. Metab. 1995, 80, 1113–1117. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bertolotti, M.; Crosignani, A.; Del Puppo, M. The Use of Stable and Radioactive Sterol Tracers as a Tool to Investigate Cholesterol Degradation to Bile Acids in Humans in Vivo. Molecules 2012, 17, 1939-1968. https://doi.org/10.3390/molecules17021939
Bertolotti M, Crosignani A, Del Puppo M. The Use of Stable and Radioactive Sterol Tracers as a Tool to Investigate Cholesterol Degradation to Bile Acids in Humans in Vivo. Molecules. 2012; 17(2):1939-1968. https://doi.org/10.3390/molecules17021939
Chicago/Turabian StyleBertolotti, Marco, Andrea Crosignani, and Marina Del Puppo. 2012. "The Use of Stable and Radioactive Sterol Tracers as a Tool to Investigate Cholesterol Degradation to Bile Acids in Humans in Vivo" Molecules 17, no. 2: 1939-1968. https://doi.org/10.3390/molecules17021939



