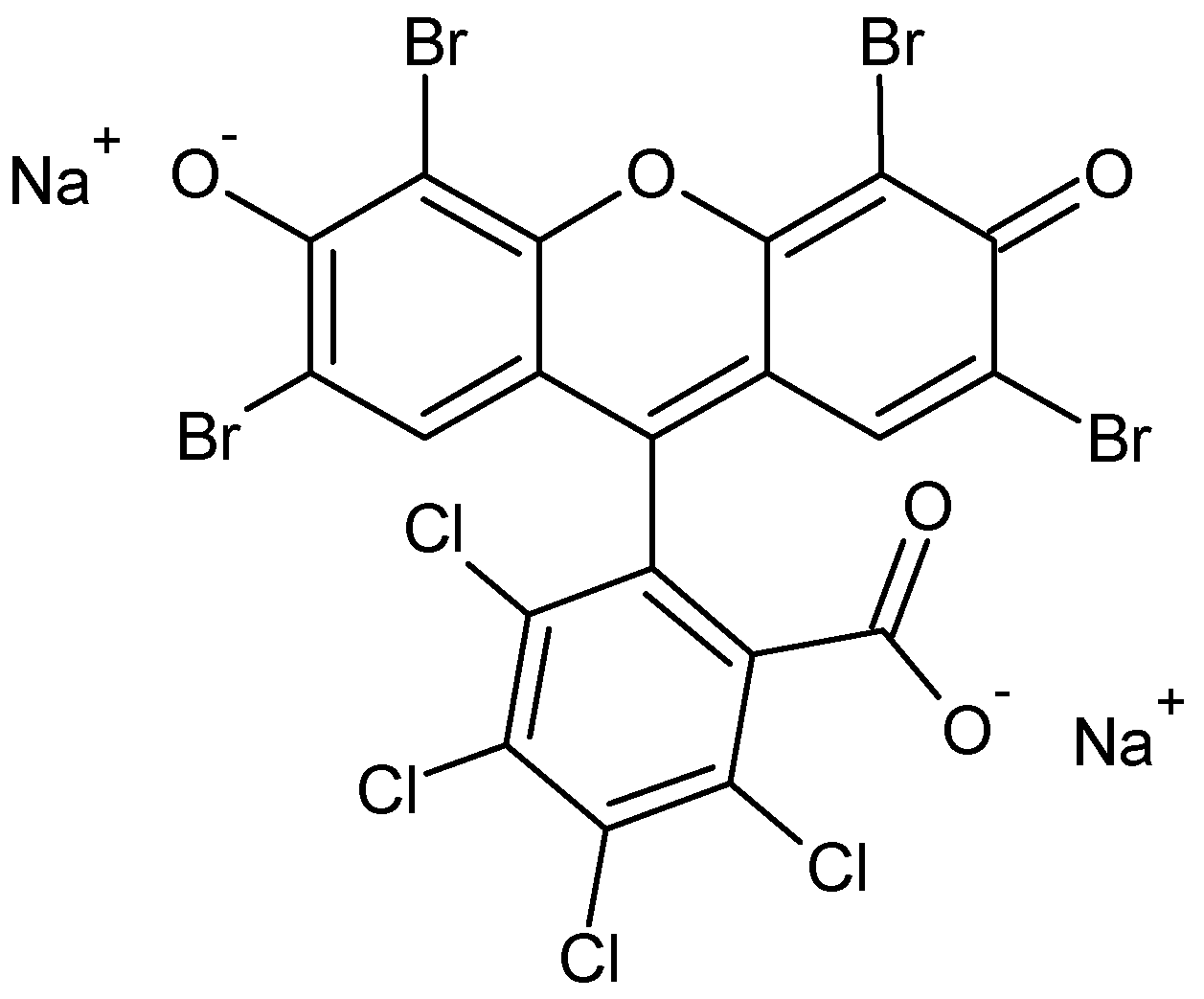
Phloxine B as a Probe for Entrapment in Microcrystalline Cellulose
Abstract
:1. Introduction
2. Results and Discussion
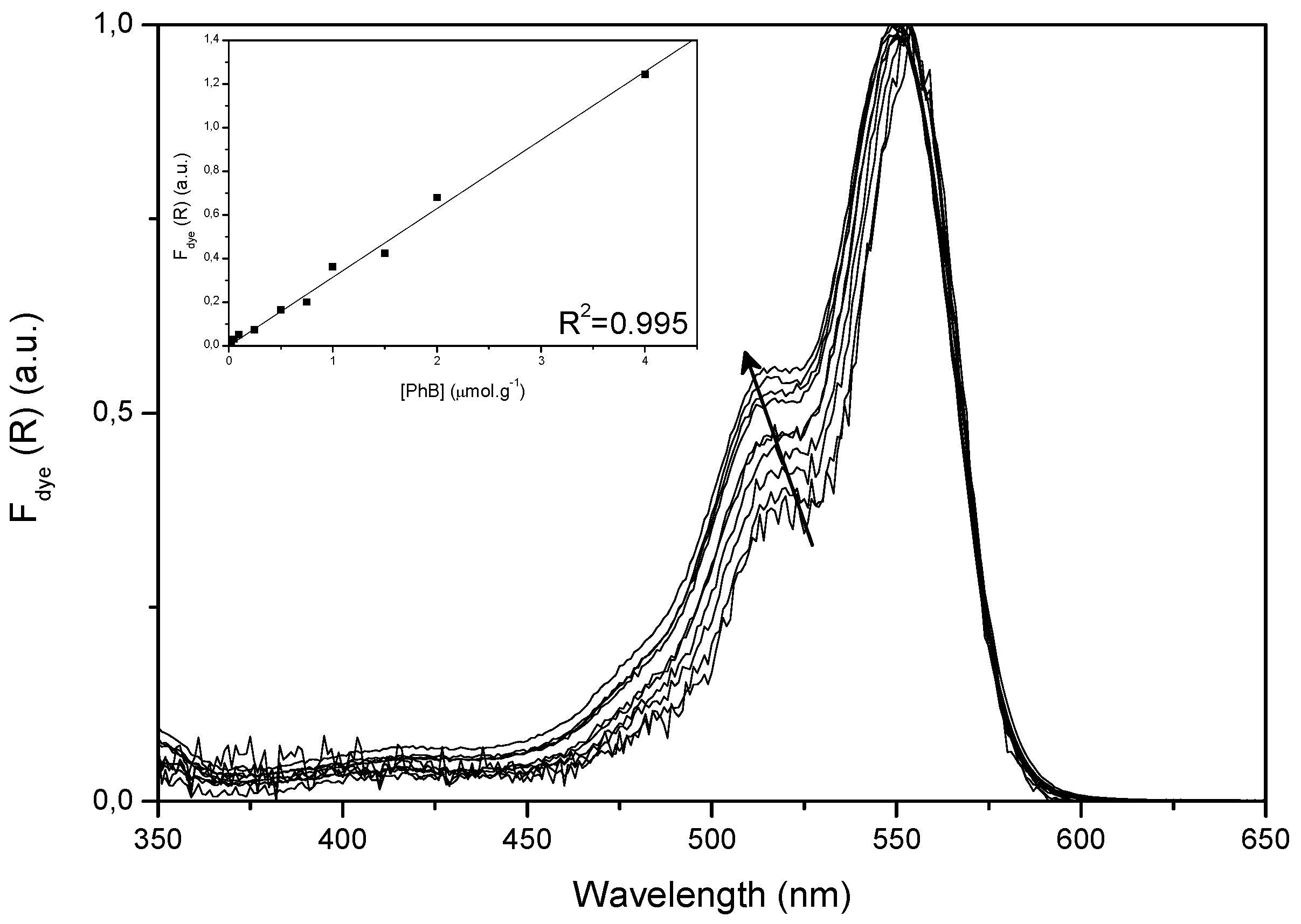
2.1. Ground State Absorption Studies
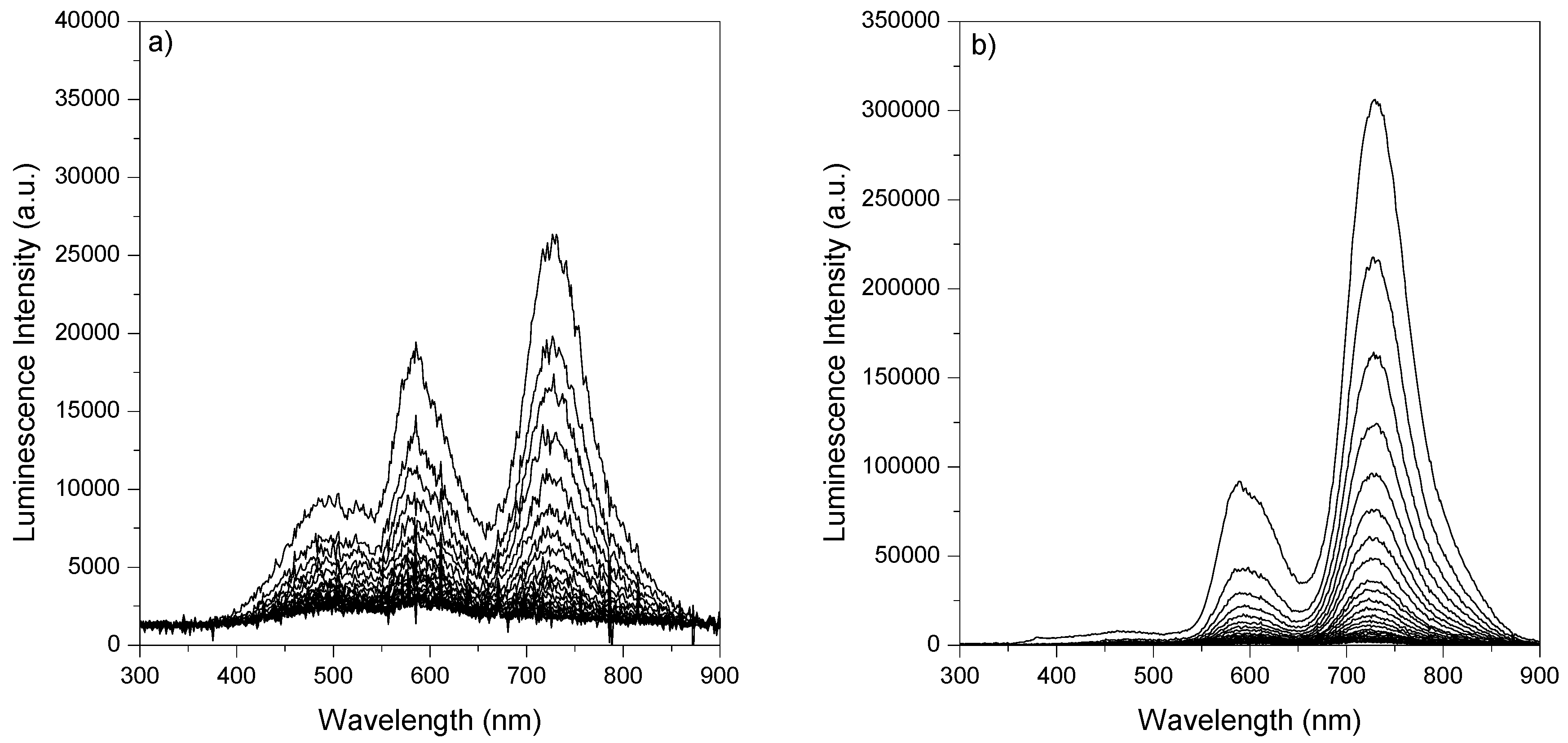
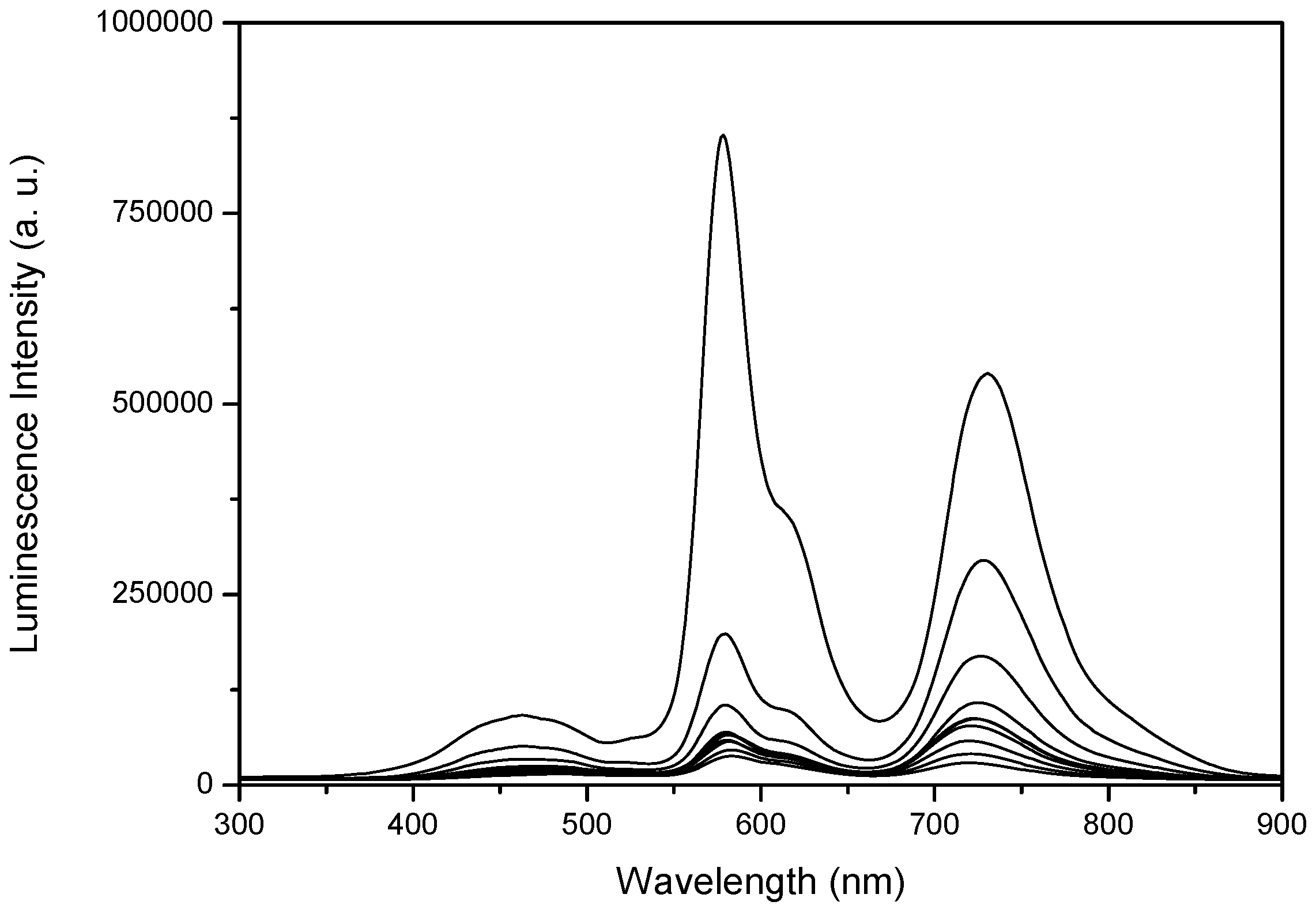
2.2. Room-Temperature Laser Induced Luminescence


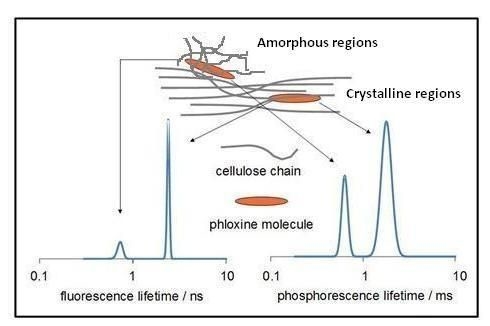
2.2.1. Lifetime Distributions Analysis (LDA)
2.2.1.1. Fluorescence Decay


2.2.1.2. Phosphorescence Decay
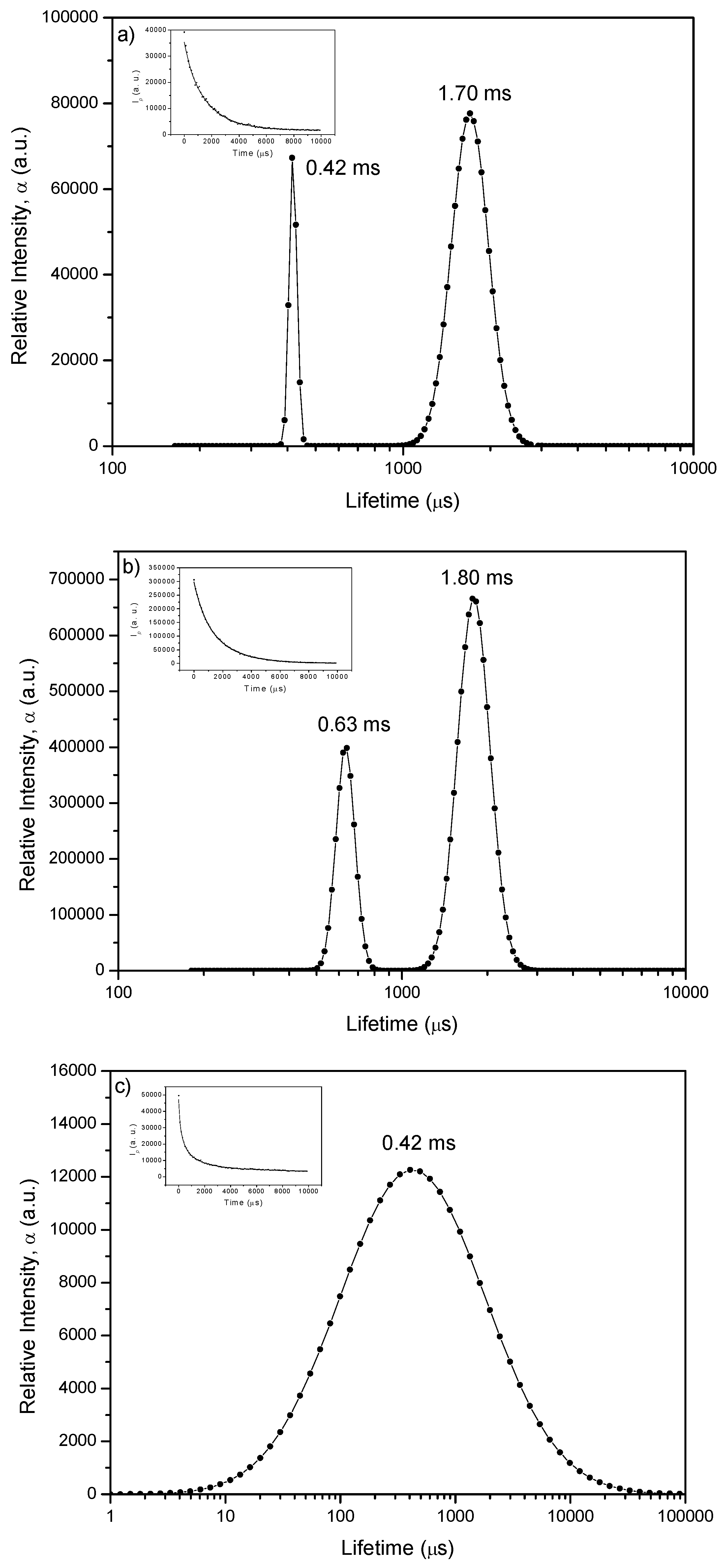
2.3. Laser Induced Luminescence at 77 K for Phloxine B Adsorbed onto Microcrystalline Cellulose
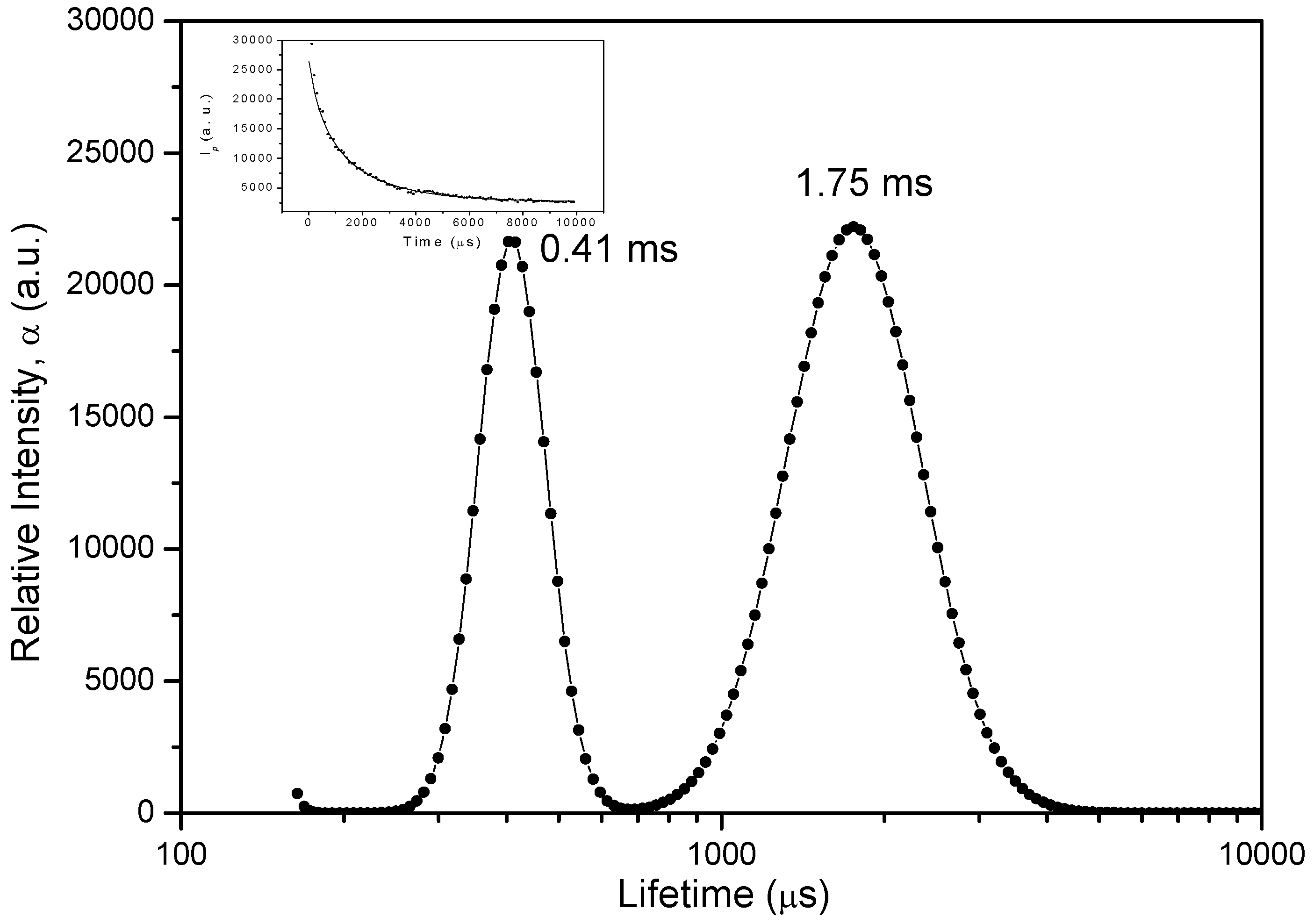
2.4. Delayed Fluorescence Decay
3. Experimental
3.1. Materials and Sample Preparation
3.2. Ground-State Diffuse Reflectance Absorption Studies
3.3. Laser Induced Luminescence (LIL) Setup
3.4. Fluorescence Lifetime Set-up
4. Conclusions
Acknowledgements
- Sample Availability: Please contact the authors.
References and Notes
- Fleming, G.R.; Knight, A.W.E.; Morris, J.M.; Morrison, R.J.S.; Robinson, G.W. Picosecond Fluorescence Studies of Xanthene Dyes. J. Am. Chem. Soc. 1977, 99, 4306–4311. [Google Scholar] [CrossRef]
- Madge, D.; Rojas, G.E.; Seybold, P.G. Solvent Dependence of the Fluorescence Lifetimes of Xanthene Dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Arbeloa, F.L.; Arbeloa, M.J.; Estévez, M.J.T.; Arbeloa, I.L. Photophysics of Rhodamines. Molecular Structure and Solvent Effects. J. Phys. Chem. 1991, 95, 2203–2208. [Google Scholar]
- Martin, M.M. Hydrogen Bond Effects on Radiationless Electronic Transitions in Xanthene Dyes. Chem. Phys. Lett. 1975, 35, 105–111. [Google Scholar]
- Penzkofer, A.; Tyagi, A.; Slyusareva, E.; Sizykh, A. Phosphorescence and delayed fluorescence properties of fluorone dyes in bio-related films. Chem. Phys. 2010, 378, 58–65. [Google Scholar] [CrossRef]
- Slyusareva, E.; Sizykh, A.; Tyagi, А.; Penzkofer, A. Spectral and photophysical properties of fluorone dyes in bio-related films and methanol. J. Photochem. Photobiol. A Chem. 2009, 208, 131–140. [Google Scholar] [CrossRef]
- Alcantara-Licudine, J.P.; Bui, N.L.; Kawate, M.K.; Li, Q.X. Analysis of Phloxine B and Uranine in Coffee by High-Performance Liquid Chromatography and Capillary Zone Electrophoresis after Solid Phase Extraction Cleanup. J. Agric. Food Chem. 1998, 46, 1005–1011. [Google Scholar] [CrossRef]
- Berni, J.; Rabossi, A.; Pujol-Lereis, L.M.; Tolmasky, D.S.; Quesada-Allué, L.A. Phloxine B affects glycogen metabolism in larval stages of Ceratitis capitata (Diptera: Tephritidae). Pestic. Biochem. Phys. 2009, 95, 12–17. [Google Scholar] [CrossRef]
- Wetzler, D.E.; García-Fresnadillo, D.; Orellana, G. A clean, well-defined solid system for photosensitized 1O2 production measurements. Phys. Chem. Chem. Phys. 2006, 8, 2249–2256. [Google Scholar]
- Pravinata, L.C.; You, Y.; Ludescher, R.D. Erythrosin B Phosphorescence Monitors Molecular Mobility and Dynamic Site Heterogeneity in Amorphous Sucrose. Biophys. J. 2005, 88, 3551–3561. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Freixo, M.R.; Garcia, A.R.; Wilkinson, F. Photochemistry on Surfaces: Fluorescence Emission Quantum Yield Evaluation of Dyes adsorbed on Microcrystalline Cellulose. J. Chem. Soc. Faraday Trans. 1992, 88, 15–22. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Garcia, A.R.; Freixo, M.R.; Costa, S.M.B. Photochemistry on Surfaces: Solvent-Matrix Effect on the Swelling of Cellulose: An Emission and Absorption Study of Adsorbed Auramine 0. J. Chem. Soc. Faraday Trans. 1993, 89, 1937–1944. [Google Scholar] [CrossRef]
- Rodríguez, H.B.; Lagorio, M.G.; San Román, E. Rose Bengal adsorbed on microgranular cellulose: evidence on fluorescent dimers. Photochem. Photobiol. Sci. 2004, 3, 674–680. [Google Scholar] [CrossRef]
- Rodríguez, H.B.; San Román, E. Energy Transfer from Chemically Attached Rhodamine 101 to Adsorbed Methylene Blue on Microcrystalline Cellulose Particles. Photochem. Photobiol. 2007, 83, 547–555. [Google Scholar] [CrossRef]
- López, S.G.; Worringer, G.; Rodríguez, H.B.; San Román, E. Trapping of Rhodamine 6G excitation energy on cellulose microparticles. Phys. Chem. Chem. Phys. 2010, 12, 2246–2253. [Google Scholar]
- Vieira Ferreira, L.F.; Netto-Ferreira, J.C.; Kmelinskii, I.V.; Garcia, A.R.; Costa, S.M.B. Photochemistry on Surfaces: Matrix Isolation Mechanisms Study of Interactions of Benzophenone Adsorbed on Microcrystalline Cellulose Investigated by Diffuse Reflectance and Luminescence Techniques. Langmuir 1995, 11, 231–236. [Google Scholar]
- Neckers, D.C.; Rose Bengal, J. Photochem. Photobiol Chem. A 1989, 47, 1–29. [Google Scholar]
- Wilkinson, F.; Leicester, P.A.; Vieira Ferreira, L.F.; Freire, V.M.M.R. Photochemistry on Surfaces: Triplet-Triplet Energy Transfer on Microcrystalline Cellulose Studied by Diffuse Reflectance Transient Absorption and Emission Spectroscopy. Photochem. Photobiol. 1991, 54, 599–608. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Ferreira Machado, I.L.; Silva, J.P.; Oliveira, A.S. A diffuse reflectance comparative study of benzil inclusion within microcrystalline cellulose and β-cyclodextrin. Photochem. Photobiol. Sci. 2004, 3, 174–181. [Google Scholar] [CrossRef]
- Murov, S.L.; Carmichael, I.; Hug, G.L. Handbook of Photochemistry, 2nd ed; Marcel Dekker: New York, NY, USA, 1993; p. 28. [Google Scholar]
- Drexhage, K.H. Structure and Properties of Laser Dyes. In Topics in Applied Physics. Dye Lasers; Shäfer, F.P., Ed.; Springer: Berlin, Germany, 1973; Volume 1, pp. 149–150. [Google Scholar]
- Kemnitz, K.; Tamai, N.; Yamazaki, Y.; Nakashima, N.; Yoshihara, K. Site-Dependent Fluorescence Lifetimes of Isolated Dye Molecules Adsorbed on Organic Single Crystals and Other Substrates. J. Phys. Chem. 1987, 91, 1423–1430. [Google Scholar] [CrossRef]
- Branco, T.J.F.; Botelho do Rego, A.M.; Ferreira Machado, I.; Vieira Ferreira, L.F. Luminescence Lifetime Distributions Analysis in Heterogeneous Systems by the Use of Excel’s Solver. J. Phys. Chem. B 2005, 109, 15958–15967. [Google Scholar] [CrossRef]
- Samoc, A.; Samoc, M.; Luther-Davies, B. Upconversion of He-Ne Laser Light in Xanthene Dye-Doped Polymer Waveguides. Polish J. Chem. 2002, 76, 345–385. [Google Scholar]
- Botelho do Rego, A.M.; Vieira Ferreira, L.F. Photonic and Electronic Spectroscopies for the Characterization of Organic Surfaces and Organic Molecules Adsorbed on Surfaces. In Handbook of Surfaces and Interfaces of Materials; Nalwa, H.S., Ed.; Academic Press: New York, NY, USA, 2001; Volume 2, pp. 275–313. [Google Scholar]
- Vieira Ferreira, L.F.; Ferreira Machado, I.L. Surface Photochemistry: Organic Molecules within Nanocavities of Calixarenes. Curr. Drug. Discov. Technol. 2007, 4, 229–245. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duarte, P.; Ferreira, D.P.; Ferreira Machado, I.; Vieira Ferreira, L.F.; Rodríguez, H.B.; San Román, E. Phloxine B as a Probe for Entrapment in Microcrystalline Cellulose. Molecules 2012, 17, 1602-1616. https://doi.org/10.3390/molecules17021602
Duarte P, Ferreira DP, Ferreira Machado I, Vieira Ferreira LF, Rodríguez HB, San Román E. Phloxine B as a Probe for Entrapment in Microcrystalline Cellulose. Molecules. 2012; 17(2):1602-1616. https://doi.org/10.3390/molecules17021602
Chicago/Turabian StyleDuarte, Paulo, Diana P. Ferreira, Isabel Ferreira Machado, Luís Filipe Vieira Ferreira, Hernan B. Rodríguez, and Enrique San Román. 2012. "Phloxine B as a Probe for Entrapment in Microcrystalline Cellulose" Molecules 17, no. 2: 1602-1616. https://doi.org/10.3390/molecules17021602