A Minor Dihydropyran Apocarotenoid from Mated Cultures of Blakeslea trispora
Abstract
:1. Introduction

2. Results and Discussion
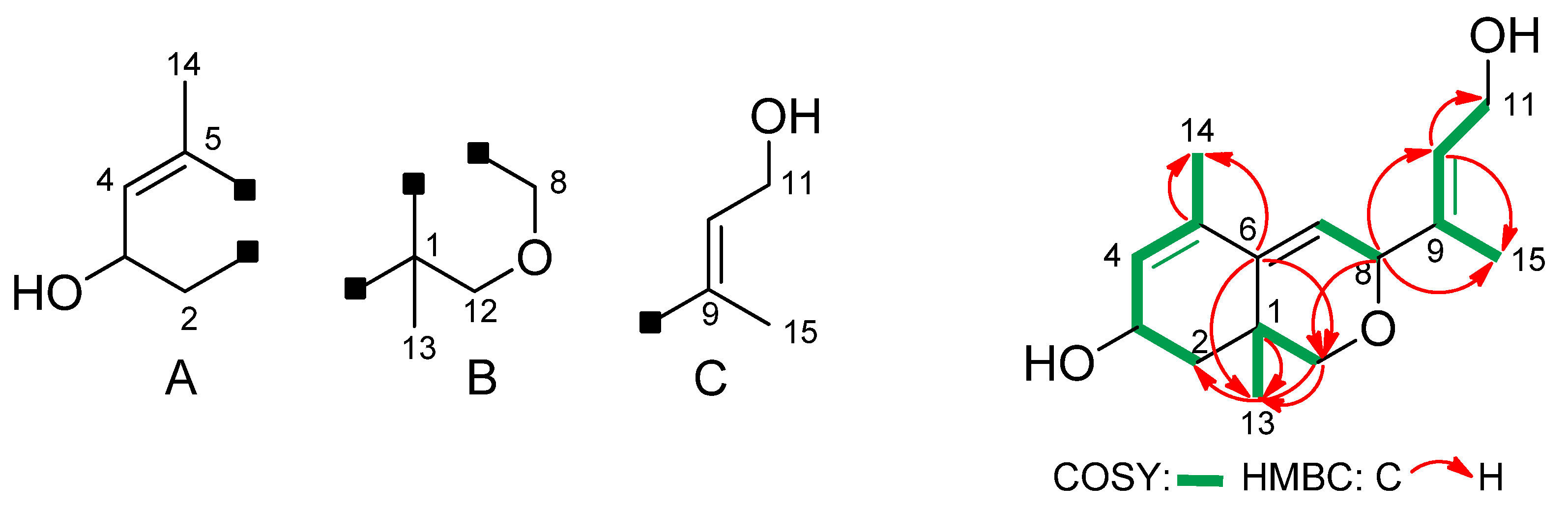
| C/H | δH | δC | COSY | HSQC | HMBC | |
|---|---|---|---|---|---|---|
| 1 | 32.1 | |||||
| 2a | 1.65–1.42 m | 38.4 | H3, H2b | C2 | C12a, C13 | |
| 2b | 1.65–1.42 m | H3, H2a | ||||
| 4.34 br s | 64.4 | H4, H2a, H2b | C3 | |||
| 4 | 5.72 d (4.6) | 125.8 | H3, H14 | C4 | ||
| 5 | 133.0 | |||||
| 6 | 140.9 | |||||
| 7 | 5.65 d (3.3) | 120.5 | H8 | C7 | ||
| 8 | 4.64 br s | 76.5 | H7 | C8 | ||
| 9 | 138.0 | |||||
| 10 | 5.54 t (6.4) | 127.3 | H11, H15 | C10 | C8, C15 | |
| 11 | 4.24 d (6.4) | 59.5 | H10 | C11 | C9, C10 | |
| 12a | 3.32 d (10.7) | 70.5 | H12b, H13 | C12 | C8, C6, C13 | |
| 12b | 3.36 d (10.7) | H12a, H13 | ||||
| 13 | 1.35 s | 24.6 | H12a, H12b | C13 | C12, C6, C2a, C1 | |
| 14 | 1.86 s | 19.4 | H4 | C14 | C6, C5, C4 | |
| 15 | 1.77 s | 14.9 | H10 | C15 | C8, C9, C10 | |
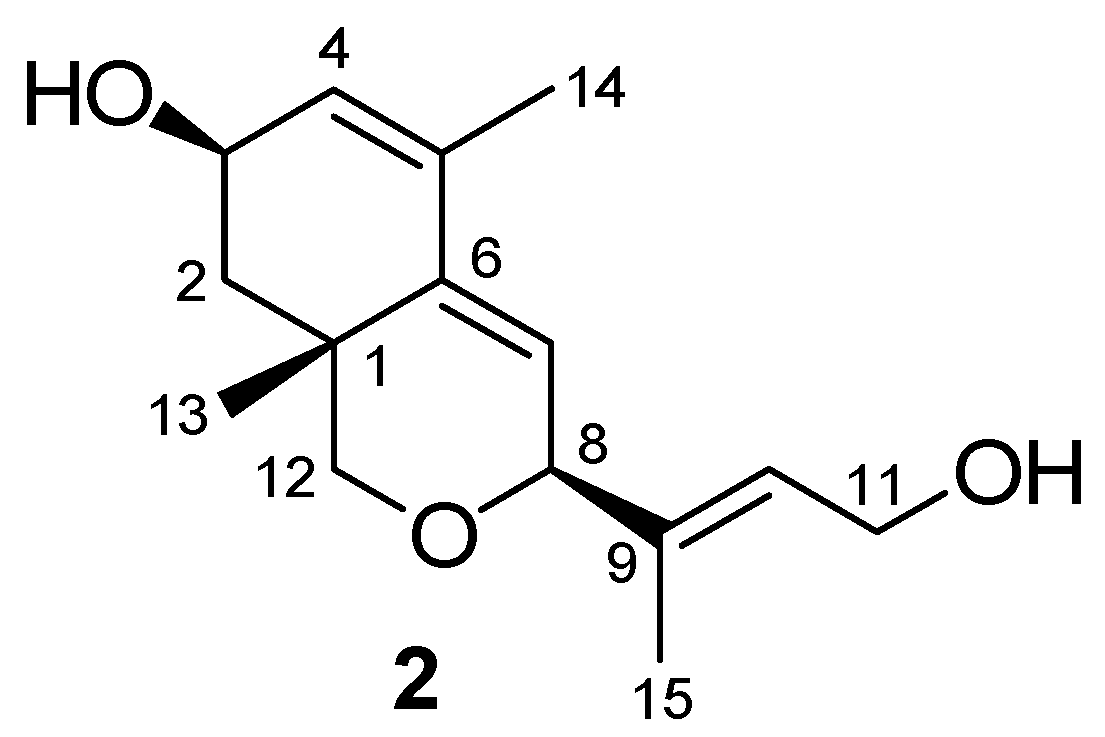

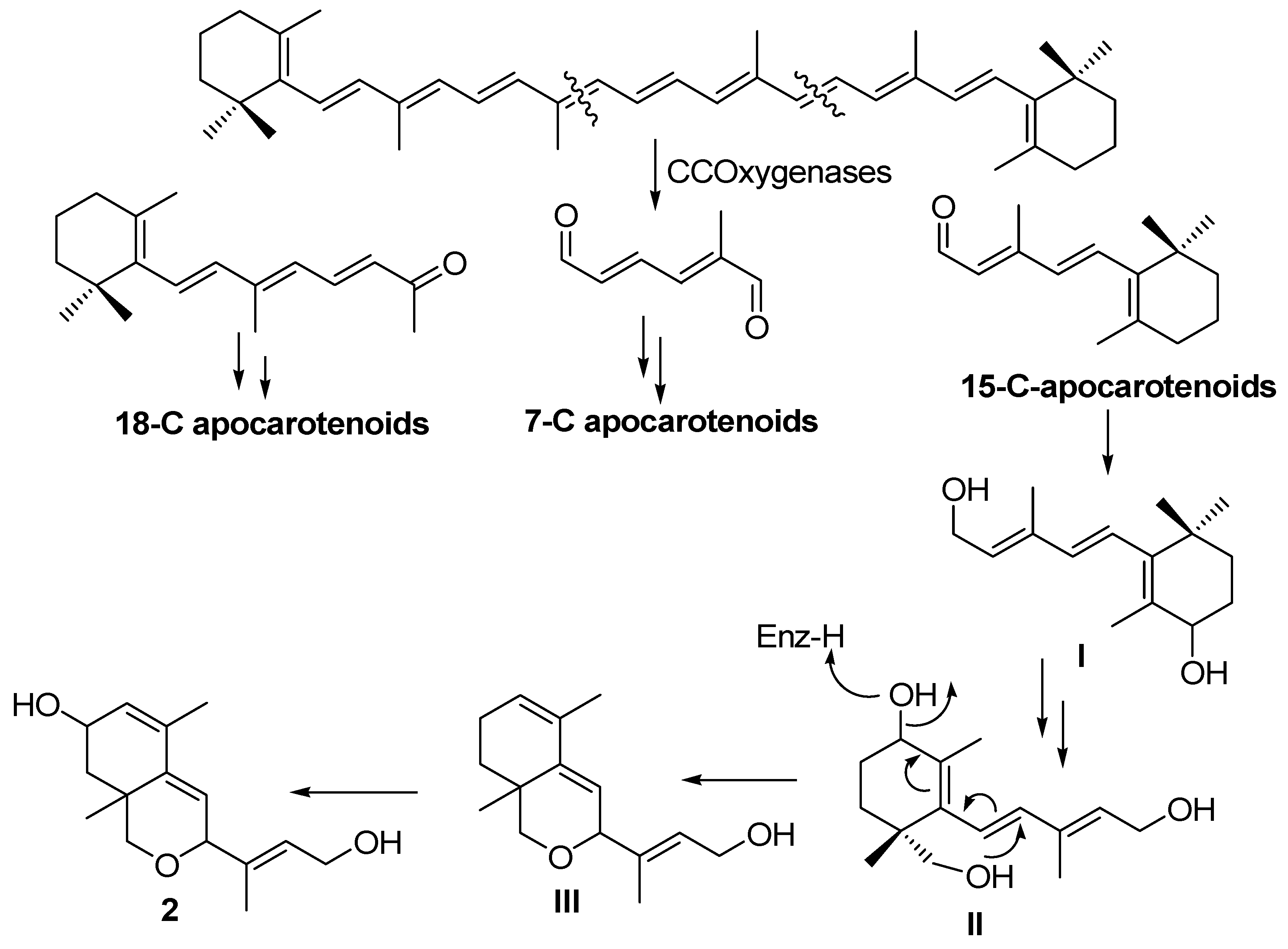
3. Experimental
3.1. General
3.2. Strains and Culture Conditions
3.3. Extraction and Fractionation of Apocarotenoids.
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Ciegler, A. Microbial carotenogenesis. Adv. Appl. Microbiol. 1965, 7, 1–34. [Google Scholar] [CrossRef]
- Avalos, J.; Cerdá-Olmedo, E. Fungal carotenoid production. In Handbook of Fungal Biotechnology; Arora, D.K., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 367–378. [Google Scholar]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef]
- Caglioti, L.; Cainelli, G.; Camerino, B.; Mondelli, R.; Prieto, A.; Quilico, A.; Salvatori, T.; Selva, A. The structure of trisporic C acid. Tetrahedron Suppl. 1966, 7, 175–187. [Google Scholar]
- Austin, D.J.; Bu’Lock, J.D.; Drake, D. The Biosynthesis of trisporic acids from β-carotene via retinal and trisporol. Experientia 1970, 26, 348–349. [Google Scholar] [CrossRef]
- Gooday, G.W. Fungal sex hormones. Ann. Rev. Biochem. 1974, 43, 35–49. [Google Scholar] [CrossRef]
- Nieuwenhuis, M.; van den Ende, H. Sex specificity of hormone synthesis in Mucor. mucedo. Arch. Microbiol. 1975, 102, 167–169. [Google Scholar] [CrossRef]
- Schachtschabel, D.; David, A.; Menzel, K.-D.; Schimek, C.; Wöstemeyer, J.; Boland, W. Cooperative biosynthesis of trisporoids by the (+) and (−) mating types of the Zygomycete Blakeslea. trispora. ChemBioChem 2008, 9, 3004–3012. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Austin, D.J.; Bu’Lock, J.D.; Gooday, G.W. Trisporic acids: Sexual hormones from Mucor. mucedo and Blakeslea. trispora. Nature 1969, 223, 1178–1179. [Google Scholar]
- Cainelli, G.; Grasselli, P.; Selva, A. Structure of trisporic acid B. Chim. Ind. 1967, 49, 628–629. [Google Scholar]
- Sutter, R.P.; Dadok, J.; Bothner-By, A.A.; Smith, R.R.; Mishra, P.K. Cultures of separates mating types of Blakeslea. trispora make D and E forms of trisporic acids. Biochemistry 1989, 28, 4060–4066. [Google Scholar]
- Bu’Lock, J.D.; Jones, B.E.; Winskill, N. The apocarotenoid system of sex hormones and prohormones in Mucorales. Pure Appl. Chem. 1976, 47, 191–202. [Google Scholar] [CrossRef]
- Sutter, R.P.; Whitaker, J.P. Zygophore-stimulating precursors (pheromones) of trisporic acids active in (−)-Phycomyces. blakesleeanus. Acid-catalyzed anhydro derivatives of methyl 4-dihydrotrisporate-C and 4-dihydrotrisporate-C. J. Biol. Chem. 1981, 256, 2334–2341. [Google Scholar]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Gil, J.; González, J.-A.; Alcalde, E.; Cerdá-Olmedo, E. New apocarotenoids and β-carotene cleavage in Blakeslea. trispora. Org. Biomol. Chem. 2011, 9, 7190–7195. [Google Scholar] [CrossRef]
- Sutter, R.P.; Zawodny, P.D. Apotrisporin: A major metabolite of Blakeslea. trispora. Exp. Mycol. 1984, 8, 89–92. [Google Scholar] [CrossRef]
- Sutter, R.P. A new sesquiterpenoid isolated from Phycomyces. blakesleeanus and Blakeslea. trispora. Exp. Mycol. 1986, 10, 256–258. [Google Scholar] [CrossRef]
- Cainelli, G.; Camerino, B.; Grasselli, P.; Mondelli, R.; Morrocchi, S.; Prieto, A.; Quilico, A.; Selva, A. Structure del trisporone e dell’anhidrotrisporone. Chim. Ind. 1967, 49, 748–751. [Google Scholar]
- Polaino, S.; González-Delgado, J.; Arteaga, P.; Herrador, M.M.; Barrero, A.F.; Cerdá-Olmedo, E. Apocarotenoids in the sexual interaction of Phycomyces. blakesleeanus. Org. Biomol. Chem. 2012, 10, 3002–3009. [Google Scholar]
- Somoza, A.D.; Lee, K.-H.; Chiang, Y.-M.; Oakley, B.R.; Wang, C.C.C. Reengineering an azaphilone biosynthesis pathway in Aspergillus. nidulans to create lipoxygenase inhibitors. Org. Lett. 2012, 14, 972–975. [Google Scholar]
- Polaino, S.; Herrador, M.M.; Cerdá-Olmedo, E.; Barrero, A.F. Splitting of β-carotene in the sexual interaction of Phycomyces. Biomol. Chem. 2010, 8, 4229–4231. [Google Scholar] [CrossRef]
- Cerdá-Olmedo, E. Standard growth conditions and variations. In Phycomyces; Cerdá-Olmedo, E., Lipson, E.D., Eds.; Cold Spring Harbor: New York, NY, USA, 1987; pp. 337–339. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barrero, A.F.; Herrador, M.M.; Artega, P.; González, J.-A.; Arteaga, J.F. A Minor Dihydropyran Apocarotenoid from Mated Cultures of Blakeslea trispora. Molecules 2012, 17, 12553-12559. https://doi.org/10.3390/molecules171112553
Barrero AF, Herrador MM, Artega P, González J-A, Arteaga JF. A Minor Dihydropyran Apocarotenoid from Mated Cultures of Blakeslea trispora. Molecules. 2012; 17(11):12553-12559. https://doi.org/10.3390/molecules171112553
Chicago/Turabian StyleBarrero, Alejandro F., M. Mar Herrador, Pilar Artega, José-Antonio González, and Jesús F. Arteaga. 2012. "A Minor Dihydropyran Apocarotenoid from Mated Cultures of Blakeslea trispora" Molecules 17, no. 11: 12553-12559. https://doi.org/10.3390/molecules171112553




