Synthetic Flavanones Augment the Anticancer Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)
Abstract
:1. Introduction

2. Results and Discussion
2.1. Cytotoxic and Apoptotic Activities of Flavanones in HeLa Cancer Cells
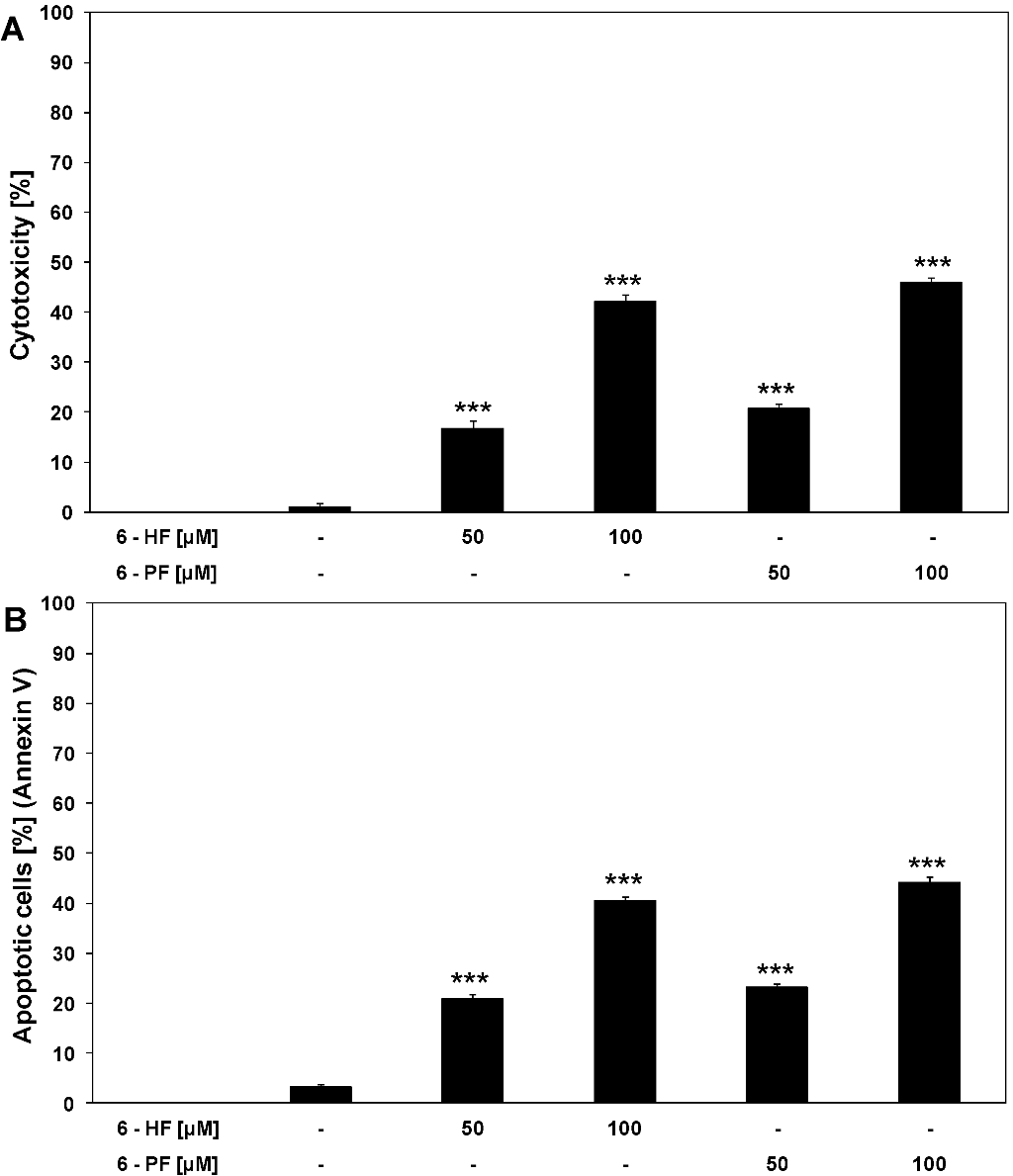
2.2. Cytotoxic and Apoptotic Activities of TRAIL in HeLa Cancer Cells
2.3. Cytotoxic and Apoptotic Activities of TRAIL in Combination with Synthetic Flavanones in HeLa Cancer Cells
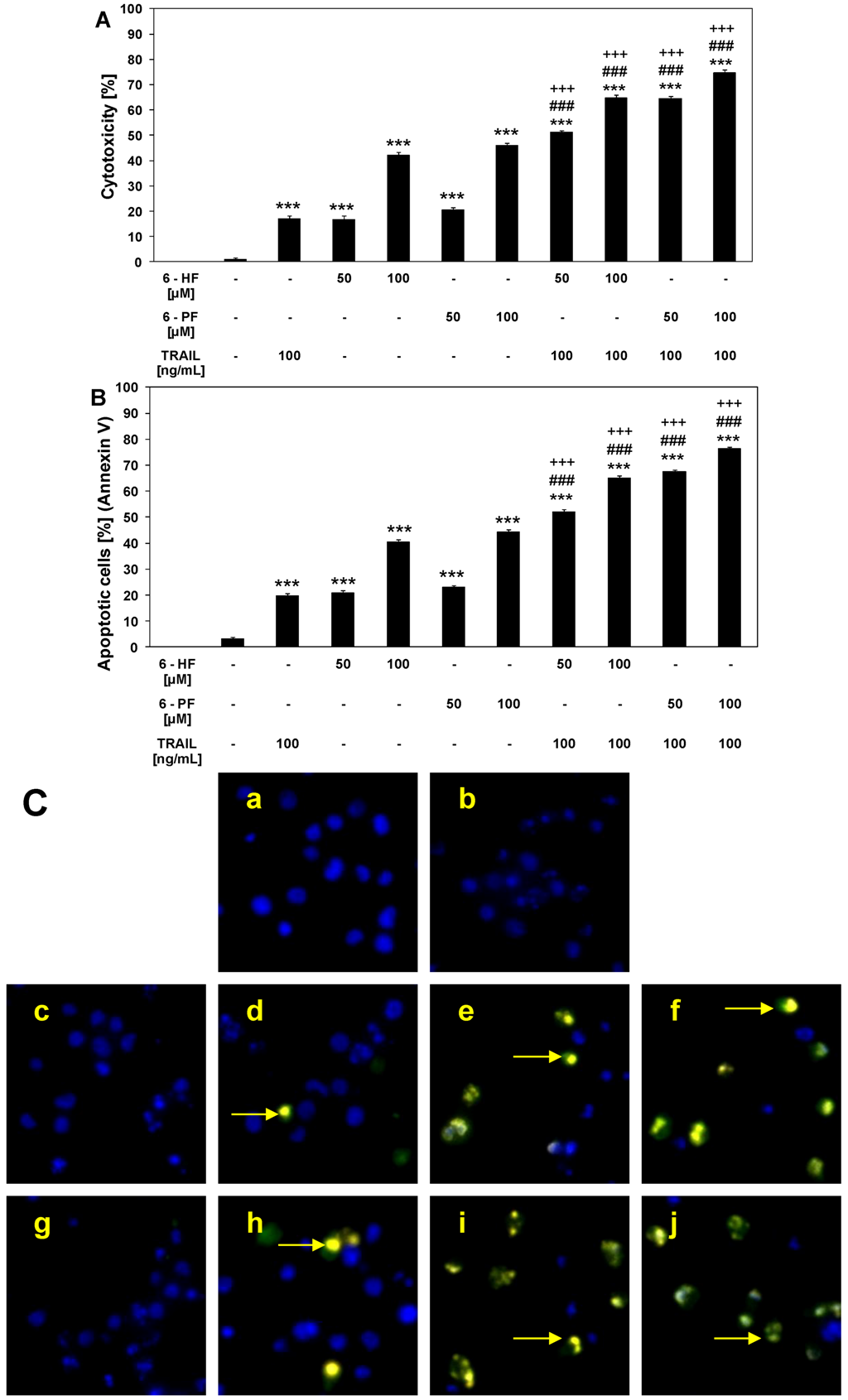
2.4. Effects of 6-HF and 6-PF on Death Receptor Expression in HeLa Cancer Cells
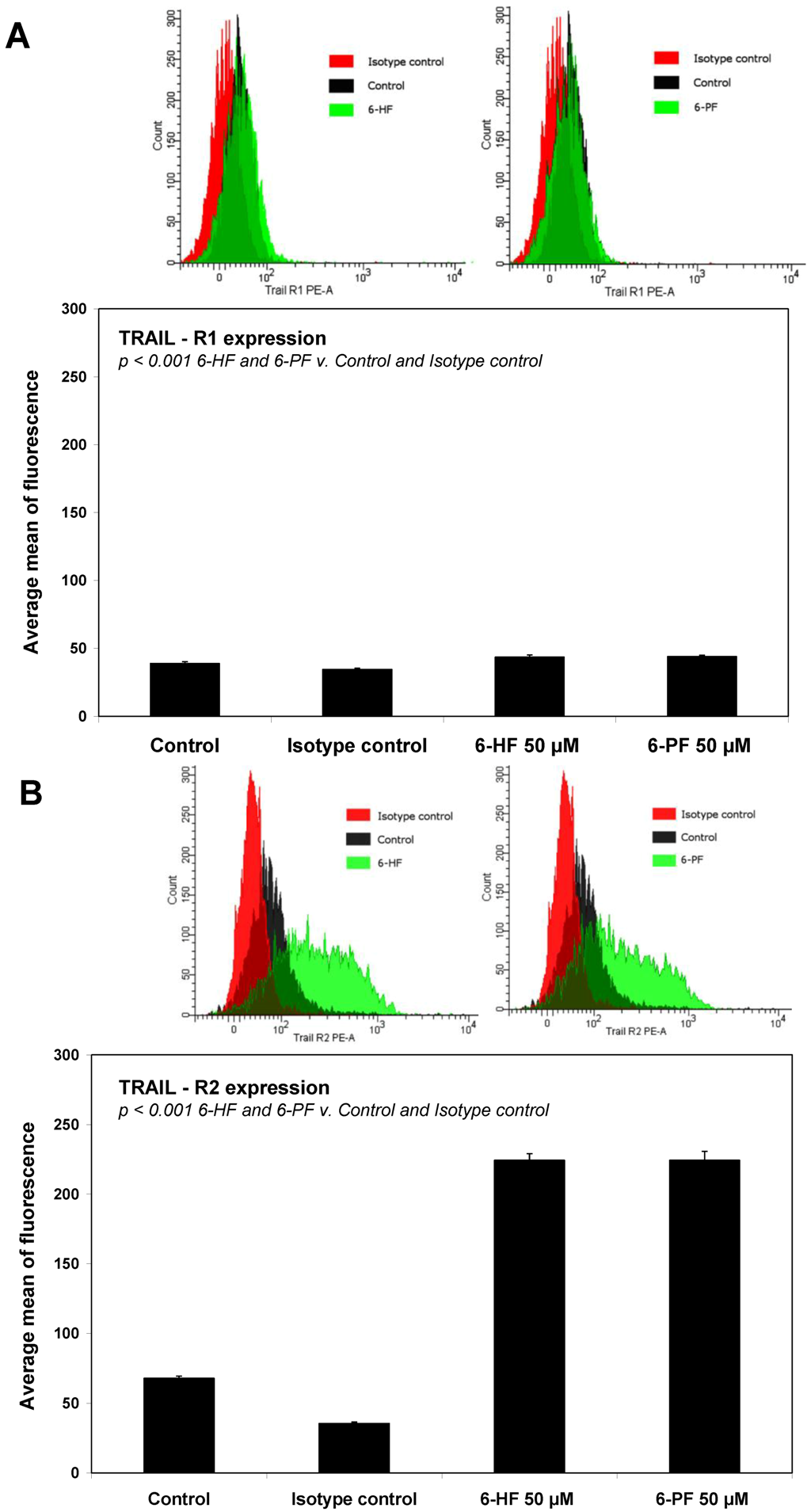
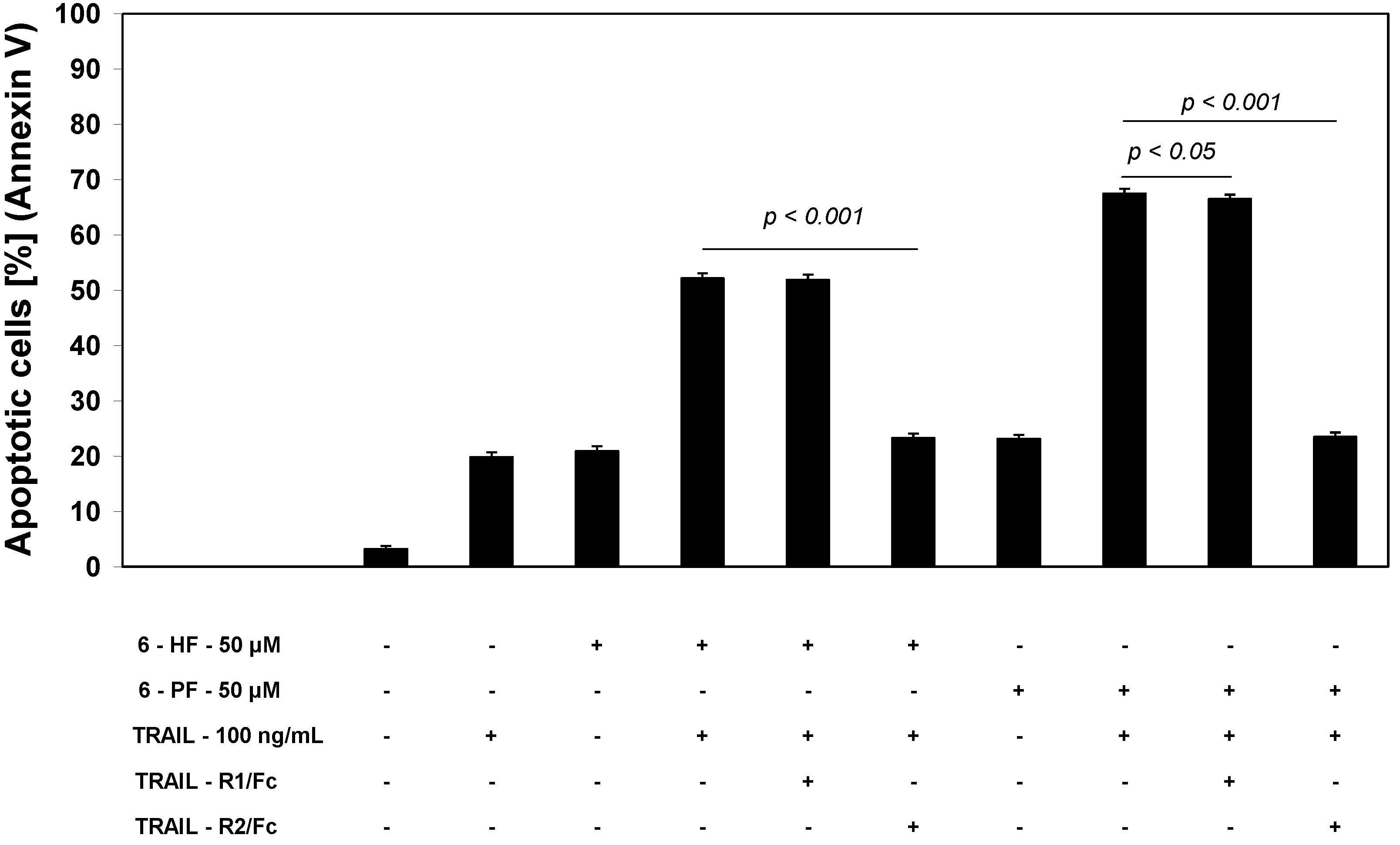
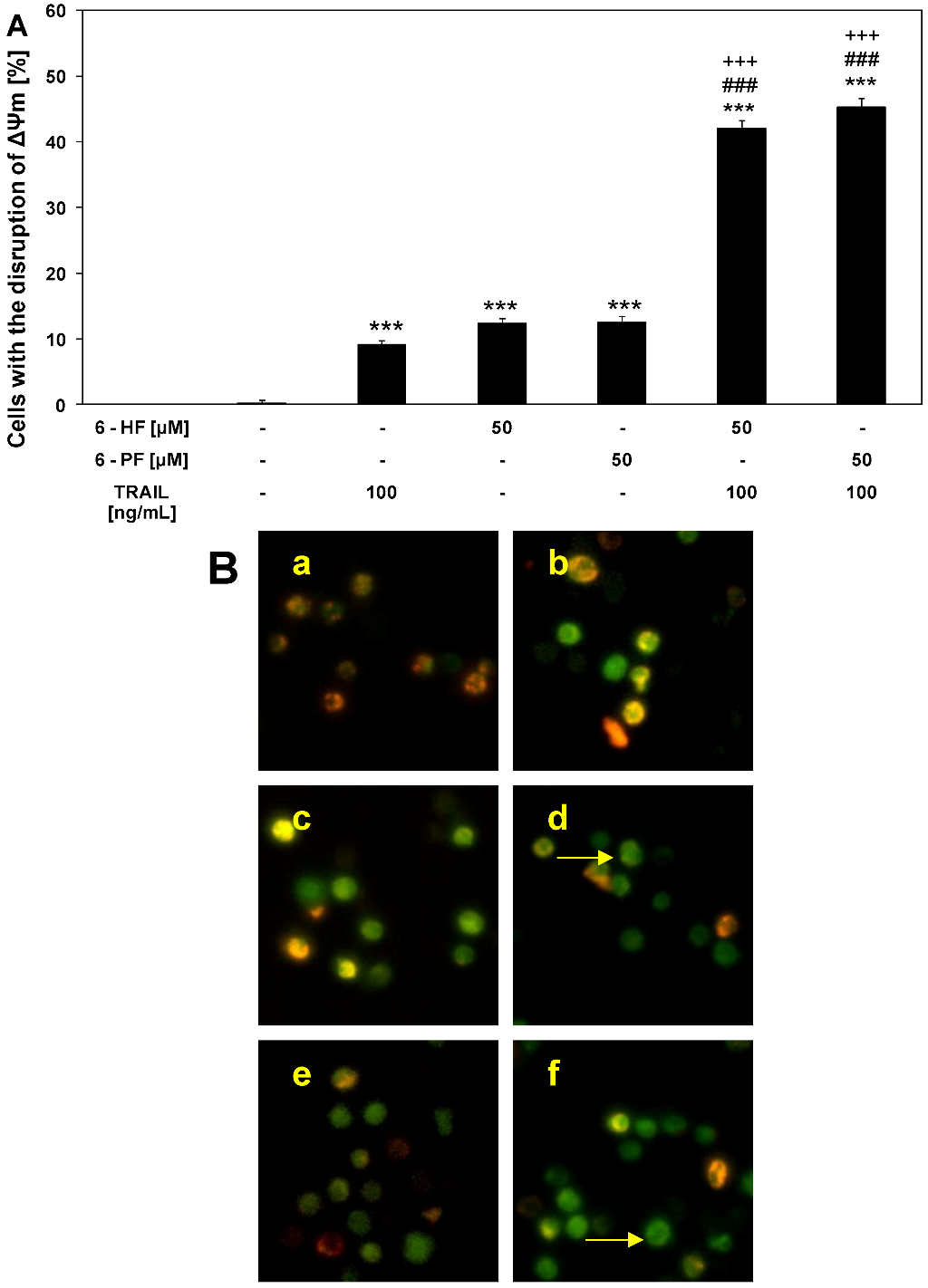
2.5. Effects of TRAIL and Synthetic Flavanones on Mitochondrial Membrane Potential (ΔΨm) in HeLa Cancer Cells
3. Experimental
3.1. Chemistry
3.1.1. General
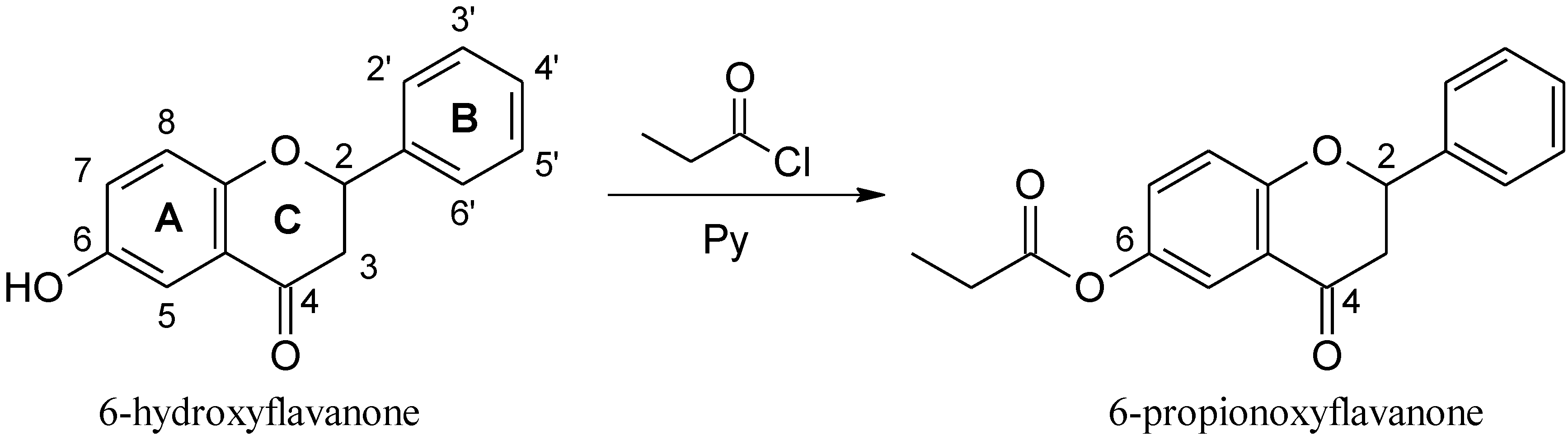
3.1.2. Synthesis of 6-PF
3.2. Biological Methods
3.2.1. Reagents
3.2.2. Cancer Cells
3.2.3. Detection of Cell Death Using MTT Colorimetric Assay
3.2.4. Lactate Dehydrogenase Release Assay
3.2.5. Determination of Apoptosis by Flow Cytometry with Annexin V-FITC Staining
3.2.6. Determination of Apoptosis by Fluorescence Microscopy with Annexin V-FITC Staining
3.2.7. Flow Cytometric Analysis of Death Receptor Expression on the Cancer Cell Surface
3.2.8. Evaluation of Mitochondrial Membrane Potential by DePsipher
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Androutsopoulos, V.P.; Papakyriakou, A.; Vourloumis, D.; Tsatsakis, A.M.; Spandidos, D.A. Dietary flavonoids in cancer therapy and prevention: Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol. Ther. 2010, 126, 9–20. [Google Scholar] [CrossRef]
- Krol, W.; Shani, J.; Czuba, Z.; Scheller, S. Modulation luminol-dependent chemiluminescence of neutrophils by flavones. Z. Naturforsch. C 1992, 47, 889–992. [Google Scholar]
- Vanamala, J.; Leonardi, T.; Patil, B.S.; Taddeo, S.S.; Murphy, M.E.; Pike, L.M.; Chapkin, RS.; Lupton, J.R.; Turner, N.D. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006, 27, 1257–1265. [Google Scholar] [CrossRef]
- Turner, N.D.; Paulhill, K.J.; Warren, C.A.; Davidson, L.A.; Chapkin, R.S.; Lupton, J.R.; Carroll, R.J.; Wang, N. Quercetin suppresses early colon carcinogenesis partly through inhibition of inflammatory mediators. Acta Hortic. 2009, 841, 237–242. [Google Scholar]
- Warren, C.A.; Paulhill, K.J.; Davidson, L.A.; Lupton, J.R.; Taddeo, S.S.; Hong, M.Y.; Carroll, R.J.; Chapkin, R.S.; Turner, N.D. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J. Nutr. 2009, 139, 101–105. [Google Scholar]
- Patil, B.S.; Jayaprakasha, G.K.; Chidambara-Murthy, K.N.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities and challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Leonardi, T.; Vanamala, J.; Taddeo, S.S.; Davidson, L.A.; Murphy, M.E.; Patil, B.S.; Wang, N.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R.; et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. 2010, 235, 710–717. [Google Scholar] [CrossRef]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264. 7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Carvalho, A.M.; Ferreira, I.C.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal home gardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef]
- Lee, J.Y.; Huerta-Yepez, S.; Vega, M.; Baritaki, S.; Spandidos, D.A.; Bonavida, B. The NO TRAIL to YES TRAIL in cancer therapy. Int. J. Oncol. 2007, 31, 685–691. [Google Scholar]
- Holoch, P.A.; Griffith, T.S. TNF-related apoptosis-inducing ligand (TRAIL): A new path to anti-cancer therapies. Eur. J.Pharmacol. 2009, 625, 63–72. [Google Scholar] [CrossRef]
- Szliszka, E.; Mazur, B.; Zydowicz, G.; Czuba, Z.P.; Krol, W. TRAIL-induced apoptosis and expression of death receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia Histochem. Cytobiol. 2009, 47, 579–585. [Google Scholar] [CrossRef]
- Mellier, G.; Huang, S.; Shenoy, K.; Pervaiz, S. TRAILing death in cancer. Mol. Aspects Med. 2010, 31, 93–112. [Google Scholar] [CrossRef]
- Russo, M.; Mupo, A.; Spagnuolo, C.; Russo, G.L. Exploring death receptor pathways as selective targets in cancer therapy. Biochem. Pharmacol. 2010, 80, 674–682. [Google Scholar] [CrossRef]
- Szliszka, E.; Krol, W. The role of dietary polyphenols in tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur. J. Cancer Prev. 2011, 20, 63–69. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Bronikowska, J.; Mertas, A.; Paradysz, A.; Krol, W. Ethanolic extract of propolis (EEP) augments TRAIL-induced apoptotic death in prostate cancer cells. Evid. Based Complement. Alternat. Med. 2011, 2011, 535172. [Google Scholar]
- Szliszka, E.; Zydowicz, G.; Janoszka, B.; Dobosz, C.; Kowalczyk-Ziomek, G.; Krol, W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2011, 38, 941–953. [Google Scholar]
- Zhang, L.; Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005, 12, 228–237. [Google Scholar] [CrossRef]
- Horinanka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervival cancer HeLa cells. Biochem. Biophys. Res. Comun. 2005, 333, 833–838. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Ther. 2006, 5, 945–951. [Google Scholar] [CrossRef]
- Son, Y.G.; Kim, E.H.; Kim, J.Y.; Kim, S.U.; Kwon, T.K.; Yoon, A.R.; Yun, C.O.; Choi, K.S. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 upregulation and downregulation of cFLIP and surviving. Cancer Res. 2007, 67, 8274–8284. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Zhuang, J.; Zhang, L.; Lin, Y. Induction of death receptor 5 and suppression of surviving contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small lung cancer cells. Carcinogenesis 2007, 28, 2114–2121. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, E.H.; Park, S.S.; Lim, J.H.; Kwon, T.K.; Choi, K.S. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 upregulation and proteasome-mediated c-FLIPS downregulation. J. Cell. Biochem. 2008, 105, 1386–1398. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Jernas, K.; Krol, W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int. J. Mol. Sci. 2008, 9, 56–64. [Google Scholar] [CrossRef]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef]
- Tanaguchi, H.; Yoshida, T.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Konishi, M.; Wakada, M.; Kataoka, K.; Yoshikawa, T.; Sakai, T. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell specific pathways in cancer cells but not in normal cells. Cancer. Res. 2008, 68, 8918–8927. [Google Scholar]
- Jung, Y.H.; Heo, J.; Lee, Y.J.; Kwon, T.K.; Kim, Y.H. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci. 2010, 86, 351–357. [Google Scholar] [CrossRef]
- Szliszka, E.; Gebka, J.; Bronikowska, J.; Krol, W. Dietary flavones enhance the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on bladder cancer cells. Cent. Eur. J. Urol. 2010, 63, 138–143. [Google Scholar] [CrossRef]
- Bronikowska, J.; Szliszka, E.; Czuba, Z.P.; Zwolinski, D.; Szmydki, B.; Krol, W. The combination of TRAIL and isoflavones enhances apoptosis in cancer cells. Molecules 2010, 15, 2000–2015. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2010, 11, 1–13. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Paradysz, A.; Krol, W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 2010, 15, 5336–5353. [Google Scholar] [CrossRef]
- Szliszka, E.; Bronikowska, J.; Czuba, Z.P.; Krol, W. Isoflavones augment the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on prostate cancer cells. Cent. Eur. J. Urol. 2010, 63, 182–186. [Google Scholar] [CrossRef]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar]
- Szliszka, E.; Krol, W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncol. Rep. 2011, 26, 533–541. [Google Scholar]
- Jin, C.Y.; Park, C.; Hwang, H.J.; Kim, G.Y.; Choi, B.T.; Kim, W.J.; Choi, Y.H. Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Mol. Nutr. Food Res. 2011, 55, 300–309. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Mertas, A.; Paradysz, A.; Krol, W. The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol. Oncol. 2011. [Google Scholar]
- Wudtiwai, B.; Sripanidkulchai, B.; Kongtawelert, P.; Banjerdpongchai, R. Metoxyflavone derivatives modulates the effect of TRAIL-induced apoptosis in human leukemic cell lines. J. Hematol. Oncol. 2011, 4, 52–63. [Google Scholar] [CrossRef]
- Ding, J.; Polier, G.; Köhler, R.; Giaisi, M.; Krammer, P.H.; Li-Weber, M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J. Biol. Chem. 2012, 287, 641–649. [Google Scholar]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones and flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Park, J.H.; Jin, C.Y.; Lee, B.K.; Kim, G.Y.; Choi, Y.H.; Jeong, Y.K. Naringenin induces apoptosis through downregulation of Akt and caspase-3 activation in human leukemia THP-1 cells. Food Chem. Toxicol. 2008, 46, 3684–3690. [Google Scholar] [CrossRef]
- Jin, C.Y.; Park, C.; Lee, J.H.; Chung, K.T.; Kwon, T.K.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Naringenin-induced apoptosis is attenuated by Bcl-2 but restored by small molecule Bcl-2 inhibitor, HA 14–1 in human leukemia U977 cells. Toxicol. In Vitro 2009, 23, 259–265. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through caspase-3 activation in human colon cells SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef]
- Ghorbani, A.; Nazari, M.; Jeddi-Tehrani, M.; Zang, H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: Involvement of PPARγ-dependent mechanism. Eur. J. Nutr. 2012, 51, 39–46. [Google Scholar]
- Zhang, S.; Zhou, Y.; Liu, Y.; Cai, Y. Effect of liquiritigenin, a flavone existed from Radix glycyrrhizae on pro-apoptotic in SMMC-7721 cells. Food Chem. Toxicol. 2009, 47, 693–701. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Xie, S.; Zhou, Y.; Ren, X.; Li, X.; Cai, Y. Liquiritigenin induces mitochondria-mediated apoptosis via cytochrome c release and caspases activation in HeLa cells. Phytother. Res. 2011, 25, 277–283. [Google Scholar]
- Ko, C.H.; Shen, S.; Chen, Y. Hydroxylation at C4' or C6 is essential for apoptosis-inducing activity of flavanone through activation of the casapase-3 cascade and production of reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 897–910. [Google Scholar] [CrossRef]
- Szliszka, Ewelina. Medical University of Silesia, Katowice, ON. Unpublished work, 2012.
- Bronikowska, J.; Szliszka, E.; Jaworska, D.; Czuba, Z.P.; Krol, W. The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 6449–6464. [Google Scholar] [CrossRef]
- Jacquemin, G.; Granci, V.; Gallouet, A.S.; Lalaoui, N.; Morle, A.; Iessi, E.; Morizot, A.; Garrido, C.; Guillaudeux, T.; Micheau, O. Quercetin-mediated Mcl-1 and surviving downregulation restores TRAIL-induced apoptosis in non-Hodgkin’s lymphoma B cells. Haematologica 2012, 97, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Białońska, A.; Ciunik, Z.; Rymowicz, W. Microbial transformations of flavanone and 6-hydroxyflavanone by Aspergillus niger strains. J. Mol. Catal. B Enzym. 2006, 39, 18–23. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Białońska, A.; Janeczko, T. 4-Oxo-2-phenylchroman-6-yl propionate. Acta Cryst. 2010, E66, o1401. [Google Scholar]
- Szliszka, E.; Majcher, A.; Domino, M.; Pietsz, G.; Krol, W. Cytotoxic activity of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) against bladder cancer cells after using chemotherapeutic drugs. Urol. Pol. 2007, 60, 138–142. [Google Scholar]
- Szliszka, E.; Bronikowska, J.; Majcher, A.; Miszkiewicz, J.; Krol, W. Enhanced sensitivity of hormone-refractory prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated cytotoxicity by taxanes. Cent. Eur. J. Urol. 2009, 62, 29–34. [Google Scholar]
- Szliszka, E.; Czuba, ZP.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Sedek, L.; Paradysz, A.; Krol, W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011, 63, 139–148. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Kawczyk-Krupka, A.; Sieron-Stoltny, A.; Sieron, A.; Krol, W. Chlorin-based photodynamic therapy enhances the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in bladder cancer cells. Med. Sci. Monit. 2012, 18, BR47–53. [Google Scholar]
- Szliszka, E.; Zydowicz, G.; Mizgala, E.; Krol, W. Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) sensitizes prostate cancer LNCaP cells to TRAIL-induced apoptosis. Int. J. Oncol. 2012, 41, 818–828. [Google Scholar]
- Sample Availability: Samples of the compounds are available by Edyta Kostrzewa-Susłow (Polish Patent, 2011, P-390768, Kostrzewa-Susłow E.; Dmochowska-Gładysz J.; Janeczko T.; Białońska A.).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szliszka, E.; Kostrzewa-Susłow, E.; Bronikowska, J.; Jaworska, D.; Janeczko, T.; Czuba, Z.P.; Krol, W. Synthetic Flavanones Augment the Anticancer Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Molecules 2012, 17, 11693-11711. https://doi.org/10.3390/molecules171011693
Szliszka E, Kostrzewa-Susłow E, Bronikowska J, Jaworska D, Janeczko T, Czuba ZP, Krol W. Synthetic Flavanones Augment the Anticancer Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL). Molecules. 2012; 17(10):11693-11711. https://doi.org/10.3390/molecules171011693
Chicago/Turabian StyleSzliszka, Ewelina, Edyta Kostrzewa-Susłow, Joanna Bronikowska, Dagmara Jaworska, Tomasz Janeczko, Zenon P. Czuba, and Wojciech Krol. 2012. "Synthetic Flavanones Augment the Anticancer Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)" Molecules 17, no. 10: 11693-11711. https://doi.org/10.3390/molecules171011693




